Abstract
Objectives
To provide students with an understanding of the principles and applications of human genetics and genomics in drug therapy optimization, patient care, and counseling.
Design
A 2-credit hour course entitled Principles of the Human Genome, Pharmacogenomics, and Bioinformatics was offered to third-professional year PharmD students. Written examinations, in-class exercises, and a written paper evaluating the current literature were used to evaluate student learning.
Assessment
Student course ratings on the pedagogical format of the course and the relevance of course material to professional practice have improved significantly since first implementation in 2002.
Conclusion
This course provided pharmacy students with an understanding of pharmacogenetics ranging from genetic principles and the inheritance of complex traits to specific examples of pharmacogenomics in drug therapy.
Keywords: pharmacogenomics, pharmacogenetics, new course, genomics, bioinformatics
INTRODUCTION
The concept that human genetic variation affects how individuals respond to drugs has been recognized for over 45 years. The term pharmacogenetics was first coined by Vogel1 in 1959 following an earlier summary by Motulsky2 concerning inter-individual drug response and its relationship to genetics (as understood to be the study of patterns of inheritance). Now, as a result of the genetic information made available through the Human Genome Project, the extent to which this information is accumulating in the biomedical and pharmaceutical literature is staggering. Equally exciting is the promise this information holds in understanding the role that encoded variation in complex biological systems has in human drug response. Such information will ultimately transform the health sciences and require health professionals to be knowledgeable about a vast new area of basic science: genomics.3 The integration of this knowledge in pharmacy education is particularly important since genomics probably will be felt most immediately in the pharmaceutical sciences since drug efficacy and toxicity are more tractable problems than many more complex, multifaceted health issues (eg, cancer, obesity, cardiovascular disease, mental disorders). Drugs having relatively specific targets, and polymorphisms in one or a few genes (coding for drug transporters or drug metabolizing enzymes) may alter drug disposition.
The need for pharmacogenetics and pharmacogenomics in pharmacy school curricula is immediate and, in fact, has been included in the recent ACPE Standards 2006.4 In a survey of 377 community pharmacists in the United States, less than 50% were satisfied with their knowledge of the Human Genome Project, genetic testing, and pharmacogenetics.5 Thus, practicing pharmacists realize the need for a better background in genetics and its application to pharmaceutical principles. This need has also been recognized among pharmacy educators.6 To date, pharmacogenomics has not been consistently incorporated into the curriculum for the doctor of pharmacy degree. In a 2004 survey of 85 colleges and schools of pharmacy in the United States, of the 41 respondents, only 16 schools provided any content on pharmacogenetics or pharmacogenomics to their professional students.7 Only 5 schools have a standalone course. Additionally, many students entering pharmacy programs have had only minimal exposure to genetics. For example, of the 1051 students who applied to the University at Buffalo's Pharmacy program in 2005, only 37% have taken a genetics course beyond what is incorporated in an introductory biology course. For these reasons we implemented a required course in pharmacogenetics and pharmacogenomics in 2002. This paper describes the topics included in this course, student assessments methods, and course evaluations since this class was offered in 2002.
DESIGN
A new course titled Principles of the Human Genome, Pharmacogenomics, and Bioinformatics was designed to be offered during the fall semester of the third-professional year. The course was first offered in spring 2002 and then every fall semester beginning in 2002. The course was assigned 2 credit hours and is a part of the required curriculum. The course was administered by 1 faculty member, but 5 faculty members from the Department of Pharmaceutical Sciences and the Department of Pharmacy Practice provided guest lectures in key areas during the final 5 weeks of the course. These guest lectures were intended to provide specific examples of the role of pharmacogenetics and pharmacogenomics technologies in clinical and research settings. The general educational outcomes of the course were based on the American Society of Human Genetics guidelines for a medical school core curriculum in genetics.8 The course outcomes for what students should know include:
What genes are and how they are organized and regulated;
How alleles segregate in and among populations;
Environmental and genetic factors that affect development of the phenotype, including drug response;
The multifactorial nature of most human traits, including drug response and the principles of multifactorial inheritance;
How polymorphisms arise and are maintained in human populations, and how gene linkage and human gene mapping are used to identify candidate genes;
How human genetic variation affects drug metabolism, activation, and disposition;
The advantages, limitations, and dangers of predictive testing for genetic disease and drug response;
How to navigate among the many comprehensive genomic databases and resources on the Internet;
The genomic technologies employed in drug discovery and development; and
Legal and ethical issues in genetic testing and patient stratification in clinical trials.
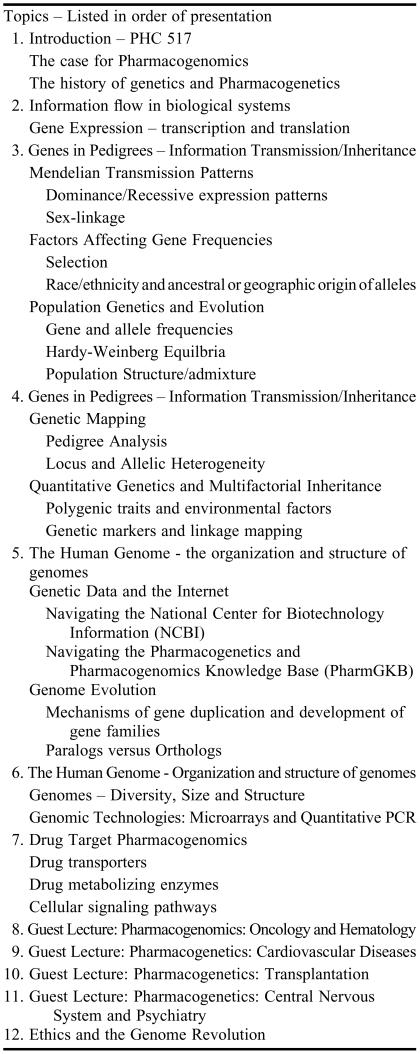
The class met once a week for 100 minutes. Lecture topics are shown in Table 1. Following an introduction to the history of pharmacogenetics and pharmacogenomics in the first class, a basic review of DNA and RNA structure leading on to mechanisms of gene expression and gene regulation was presented in the second week. During the third week of the course, how genetic traits are acquired, maintained, and distributed among populations (traditional population genetics) was discussed. Complex or multifactorial patterns of inheritance were covered in the fourth week, along with issues relating to genetic mapping of complex traits. In the first 4 weeks of the course, each of these basic genetic concepts were presented using examples from the pharmaceutical sciences. For example, population genetics and allelic differences among populations were discussed in the context of the geographic distribution of variants for many drug metabolizing enzymes.9 In the fifth and sixth weeks, the structure, evolution, and organization of genomes and the sorts of bioinformatic tools available on the Internet were covered. Also covered was a brief discussion of the most important genomic technologies involved in drug discovery, drug development, and genotyping, including DNA sequencing, microarrays, and the quantitative polymerase chain reaction (QPCR). These technologies were discussed in the specific context of drug discovery and development, and in human genotyping of genes involved in drug metabolism and transport. The final 5 weeks were used to review current case studies or examples of pharmacogenetics and drug response. Included were discussions of the pharmacogenetics of drug metabolizing enzymes, drug transporters, cancer therapeutics, organ transplantations, gene therapies, and multiple sclerosis. Guest faculty members were encouraged to use examples of pharmacogenetic principles or methodologies from their research. The final class period was devoted to a discussion of the ethical issues involved in genetic testing, patient stratification, and clinical trials.
Table 1.
Lecture Topics in Principles of the Human Genome, Pharmacogenomics, and Bioinformatics Course*
*The course meets once a week for 100 minutes
One textbook was required, Pharmacogenomics. Applications to Patient Care Modules 1, 2, and 3.10 A second text, Human Molecular Genetics, Third Edition11 was strongly recommended. Readings from the current literature were assigned to provide additional background and relevancy to the lecture topics.
Since this was a third-year course for students who would soon begin their advanced pharmacy practice experiences, the course was developed with a format including didactic lectures with active learning in-class activities, a written paper, and examinations composed entirely of written essay questions (Table 2). A unique feature implemented in the development of this course was the decision to minimize the distribution of prepared class notes by faculty members. As such, students were not provided with notes or note packets prior to each class. The rationale for this decision was that the ability to listen carefully and take accurate notes was an important skill that our students needed to develop. Providing students with the notes prior to each class would not have furthered this goal.12
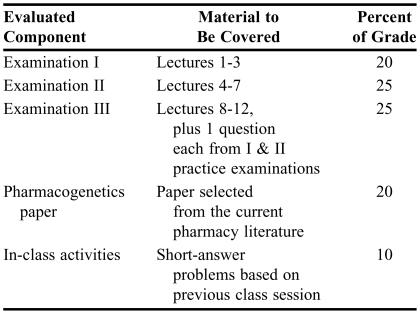
Table 2.
Student Assessment Criteria Used in a Pharmacogenomics Course
Examinations covered approximately one third of the material each and were entirely short answer and essay. Each of the guest faculty members submitted questions for the examinations, which were compiled by the course coordinator. Many of the questions utilized a case-based approach. All examination questions from the previous years were posted online as “practice examinations.” Approximately 100 questions for the 3 practice examinations were available to the students. To encourage student-centered learning, the answers were not provided. The students were encouraged to work out the answers in groups and to meet with faculty members to discuss their answers. Each examination covered material presented since the last examination, though the third examination did include 1 question each from earlier course material. These “comprehensive” questions were taken verbatim from the practice examinations.
In-class exercises were given nearly every week and consisted of short-answer problems concerning material covered in the previous lecture or in the assigned readings. The educational goal of the in-class exercises was to keep the students current with the lecture materials and readings and to promote active learning and life-long learning of the topic. An additional benefit was that the in-class work assured nearly 100% attendance. Finally, students were required to write a 2-3 page paper summarizing a current pharmacogenetics paper from the primary literature (Appendix 1). This paper was to be a summary of the recent research, explained at a level such that their fellow students would understand what was done and why it was done. The students had to choose a paper from the current calendar year. This eliminated the possibility of copying the work of students who had taken the course in previous years. Students had no difficulty in finding papers within the current year as the number of publications in the area of pharmacogenomics, pharmacogenetics, and bioinformatics is expanding rapidly. Students had to choose their paper within the first 5 weeks of the course and confirm their choice with the course coordinator in order to avoid multiple students choosing the same paper. Chosen papers were posted weekly so that students could avoid choosing a paper already selected by a classmate. In order to assist students in their writing skills, the instructor provided comments on draft papers. On the due date, students had to submit both a hard copy and an electronic copy of their paper. Students were informed that the electronic copy would be checked for plagiarism using Turnitin (iParadigms, Oakland, Calif, 2006).
Feedback from the students was solicited using CourseEval (Academic Management Systems, Buffalo, NY, 2006). Students could evaluate the entire course and each instructor. The evaluation process was done online and all responses were anonymous. Students could submit their evaluations as early as the tenth week of the course, though most did not complete the process until the final weeks. Significance of student responses was evaluated using the Student's t test, corrected for multiple comparisons.
RESULTS
Five hundred twenty-six students have taken the course since spring of 2002. Approximately 95% were in the PharmD program, and the remaining students were bachelor of science, master of science, and doctor of philosophy students in the pharmaceutical sciences.
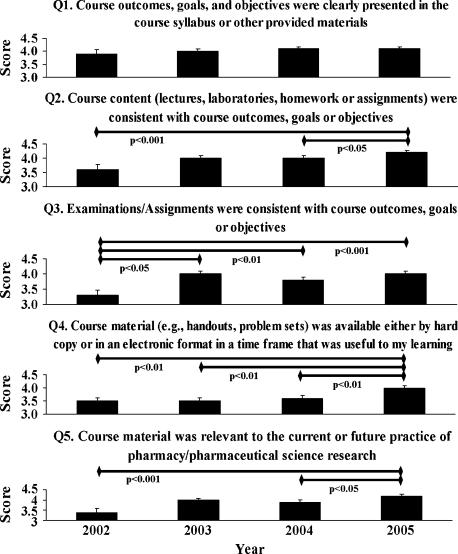
Students’ scores exhibit the typical bell-shaped curve, with approximately 57% scoring 80% or higher. Student course evaluations for the 2002-2005 fall semesters were evaluated and the results are shown in Figure 1. Between 2003-2005, approximately 83% (range 77% to 88%) of students completed the course evaluation. In 2002, only 48% of the students completed the required course evaluation. However, this was the first year online course evaluations were utilized in the School and student response rates were low for all courses as students became familiar with the program. Over the 4 years since this course was offered in the fall of the third-professional year, on average, students rated this course a 4 (Likert scale of 1 to 5) with respect to the course outcomes, goals, and objectives being clearly presented in the course syllabus or other provided materials (Q1, Figure 1). Specifically, approximately 76%, 83%, 87%, and 87% of students agreed or strongly agreed that the course outcomes, goals, and objectives were clearly presented in 2002, 2003, 2004, and 2005, respectively. There was a progressive increase in the student ratings with respect to the course content being consistent with the outcomes, goals, and objectives as shown by the mean values and percentage of students who agreed or strongly agreed (Q2, Figure 1). Student ratings increased significantly from 2002 to 2005 (mean 3.6 to 4.2 respectively; p < 0.001, Figure 1) and from 2004 to 2005 (mean 4.0 to 4.2, respectively; p < 0.05, Figure 1).
Figure 1.
Course Evaluations from 2002-2005. Mean and standard errors of student response are based upon a score of 1 = strongly disagree, 2 = disagree, 3 = neither disagree or agree, 4 = agree, and 5 = strongly agree. Evaluations are done online and can be completed at anytime following the tenth week of the semester. Evaluations are voluntary; students are not required to complete the survey. Horizontal lines denote significant pair wise differences between years.
With respect to examinations and course assignments being consistent with course outcomes, goals, or objectives, there was a significant increase in the mean value of 3.3 in 2002 to an approximate value of 3.9 from 2003 though 2005 (Q3, Figure 1). The same trend is observed when evaluating the percentage of students who agreed or strongly agreed with this statement being 54% in 2002, 82% in 2003, 72% in 2004, and 76% in 2005. Based on student written comments, the written paper and essay examination format was one area of controversy as students did not see the relevance of the writing assignment and found the essay examination format challenging based upon their previous educational experiences in the curriculum (multiple-choice examinations). Student evaluations with respect to the availability of course materials by hard copy or an electronic format similarly increased significantly from 3.5 in 2002 to 4.0 in 2005 (Q4, Figure 1). Since many of the required courses provided written notes either online or in a packet, students understandably came to expect these and expressed their concern when this material was not provided.
Finally, there was a consistent significant increase in student ratings in the perceived relevancy of this course over the 4 years (Q5, Figure 1). Mean student ratings of the relevance of this course material to the future practice of pharmacy and research in the pharmaceutical sciences were 3.4, 4.0, 3.9, and 4.2 from 2002 through 2005, respectively. These findings are more dramatically illustrated by looking at the percentage of students who agreed or strongly agreed with this statement: 54% in 2002, 77% in 2003, 75% in 2004, and 89% in 2005.
DISCUSSION
The principal criticism of the course by students has been the relevancy of the material. Many of the students did not see the importance of genetics as it pertains to the current perceived daily activities of the pharmacist. In some respects this criticism is unavoidable given the newness of this field. Applications of pharmacogenetic data and concepts are just beginning to appear in clinical settings (eg, drug labeling, including statements related to patient specific genetics) and are seldom encountered in community pharmacies. Impressing upon students that their training must anticipate future developments as well as current issues has been challenging. The course has continually been fine tuned to included materials and case studies demonstrating the growing importance of pharmacogenetics in the practice of pharmacy. For example, specific cases from local and national newspapers describing the application of genomics to patient care were highlighted each week. Student evaluations indicated that relevancy was becoming less of an issue, probably due to course improvements and an increasing number of references to this material in other courses, as well as increasing publications in the professional literature and media coverage of pharmacogenetic issues. Similarly, student evaluations concerning course assignments and examinations have also improved. The course has been offered now for more than 5 years and apparently written examinations and course assignments have become more accepted.
CONCLUSIONS
A new required course entitled Principles of the Human Genome, Pharmacogenomics, and Bioinformatics was introduced to the third-year professional PharmD curriculum to strengthen the backgrounds of our students in the latest genomic and bioinformatics technologies and data. Evidence that the objectives of the course are being met are seen in improving students evaluations as well as anecdotal comments from former students who note they are among the few with pharmacogenomic training among their peers.
The course will continue to be offered as a required course in the third-professional year. The relevance of course topics to professional practice will continue to be emphasized, as will ways in which pharmacists can collaborate with other health care professionals both as a resource on pharmacogenetic information and in patient counseling.
Appendix 1. Directions for the written assignment for the Principles of the Human Genome, Pharmacogenomics, and Bioinformatics course
Principles of the Human Genome, Pharmacogenomics, and Bioinformatics Written Assignment
Choose a scientific research paper (the “foundation” paper) in Pharmacogenomics or Pharmacogenetics from the primary literature and write a brief review of the findings of the paper and its importance in pharmaceutical sciences. In doing this you should find (and read) at least three other papers in the same area to help you summarize the important findings of your “foundation” paper. The length of paper must be no less than two pages, and no more than three pages (excluding the title page and references). Manuscripts must be double-spaced, and have the right justification removed. Margins must be 1 inch on all sides, top and bottom. Font must be 12 pt. You must cite at least 3 other relevant references in your paper.
Choosing a “foundation” paper – This is the paper you have chosen to use as the focus of your mini-review. Rules for selection of your “foundation” paper:
The paper must be from the primary literature and report on experimental data concerning some aspect of pharmacogenomics or pharmacogenetics. No review papers can be used.
The paper must have been published this year!!!!
Each student will have to choose a different paper (i.e. two students cannot use the same paper). Papers will be accepted on a first come basis. You may submit the complete citation at any time. If two students submit the same paper the student's paper with the earliest submittal time will be accepted. The other student must find another paper and submit again. Paper submittals must be emailed to the course teaching assistant. We will post submitted paper titles on the course website weekly.
Scoring is as follows (100 points total):
-
Introduction 30 pts
Provide a brief statement and explanation of the nature of the problem/question being addressed in the paper. What is the hypothesis being tested in the paper?
-
Methods 20 pts
Provide a short description of the experimental methods being used. For example, exclusion criteria for clinical trials, sample sizes, genomic technologies employed
-
Discussion 40 pts
What are the primary conclusions from the paper? How are they significant? How does this paper agree or disagree with other studies? Do you think this is an important study?
Grammar & Syntax 10 pts
REFERENCES
- 1.Vogel F. Moderne probleme der humangenetik. Ergebnisse der inneren Medizin und Kinderheilkunde. Adv Int Med Pediatr. 1959;12:65–126. [Google Scholar]
- 2.Motulsky AG. Drug reactions, enzymes, and biochemical genetics. JAMA. 1957;165:835–837. doi: 10.1001/jama.1957.72980250010016. [DOI] [PubMed] [Google Scholar]
- 3.Korf BR. Integration of genetics into clinical teaching in medical school education. Genet Med. 2002;4:S33–38. doi: 10.1097/00125817-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 4. Revised Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Available at: http://www.acpe-accredit.org/standards/default.asp.
- 5.Sansgiry SS, Kulkarni AS. The Human Genome Project: assessing confidence in knowledge and training requirements for community pharmacists. Am J Pharm Educ. 2003;67(2) Article 39. [Google Scholar]
- 6. AACP Final Report of the 2001-02 Academic Affairs Committee. Pharmacogenomics: a scientific revolution in pharmaceutical sciences and pharmacy practice. Available at: http://www.aacp.org/Docs/AACPFunctions/Governance/6103_AcademicAffrsfinalreport.pdf.
- 7.Latif DA, McKay AB. Pharmacogenetics and Pharmacogenomics Instruction in colleges and schools of Pharmacy in the United States. Am J Pharm Educ. 2005;69(2) Article 23. [Google Scholar]
- 8.ASHG Report. Report from the ASHG Information and Education Committee: medical school core curriculum in genetics. ASHG Information and Education Committee. Am J Hum Genet. 1995;56:535–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JF, Weale ME, Smith AC, et al. Population genetic structure of variable drug response. Nat Genetics. 2001;29:265–29. doi: 10.1038/ng761. [DOI] [PubMed] [Google Scholar]
- 10. Pharmacogenomics. Applications to Patient Care. American College Clinical Pharmacy, Kansas City, MO. 2004.
- 11.Strachan T, Read AP. Human Molecular Genetics. 3rd ed. New York, NY: Garland Science; 2004. [Google Scholar]
- 12.Brazeau G. Handouts in the classroom: is note taking a lost skill? [editorial] Am J Pharm Educ. 2006;70(2) doi: 10.5688/aj700238. Article 38. [DOI] [PMC free article] [PubMed] [Google Scholar]