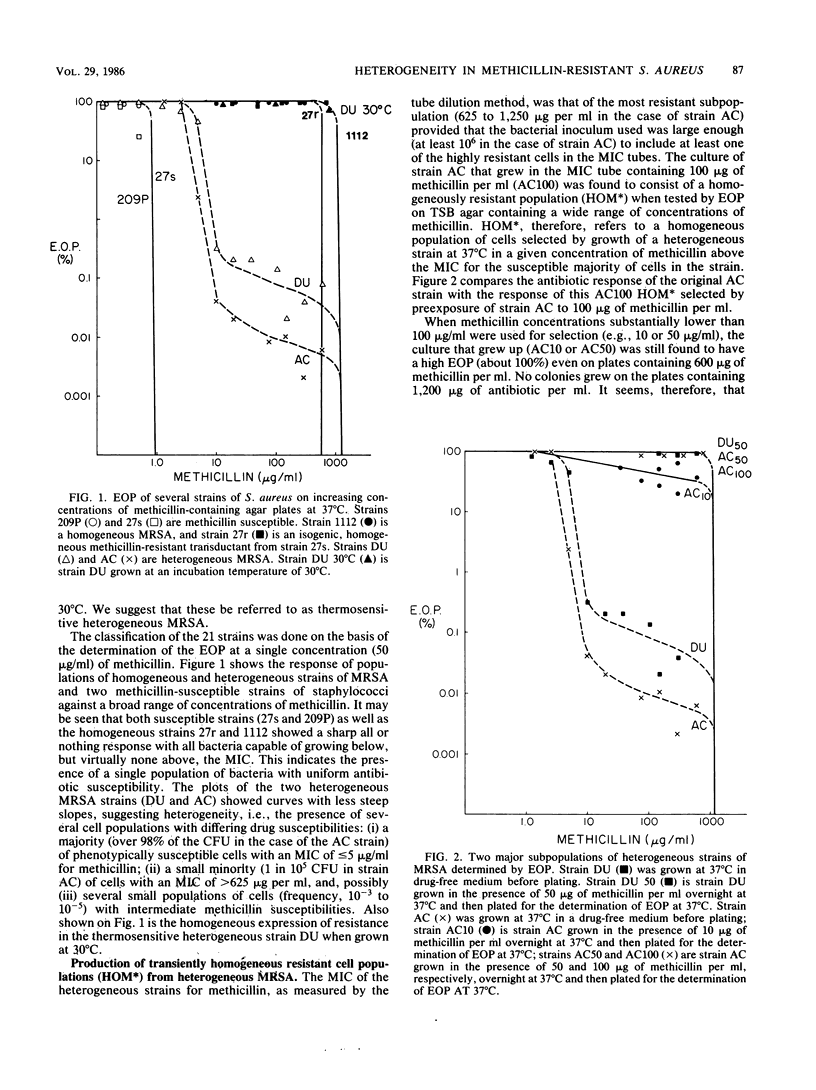
Abstract
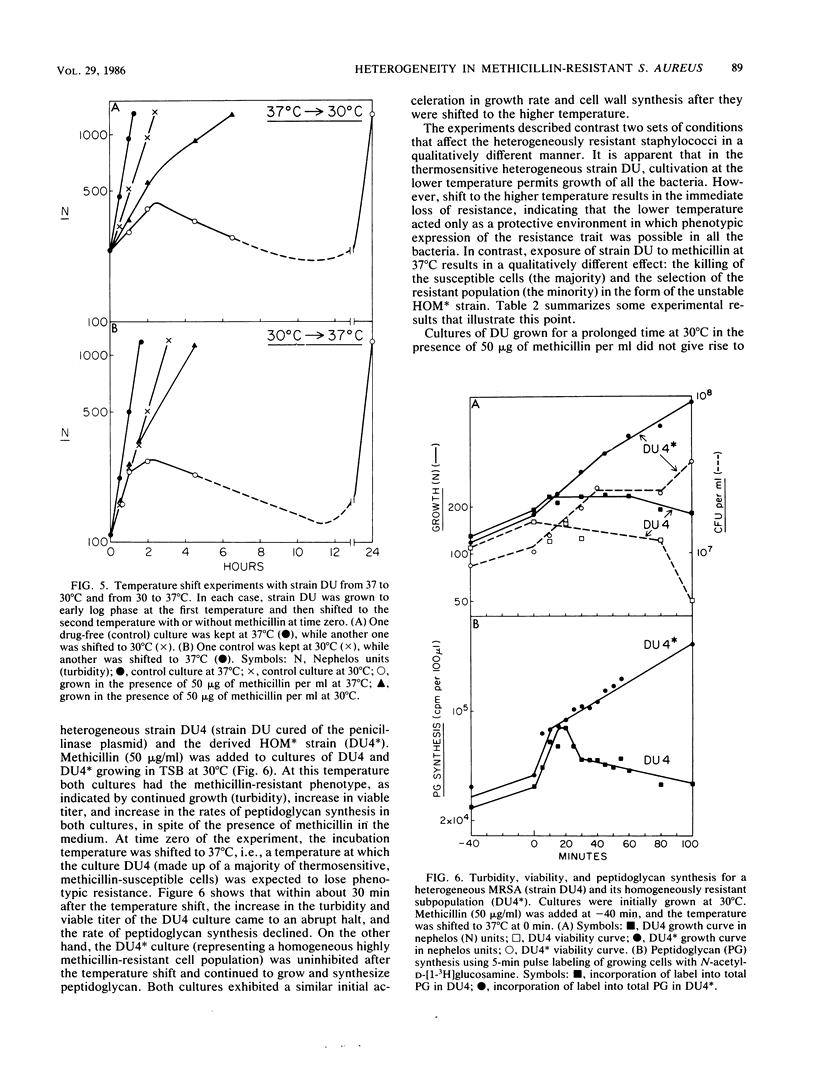
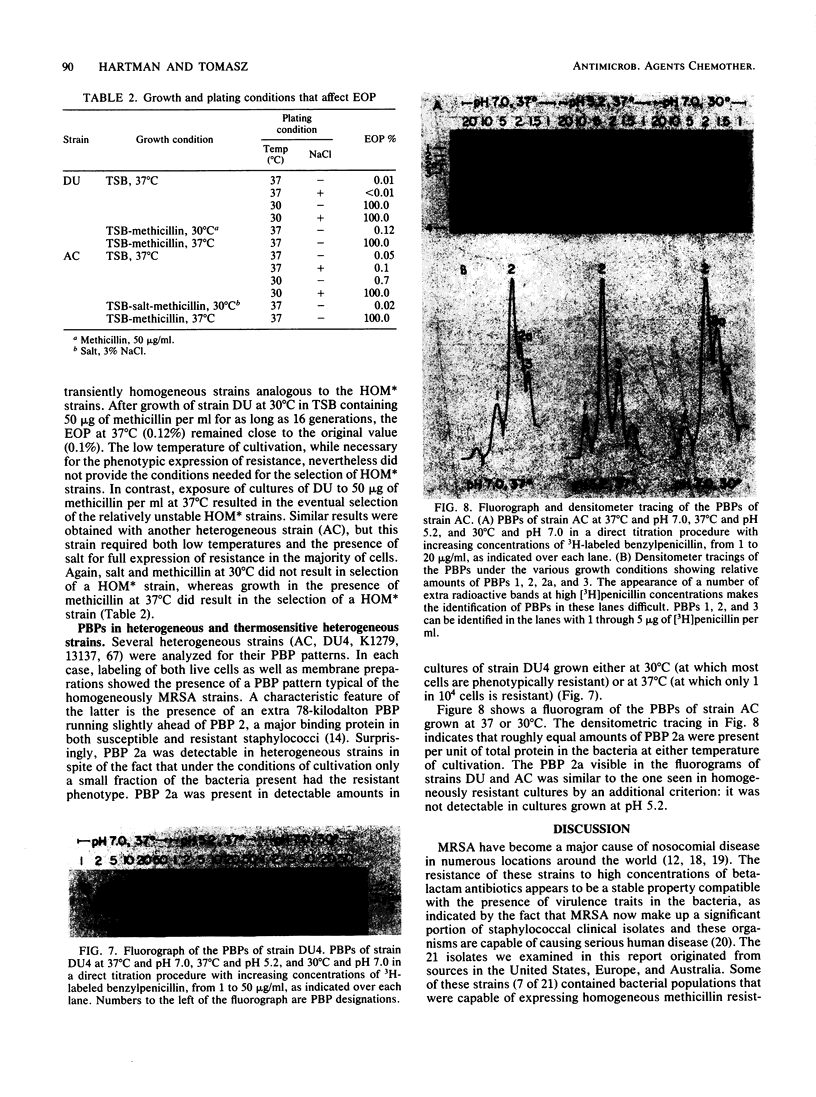
The phenotypic expression of methicillin resistance was studied in a number of clinical isolates and laboratory strains of Staphylococcus aureus. The methicillin-resistant S. aureus strains could be divided into three classes, homogeneous, heterogeneous, and thermosensitive heterogeneous methicillin-resistant S. aureus, on the basis of their plating efficiencies at 30 or 37 degrees C on methicillin-containing agar plates. Heterogeneous strains of methicillin-resistant S. aureus were composed of two subpopulations: a small minority of cells (10(-5) to 10(-3); MIC, 600 to 1,000 micrograms/ml) that expressed resistance to high concentrations of methicillin at 37 degrees C, and a majority of cells (MIC, 5 micrograms/ml) that remained susceptible to the drug at 37 degrees C. Cultures of a thermosensitive heterogeneous strain were able to grow in the presence of high concentrations of methicillin, provided that the growth temperature was 30 degrees C. Such cultures lost their phenotypic resistance within 30 min (i.e., in less than one doubling time) after the growth temperature was shifted to the nonpermissive 37 degrees C. Shift of the temperature of the culture in the reverse direction (37 to 30 degrees C) resulted in an equally rapid expression of phenotypic resistance. The majority of the cells in such heterogeneous strains may be considered heat (or salt) conditional in their phenotypic expression of methicillin resistance. Both heterogeneous and thermosensitive heterogeneous strains, irrespective of their temperature of cultivation and degree of phenotypic resistance, contained detectable quantities of the 78-kilodalton penicillin-binding protein 2a (PBP 2a) that previous studies have suggested is a biochemical correlate of methicillin resistance in homogeneous strains of methicillin-resistant S. aureus. However, in contrast to the homogeneous stains, in heterogeneous and thermosensitive heterogeneous isolates the ability to synthesize PBP 2a is apparently not sufficient to provide a resistant phenotype. In these strains some additional, as yet undefined factor(s) is also needed for the expression of methicillin resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annear D. I. The effect of temperature on resistance of Staphylococcus aureus to methicillin and some other antibioics. Med J Aust. 1968 Mar 16;1(11):444–446. [PubMed] [Google Scholar]
- BARBER M. NATURALLY OCCURING METHICILLIN-RESISTANT STAPHYLOCOCCI. J Gen Microbiol. 1964 May;35:183–190. doi: 10.1099/00221287-35-2-183. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Bruns W., Keppeler H. Mechanism of intrinsic penicillin-resistance in Staphylococcus aureus. Binding of penicillin G to the cytoplasmic membrane of resistant staphylococci. Arzneimittelforschung. 1980;30(9):1469–1475. [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):261–278. doi: 10.1099/00222615-2-3-261. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. T., Hewitt J. H. Methicillin resistance in Staphylococcus aureus. Lancet. 1970 Apr 18;1(7651):800–804. doi: 10.1016/s0140-6736(70)92408-6. [DOI] [PubMed] [Google Scholar]
- Pavillard R., Harvey K., Douglas D., Hewstone A., Andrew J., Collopy B., Asche V., Carson P., Davidson A., Gilbert G. Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust. 1982 May 29;1(11):451–454. [PubMed] [Google Scholar]
- Peacock J. E., Jr, Moorman D. R., Wenzel R. P., Mandell G. L. Methicillin-resistant Staphylococcus aureus: microbiologic characteristics, antimicrobial susceptibilities, and assessment of virulence of an epidemic strain. J Infect Dis. 1981 Dec;144(6):575–582. doi: 10.1093/infdis/144.6.575. [DOI] [PubMed] [Google Scholar]
- Sabath L. D. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):47–51. doi: 10.1093/jac/3.suppl_c.47. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Byers K., Toftegaard I. Resistance of Staphylococcus aureus to penicillins and cephalosporins: reversal of intrinsic resistance with some chelating agents. Ann N Y Acad Sci. 1974 Jul 31;236(0):435–443. doi: 10.1111/j.1749-6632.1974.tb41508.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Gerstein D. A. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Nov;2(5):350–355. doi: 10.1128/aac.2.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J. The problems of drug-resistant pathogenic bacteria. Factors influencing methicillin resistance in staphylococci. Ann N Y Acad Sci. 1971 Jun 11;182:258–266. doi: 10.1111/j.1749-6632.1971.tb30662.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Wong W., Chatterjee A. N., Young F. E. Regulation of bacterial cell walls: correlation between autolytic activity and cell wall turnover in Staphylococcus aureus. J Bacteriol. 1978 May;134(2):555–561. doi: 10.1128/jb.134.2.555-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]