Abstract
The Arabidopsis (Arabidopsis thaliana) SPINDLY (SPY) protein negatively regulates the gibberellin (GA) signaling pathway. SPY is an O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) with a protein-protein interaction domain consisting of 10 tetratricopeptide repeats (TPR). OGTs add a GlcNAc monosaccharide to serine/threonine residues of nuclear and cytosolic proteins. Determination of the molecular defects in 14 new spy alleles reveals that these mutations cluster in three TPRs and the C-terminal catalytic region. Phenotypic characterization of 12 spy alleles indicates that TPRs 6, 8, and 9 and the catalytic domain are crucial for GA-regulated stem elongation, floral induction, and fertility. TPRs 8 and 9 and the catalytic region are also important for modulating trichome morphology and inflorescence phyllotaxy. Consistent with a role for SPY in embryo development, several alleles affect seedling cotyledon number. These results suggest that three of the TPRs and the OGT activity in SPY are required for its function in GA signal transduction. We also examined the effect of spy mutations on another negative regulator of GA signaling, REPRESSOR OF ga1-3 (RGA). The DELLA motif in RGA is essential for GA-induced proteolysis of RGA, and deletion of this motif (as in rga-Δ17) causes a GA-insensitive dwarf phenotype. Here, we demonstrate that spy partially suppresses the rga-Δ17 phenotype but does not reduce rga-Δ17 or RGA protein levels or alter RGA nuclear localization. We propose that SPY may function as a negative regulator of GA response by increasing the activity of RGA, and presumably other DELLA proteins, by GlcNAc modification.
GAs are tetracyclic diterpenoid compounds that affect many aspects of plant growth and development, including seed germination, stem elongation, flowering, and fruit and seed development (for review, see Olszewski et al., 2002; Sun and Gubler, 2004; Fleet and Sun, 2005; Swain and Singh, 2005). In Arabidopsis (Arabidopsis thaliana), spindly (spy) mutants were first identified by virtue of their resistance to both the germination inhibiting and dwarfing effects of paclobutrazol, a gibberellin (GA) biosynthesis inhibitor (Jacobsen and Olszewski, 1993). All the known spy alleles are recessive and exhibit some phenotypes similar to those of wild-type plants repeatedly treated with GA (Jacobsen and Olszewski, 1993). To varying degrees, spy mutants also suppress all of the phenotypes caused by GA deficiency (Jacobsen and Olszewski, 1993; Silverstone et al., 1997). Therefore, SPY has been hypothesized to negatively regulate GA signal transduction. However, not all of the spy mutant phenotypes (e.g. altered phyllotaxy) mimic wild-type plants that are overdosed with GA, indicating that SPY regulates additional cellular pathways (Swain et al., 2001, 2002; Tseng et al., 2004; Greenboim-Wainberg et al., 2005).
SPY encodes a 104-kD peptide with similarity to O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) from human, rat, and Caenorhabditis elegans (Jacobsen et al., 1996; Kreppel et al., 1997; Lubas et al., 1997), and SPY expressed in insect cells possesses OGT activity (Thornton et al., 1999). Both SPY and animal OGTs contain multiple copies of N-terminal tetratricopeptide repeats (TPR). TPR motifs are highly degenerate 34-amino acid repeats with eight loosely conserved residues (Blatch and Lässle, 1999). TPRs are usually present in multiple copies and act as scaffolds for assembly of multiprotein complexes with specific blocks of TPR motifs mediating the interaction with different proteins (Tzamarias and Struhl, 1995; Moir et al., 1997; Ollendorff and Donoghue, 1997; Young et al., 1998). The crystal structure of the TPR domain of human OGT indicates that, like other TPR proteins, each TPR is composed of one pair of α helices, which are packed in an antiparallel arrangement to the neighboring helices, with the domain folded into a right-handed superhelix (Jinek et al., 2004). The TPR motifs of SPY and animal OGT mediate protein-protein interactions that are important for both the substrate specificity and the assembly of the enzyme (Kreppel and Hart, 1999; Lubas and Hanover, 2000; Tseng et al., 2001, 2004). Protein interactions at the TPR domain are likely to be important for GA signaling, because ectopic expression of the SPY TPR domain alone causes changes in GA response (Tseng et al., 2001).
The C terminus of OGT is the catalytic domain. Two conserved domains (CDI and CDII) are present in the C termini of SPY and animal OGTs (Fig. 1A). Mutational analysis suggests that CDI and CDII are part of a UDP-GlcNAc binding pocket and participate in the transfer of GlcNAc (Lazarus et al., 2005).
Figure 1.
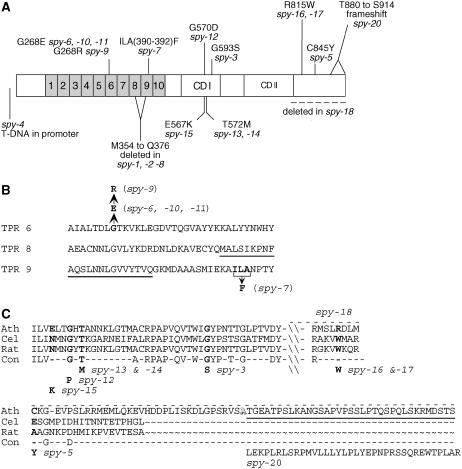
The protein defects caused by 19 spy alleles. A, Schematic representation of the SPY protein with the nature and location of 19 spy mutations indicated. The numbered boxes represent the TPRs. CDI and CDII are conserved domains found in OGTs (Roos and Hanover, 2000). The dotted line indicates the portion of SPY encoded by the PstI fragment affected in spy-18. The defects in spy-1 to spy-5 were determined previously (Jacobsen et al., 1996). B, Locations of the mutations affecting TPRs 6, 8, and 9. The underlined amino acids of TPR 8 and 9 are predicted to be deleted in spy-1, spy-2, and spy-8. spy-7 causes replacement of the bolded amino acids with Phe. C, C-terminal alignment of SPY (Ath) and OGTs from C. elegans (Cel) and rat (Rat). Consensus residues (Con) represent conserved amino acids among the three sequences. The amino acid changes caused by the spy alleles are indicated below the consensus. The portion of SPY encoded by the PstI fragment that was affected in spy-18 is indicated by the dotted line above the SPY sequence. The spy-20 mutation deleted one nucleotide of codon 879 (Table I), which affects the amino acids after Ala-879 (underlined). The new amino acids encoded by the frame-shifted region are shown below the consensus. Due to a single nucleotide polymorphism, position 879, A, is an Ala in Ler and Ser in Col-0.
OGTs transfer a GlcNAc monosaccharide in O-linkage to Ser/Thr of nuclear and cytosolic proteins (Love and Hanover, 2005). In animals, the modification is believed to regulate diverse processes. More than 100 O-GlcNAc modified proteins have been identified. These proteins include regulatory proteins, enzymes, and structural proteins. Many of the proteins are phosphoproteins, and in some cases, O-GlcNAc modification and phosphorylation can occur at the same site (Comer and Hart, 2001; Wells et al., 2001; Slawson and Hart, 2003; Love and Hanover, 2005).
While animals have a single OGT gene, Arabidopsis contains a second OGT gene (SECRET AGENT [SEC]; Hartweck et al., 2002). Although sec plants do not exhibit dramatic defects, the spy sec double mutant has an embryo-lethal phenotype. Deleting the mouse OGT gene also causes lethality (Shafi et al., 2000). Interestingly, loss of OGT activity does not cause lethality in C. elegans (Hanover et al., 2005).
In addition to SPY, the DELLA proteins (REPRESSOR OF ga1-3 [RGA], GA INSENSITIVE [GAI], RGA-LIKE1 [RGL1], and RGL2) are also negative regulators of GA signaling in Arabidopsis (Olszewski et al., 2002; Peng and Harberd, 2002). The DELLA proteins are thought to be nuclear transcriptional regulators. RGA and GAI play a major role in repressing stem elongation and floral induction (Dill and Sun, 2001; King et al., 2001), and RGL2 is important in inhibiting seed germination (Lee et al., 2002), whereas a combination of RGA, RGL1, and RGL2 modulates floral development (Cheng et al., 2004; Tyler et al., 2004; Yu et al., 2004). It has been demonstrated that GA derepresses its signaling pathway by inactivating the DELLA proteins, and the conserved DELLA motif, which is located near the N terminus of these proteins, is required for GA-dependent proteolysis of these repressors (Sun and Gubler, 2004). GA has been shown to induce degradation of RGA, GAI, and RGL2 (Silverstone et al., 2001; Dill et al., 2004; Tyler et al., 2004), and mutant alleles (gai-1, rga-Δ17, and rgl1-Δ17) that encode DELLA-motif deleted mutant DELLA proteins confer a GA-insensitive dwarf phenotype (Peng et al., 1997; Dill et al., 2001; Wen and Chang, 2002). This proteolysis event is likely to be targeted by a ubiquitin E3 ligase complex SCFSLY1 to the 26S proteasome (McGinnis et al., 2003; Dill et al., 2004; Fu et al., 2004; Tyler et al., 2004). The DELLA proteins and their function in GA signaling are conserved in plants (Thomas and Sun, 2004). For example, the DELLA proteins in barley (Hordeum vulgare; SLN1) and in rice (Oryza sativa; SLR1) are also GA signaling repressors and are responsive to GA-induced degradation by the ubiquitin-proteasome pathway (Fu et al., 2002; Gubler et al., 2002; Itoh et al., 2002; Sasaki et al., 2003). Mutations in the DELLA motif of SLN1 and SLR1 also confer GA-insensitive dwarf phenotypes. Recently, the rice GID1 protein and its Arabidopsis orthologs (AtGID1a, AtGID1b, and AtGID1c) have been shown to function as GA receptors (Ueguchi-Tanaka et al., 2005; Nakajima et al., 2006). In the presence of bioactive GA, GID1 interacts with DELLA proteins in yeast (Saccharomyces cerevisiae) two-hybrid assays, suggesting that a GA-GID1-DELLA complex may be targeted for degradation by the SCFSLY1/GID2 complex.
The relationship between the DELLA proteins and SPY in GA signaling is not clear. The DELLA proteins contain sequences that are rich in Ser and Thr residues, which may be target sites for OGT (Peng et al., 1997; Silverstone et al., 1998). Therefore, SPY may activate the DELLA proteins by GlcNAc modification, which has been shown in animal systems to affect nuclear localization, protein stability, and/or activity of target proteins (Love and Hanover, 2005). Consistent with this model, the dwarf phenotype of the gain-of-function gai-1 mutant is largely suppressed by spy alleles (Wilson and Somerville, 1995; Jacobsen et al., 1996; Peng et al., 1997).
Previously, the mutations of five spy alleles have been identified by DNA sequence analysis (Jacobsen et al., 1996). More spy alleles have been isolated through suppressor screens of the ga1-3 and gai-1 mutants (Carol et al., 1995; Wilson and Somerville, 1995; Silverstone et al., 1997). Here, we report the characterizations of 15 new spy alleles. Identifying the mutations in spy alleles helped us define the putative functional motifs of the SPY protein and the significance of these motifs in regulating pathways related and unrelated to GA signal transduction. We also showed that, similar to gai-1, the phenotypes of rga-Δ17 were partially suppressed by a spy allele. The spy mutation does not cause a reduction in rga-Δ17 or RGA protein levels, nor does it alter the nuclear localization of these proteins, suggesting that SPY may increase the activity of the RGA protein.
RESULTS
The spy mutants exhibit pleiotropic phenotypes, some of which are related to elevated GA response (early floral induction, reduced fertility, and increased trichome branching), but others are not (such as altered phyllotaxy, cytokinin response, light responses, and circadian rhythms, and reduced hypocotyl and rosette growth; Thornton et al., 1999; Swain et al., 2001; Tseng et al., 2004; Greenboim-Wainberg et al., 2005). To determine the functional motifs in SPY and identify new spy phenotypes, we identified 13 new recessive spy alleles (spy-8–spy-20) that suppress dwarfism caused by GA deficiency (see “Materials and Methods”). The mutations responsible for 12 of these alleles and two previously identified alleles were determined. The GA signaling defects of 10 of the new mutants and two previously characterized mutants were compared. In addition, these mutants were examined for new phenotypes.
Characterization of Molecular Defects of spy Alleles
The transcribed portion of the SPY locus (At3g11540) contains 18 exons and 17 introns that span 5.8 kb. We first attempted to map the mutations of some of the spy alleles by examining restriction digests of PCR products of the exons of these alleles for single-stranded conformational polymorphisms (SSCP; Hayashi, 1992; Bailey, 1995). SSCPs were detected in exon 9 of spy-7, exon 13 of spy-13 and spy-14, and exon 18 of spy-16 and spy-17 (data not shown). DNA sequencing confirmed that each of the exons exhibiting a SSCP contained a mutation (Table I; Fig. 1). SSCPs were not detected in any of the exons of spy-6, spy-8, spy-9, spy-10, spy-11, and spy-15.
Table I.
Mutations of new spy alleles
| Allele | DNA Mutationa | Protein Mutation | Mutagen | Background |
|---|---|---|---|---|
| spy-6 | G (1823) → A | Gly-268 → Glu | EMS | Col-0 |
| spy-7 | ATACTTGC (2902–2909) → TT | Ile-Leu-Ala (390–392) → Phe | γ-Radiation | Ler |
| spy-8 | G (2778) → A; skipping of exon 8 (2710–2788)b | Partial deletion of TPR8 → TPR9 (354–376) | EMS | Ler |
| spy-9 | G (1822) → A | Gly-268 → Arg | EMS | Ler |
| spy-10 | G (1823) → A | Gly-268 → Glu | EMS | Ler |
| spy-11 | G (1823) → A | Gly-268 → Glu | EMS | Ler |
| spy-12 | G (3893) → A | Gly-570 → Asp | EMS | Ler |
| spy-13 | C (3899) → T | Thr-572 → Met | EMS | Ler |
| spy-14 | C (3899) → T | Thr-572 → Met | EMS | Ler |
| spy-15 | G (3883) → A | Glu-567 → Lys | EMS | Ler |
| spy-16 | C (5076) → T | Arg-815 → Trp | EMS | Ler |
| spy-17 | C (5076) → T | Arg-815 → Trp | EMS | Ler |
| spy-18 | Deletion of 3′ endc | Leu-782 to Ser-914 deleted | Fast neutron | Ler |
| spy-19 | n.i.d | n.i. | Fast neutron | Ler |
| spy-20 | T (5270) deleted | Frameshift of Thr-880 to Ser-914 | Fast neutron | Ler |
The location of each mutation in SPY genomic DNA is indicated relative to the start of transcription (GenBank accession no. NM_111987).
The spy-8 mutation is predicted to cause missplicing leading to deletion of exon 8 from the mature mRNA and amino acids 354 to 376 from SPY.
The precise 5′ end of the deletion is not known but could cause a deletion from Leu-782 to the end of SPY.
Not identified.
The mutations of spy-6, spy-8, spy-9, spy-10, spy-11, spy-12, spy-15, spy-18, spy-19, and spy-20 were investigated by sequencing their exons (Table I; Fig. 1). The mutations of spy-6, spy-8, spy-9, spy-10, and spy-11 affected the N terminus of SPY, while spy-12, spy-15, and spy-20 represented mutations in the C-terminal region of SPY (Fig. 1). The spy-8 mutation is identical to that of spy-1, and the last G of exon 8 is mutated to an A. The spy-1 mutation causes frequent missplicing and deletion of exon 8 (Jacobsen et al., 1996). Although this has not been confirmed experimentally, spy-8 mRNA is expected to have the same splicing defect as spy-1. Skipping of exon 8 results in an in-frame deletion of Met-354 to Gln-376.
Hybridization of Southern blots containing restriction digests of spy-18 genomic DNA (data not shown) indicated that the last PstI fragment of the gene was missing (Fig. 1). With the exception of the last exon of the gene, all of the exons were amplified by PCR. Therefore, the deletion must begin within the last exon of the gene or the intron that precedes this exon. The extent of the deletion beyond the SPY locus has not been determined, but it must extend into the PstI fragment following the gene, because that fragment was not detected. Based on this analysis, the deletion in spy-18 could begin at Leu-782, the first amino acid encoded in this exon or somewhere downstream of this.
We did not detect any mutation in any of the exons in the spy-19 locus by DNA sequence analysis. This mutant allele did not show visible deletions, insertions, or DNA arrangements around the spy locus by genomic DNA-blot analysis (data not shown). It is possible that the mutation is localized in the promoter or an intron.
Phenotypic Characterization of the spy Alleles
To investigate the role of different domains of SPY in GA-related and unrelated functions, we analyzed and compared the phenotypes of 12 spy mutants: spy-2, -4, -8, -9, -11, -12, -14, -15, -16, -18, -19, and -20. spy-4 contains a T-DNA insertion that is 13 bp upstream of the SPY transcriptional start site, which greatly reduces the abundance of the SPY transcript (Jacobsen et al., 1996; Filardo, 2004). spy-9 and -11 affect TPR6, spy-2 and -8 result in partial deletion of TPR8 and TPR9, and spy-12, -14, -15, -16, -18, and -20 encode spy proteins with missense or deletion mutations in the catalytic region. The unidentified mutation in spy-19 is likely to be in the promoter or intron sequence. Most of these mutants (spy-8 to spy-20) were isolated as suppressors of ga1-3 in the Landsberg erecta (Ler) background (Silverstone et al., 1997, 1998). The homozygous spy-8 to spy-20 mutants (backcrossed once) were identified in the F2 generation of crosses between the spy ga1-3 double mutants and Ler. The spy-2 (originally in Columbia [Col-0]) and spy-4 (originally in Wassilewskija) mutants had been crossed for three or four generations, respectively, into Ler wild-type plants that were also included in these analyses for comparison.
The following sets of parameters were measured for these spy mutants and wild type: flowering time (in days and in leaf number), final stem height, number of siliques on the primary inflorescence stem, and the average number of seeds in the first 15 siliques as a measure of fertility. In addition, we noted any other developmental abnormalities, including alterations in phyllotaxy, trichome branching, and cotyledon number.
Flowering Time
Flowering time can be measured both in days to flower (a chronological measurement) and developmental age, by scoring the number of both rosette and cauline leaves produced. Bioactive GAs are an essential part of the autonomous flowering pathway. The ga1-3 mutant contains extremely low levels of GAs (Silverstone et al., 2001) and flowers very late in long day (LD) and not at all under short day (SD) consisting of fluorescent light (Wilson et al., 1992). Thus, GA is required for floral induction in SD in Arabidopsis under specific laboratory conditions. In addition, GA also promotes the transition from vegetative-to-reproductive phase in LD. Several spy alleles (spy-1 to spy-5 in a wild-type background, and spy-2 to spy-4 and spy-8 to spy-9 in a ga1 background) confer an earlier flowering phenotype, consistent with the role of SPY as a negative regulator of GA signaling (Jacobsen and Olszewski, 1993; Jacobsen et al., 1996; Silverstone et al., 1997; Swain et al., 2001). However, it has been proposed that spy alleles also promote flowering through action in the LD flowering pathway (Tseng et al., 2004).
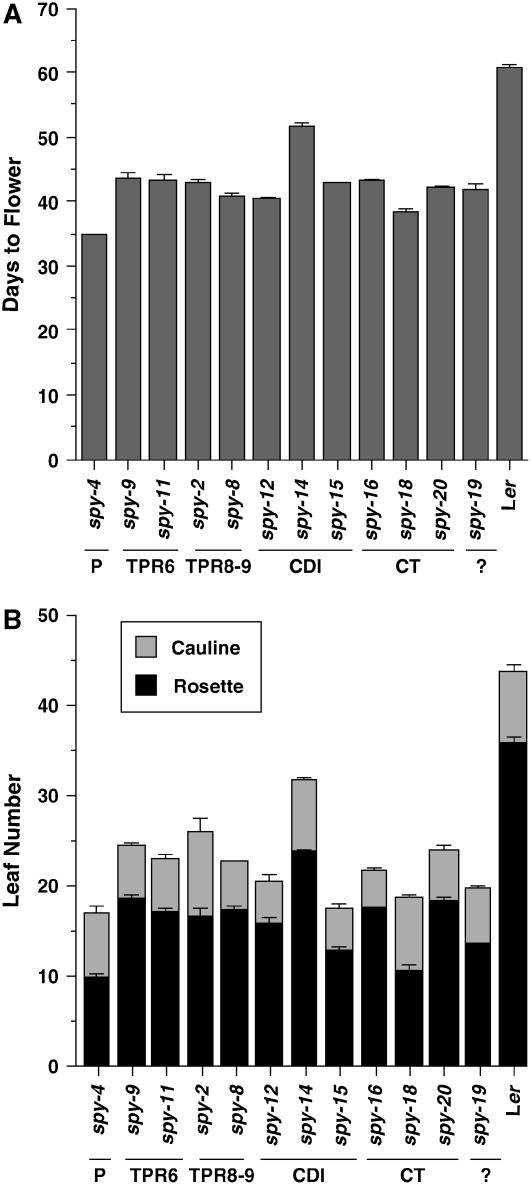
In SD, we found that almost all of the spy alleles (except spy-14) flowered significantly earlier than wild type (17–23 d earlier with 18–28 fewer leaves; Fig. 2). The spy-14 mutant only flowered 9 d earlier and produced 12 fewer leaves than wild type, indicating that this allele has a milder defect in repressing GA-promoted flowering. We also measured flowering time under LD conditions, and the results are similar, but with a smaller reduction in flowering time relative to wild type compared to SD (data not shown). These data indicated that TPRs 6, 8, and 9, CDI and the C terminus of SPY are all crucial for repressing floral induction.
Figure 2.
The spy alleles flowered earlier than wild type in SD. A, Number of days from sowing until floral buds are clearly visible. B, Number of rosette and cauline leaves produced by the primary inflorescence stem after bolting. The values plotted are the means ± se of 10 plants. Some error bars are too small to be seen. The spy alleles are organized in the order of the mutations in the SPY genomic sequence. P, Promoter; CT, C terminus.
Fertility
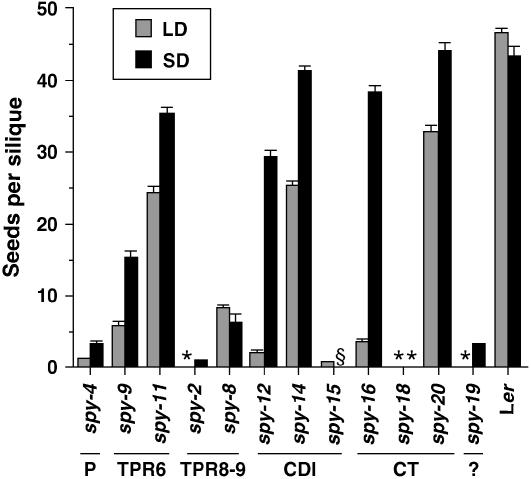
Bioactive GAs are essential for flower development in Arabidopsis. The GA-deficient mutant ga1-3 is male sterile (Koornneef and van der Veen, 1980). This fertility defect can be partially rescued by derepression of the GA signaling pathway due to loss-of-function mutations in SPY or DELLA protein genes (Silverstone et al., 1997; Cheng et al., 2004; Tyler et al., 2004; Yu et al., 2004). However, an optimal concentration of GA is important. Whereas an absence of GA results in male sterility, an excess of GA or derepressed GA signaling also leads to partial-to-complete male sterility (Jacobsen and Olszewski, 1993; Dill and Sun, 2001). In LD, all the spy mutant lines had some degree of reduced fertility when measuring seeds/silique on the primary inflorescence, from a moderate lowering of between 50% and 70% of wild type in spy-11, -14, and -20 to almost completely sterile in spy-2, -18, and -19 (Fig. 3). However, spy-2, -18, and -19 plants did produce a small amount of viable seeds from their axillary inflorescence stems. Day length appeared to have an affect on fertility. SD conditions were able to increase the fertility of most spy lines, although spy-18 still remained highly infertile (Fig. 3).
Figure 3.
SD conditions partially restored the fertility of the spy mutants. Fertility was measured as the average number of seeds per silique in the first 15 siliques on the primary inflorescence stems of 10 plants that were grown in LD or SD conditions. The values plotted are the means ± se of 10 plants. The spy alleles are organized in the order of the mutations in the SPY genomic sequence. *, Sterile; §, not determined.
As evident by the reduced fertility of spy-2, -8, -9, -12, -16, and -18 mutations, TPRs 6, 8, and 9, and catalytic region are important for SPY's function in regulating fertility. Interestingly, the C-terminal-most mutation, spy-20, did not have a large effect on this developmental process.
Height
GA promotes stem elongation by increasing cell elongation and cell division (Davies, 1995; Fabian et al., 2000). In the GA-deficient ga1-3 background, all of the spy alleles increase stem elongation (up to one-third to one-fourth of wild type), by increasing cell length and the number of internodes (Silverstone et al., 1997; data not shown). However, in the presence of the erecta (er) mutation (in either the Col-0 or Ler background), the spy-2 and spy-4 mutants (in the wild-type GA1 background) were reported to have significantly shorter internodes than wild type (Swain et al., 2001). This reduced internode length phenotype is likely due to an interaction between er and spy and may not be directly related to an alteration in GA response. The reduced fertility of spy mutants further complicate the effect of these alleles on stem height, because an increase in sterility leads to delayed cessation of proliferation of the inflorescence meristem and, hence, an increase in silique number and stem height (Hensel et al., 1994). We analyzed the final primary stem heights of the spy alleles to determine whether the tallest plants were the most sterile and produced the most siliques (Table II). This was the case for spy-2 and -18, which were sterile and 22% to 28% taller than wild type in LD. Nevertheless, spy-19, which was also sterile in LD, was actually shorter than wild type, whereas in SD it is only 25% taller compared to wild type. By examining the average length of the internodes between siliques, it is possible to differentiate between growth of internodes and production of nodes. For all the spy alleles, there was a reduction of average internode length (height/node) and an increase in number of nodes. These results indicated that the effect of spy on stem height is complex and cannot be explained simply by an alteration of GA signaling.
Table II.
Final stem height, silique number, and internode length of wild type and the spy alleles in LD and SD conditions
Values for height and silique number are means ± se.
| spy Allele | Affected Regiona | Height | Silique | Average Internode Length |
|---|---|---|---|---|
| cm | n | mm | ||
| LD | ||||
| spy-4 | P | 14.3 ± 0.2 | 50.6 ± 1.7 | 2.8 |
| spy-9 | TPR6 | 15.4 ± 1.1 | 35.7 ± 1.9 | 4.3 |
| spy-11 | TPR6 | 11.7 ± 0.9 | 20.6 ± 2.1 | 5.7 |
| spy-2 | TPR8-9 | 25.8 ± 0.6 | 72.2 ± 2.3 | 3.6 |
| spy-8 | TPR8-9 | 22.0 ± 0.9 | 59.3 ± 3.0 | 3.7 |
| spy-12 | CDI | 18.1 ± 0.7 | 41.8 ± 2.2 | 4.3 |
| spy-14 | CDI | 14.2 ± 0.4 | 35.7 ± 1.7 | 4.0 |
| spy-15 | CDI | 17.2 ± 1.1 | 34.6 ± 3.7 | 5.0 |
| spy-16 | CT | 19.7 ± 0.7 | 53.8 ± 2.8 | 3.7 |
| spy-18 | CT | 24.6 ± 2.0 | 64.5 ± 5.7 | 3.8 |
| spy-20 | CT | 16.4 ± 0.5 | 30.0 ± 1.1 | 5.5 |
| spy-19 | ? | 17.6 ± 0.3 | 56.3 ± 3.2 | 3.1 |
| Ler | 20.2 ± 0.5 | 30.9 ± 1.1 | 6.5 | |
| SD | ||||
| spy-4 | P | 13.2 ± 1.1 | 66.8 ± 6.5 | 2.0 |
| spy-9 | TPR6 | 18.1 ± 0.8 | 59.9 ± 2.8 | 3.0 |
| spy-11 | TPR6 | 14.5 ± 0.8 | 68.1 ± 3.0 | 2.1 |
| spy-2 | TPR8-9 | 28.5 ± 1.7 | 129.9 ± 8.0 | 2.2 |
| spy-8 | TPR8-9 | 11.5 ± 0.7 | 65.7 ± 4.3 | 1.8 |
| spy-12 | CDI | 15.9 ± 0.6 | 58.2 ± 3.0 | 2.7 |
| spy-14 | CDI | 15.2 ± 0.6 | 47.6 ± 1.4 | 3.2 |
| spy-15 | CDI | n.d.b | n.d. | n.d. |
| spy-16 | CT | 23.6 ± 1.1 | 65.4 ± 3.0 | 3.6 |
| spy-18 | CT | 32.7 ± 1.4 | 130.9 ± 5.7 | 2.5 |
| spy-20 | CT | 17.9 ± 1.0 | 48.8 ± 2.2 | 3.7 |
| spy-19 | ? | 24.4 ± 1.1 | 74.0 ± 5.6 | 3.3 |
| Ler | 18.0 ± 0.5 | 41.2 ± 2.3 | 4.4 | |
The alleles are organized in the order of the mutations in the SPY genomic sequences. P, Promoter; CT, C terminus.
Not determined.
Trichomes
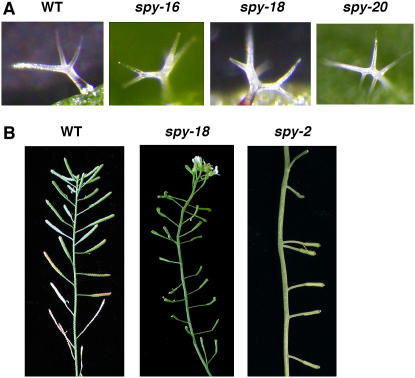
GA induces the expression of a MYB transcription factor, GL1, which is essential for trichome initiation (Perazza et al., 1998). The GA-deficient ga1-3 mutant has reduced adaxial trichomes and does not produce any abaxial trichomes (Chien and Sussex, 1996). GA also affects trichome morphogenesis. In Arabidopsis, 80% of trichomes on the rosette leaves of wild-type plants (Ler) have three branches and 20% have four branches (Perazza et al., 1998). The spy-5 allele was reported to cause a 2-fold increased production of the four branch trichomes (Perazza et al., 1998). Among the 12 spy alleles examined, we found that five additional spy alleles also exhibited an increase in trichome branching (Fig. 4A; data not shown). The effect of spy-4, -16, and -20 is similar to spy-5, whereas spy-2 and spy-18 produced predominantly four branch trichomes (data not shown). The spy-2 allele results in deletion of the TPR-8 and -9 motifs, and mutations in spy-5, -16, -18, and -20 are all localized in the C-terminal coding region, suggesting that these regions are important for SPY's function in regulating trichome development.
Figure 4.
Abnormal trichome branching and inflorescence phyllotaxis in the spy mutants. A, Most of the trichomes on wild-type Ler leaves have three branches, whereas spy-16, -18, and -20 produce higher percentages of four branched trichomes. Examples of three- (wild type), four- (spy-16 and -18), and five-branch trichomes are shown. B, spy-2 and spy-18 showed abnormal inflorescence phyllotaxy in comparison to wild-type Ler. [See online article for color version of this figure.]
Phyllotaxy
Arabidopsis normally produces plants with a spiral phyllotaxy for its flowers on the inflorescence stems. Previous studies indicated that the spy-4 allele in the Col-0 background showed altered inflorescence phyllotaxy (Swain et al., 2001). In the Ler background, spy-2 and spy-18, in addition to spy-4, also exhibited this phenotype (Fig. 4B). The flowers of these alleles often initiated in a disordered fashion and sometimes with multiple flowers appearing to initiate from the same node. These observations indicated that TPR8, TPR9, and the C-terminal region of SPY are required for regulating the phyllotaxy of flowers on the inflorescence stem.
Embryogenesis
Arabidopsis seedlings have two opposite cotyledons that are produced during embryogenesis. During the course of this work, it was noted that some spy alleles caused a partially penetrant defect in seedling cotyledon number. Therefore, several of the spy alleles were examined in more detail for this phenotype (Table III). The effect of spy in both Col-0 and Ler backgrounds was examined to determine if the phenotype exhibited any background dependence. To varying extents, the spy alleles examined increased the frequency of seedlings with one, two fused, or three cotyledons relative to the corresponding wild type. While no wild-type Col-0 or gai-1 seedlings exhibited this phenotype, a proportion of spy-1, -2, -3, and -4 seedlings had abnormal cotyledons, with the phenotype being most penetrant in spy-2. Cotyledon defects were also observed in gai-1 spy-2 double mutants but were complemented by the addition of a genomic clone containing the entire SPY gene (data not shown). Although some abnormal cotyledons were observed in Ler plants, the frequency was higher when a spy allele was present.
Table III.
Several spy alleles increase the frequency of seedlings with abnormal cotyledons
| Genotype | Background | Total Seed No.a | Abnormal Seedlings | % Abnormal Seedlings |
|---|---|---|---|---|
| Wild type | Col-0 | 850 | 0 | 0.00 |
| spy-1 hy-2 | Col-0 | 760 | 27 | 3.55 |
| spy-2 | Col-0 | 456 | 21 | 4.61 |
| spy-3 | Col-0 | 1,849 | 7 | 0.38 |
| spy-4 | Col-0 (BC6)b | 598 | 4 | 0.67 |
| gai-1 | Col-0 (BC3) | 291 | 0 | 0.00 |
| Wild type | Ler | 904 | 2 | 0.22 |
| spy-2 | Ler (BC3) | 163 | 16 | 9.82 |
| spy-4 | Ler (BC3) | 227 | 1 | 0.44 |
| spy-5 | Ler | 986 | 5 | 0.71 |
| spy-18 | Ler | 405 | 52 | 12.83 |
| spy-19 | Ler | 127 | 15 | 11.84 |
For spy-2 (Col-0 and Ler), spy-4 (Ler), spy-18, and spy-19, a segregating population was analyzed, and the number of seeds examined represents 25% of the seeds analyzed from heterozygous SPY spy parents. The number reported for the other genotypes is the actual number of homozygous seeds analyzed.
The backcross (BC) generation into this background is indicated.
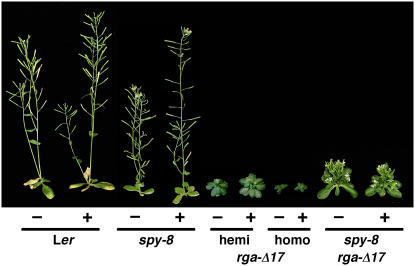
spy Partially Suppresses the Dwarf Phenotype of rga-Δ17
The mechanism of the interaction between SPY and the DELLA proteins (RGA, GAI, and RGLs) in GA signaling is not clear, although genetic studies indicated that these proteins are all negative regulators of GA signaling. The loss-of-function rga and spy-8 alleles have a synergistic effect in rescuing the dwarf phenotype of the GA-deficient mutant ga1-3 (Silverstone et al., 1997). Because spy-8 is not a null allele, RGA and SPY may act in the same pathway or in two parallel pathways. Previous studies also showed that spy mutations suppress the dwarf phenotype of the gain-of-function gai-1 allele (Wilson and Somerville, 1995; Jacobsen et al., 1996; Peng et al., 1997). To investigate further the relationship between RGA and SPY in GA signaling, we tested whether spy suppresses the gain-of-function rga-Δ17 allele. When comparing phenotypes of the homozygous rga-Δ17 line and the double homozygous spy-8 rga-Δ17 plant (Fig. 5; Table IV), we found that spy-8 partially rescued all the phenotypes of rga-Δ17 but did not restore its GA responsiveness. These results are consistent with the hypothesis that SPY may activate the DELLA proteins by GlcNAc modification. In animal systems, GlcNAc modification can affect target proteins at multiple levels, including inhibition of phosphorylation, increased protein stability, altered nuclear localization, or protein activity (Wells et al., 2001). We therefore analyzed the effect of spy-8 on the levels and localization of the RGA and/or rga-Δ17 proteins. Unexpectedly, immunoblot analysis demonstrated that the spy-8 mutation causes an increase (instead of a reduction) in the amount of RGA and rga-Δ17 proteins (Fig. 6, A and B). The spy-8 mutation did not affect GA-induced degradation of RGA (Fig. 6A), consistent with the previous observation that spy mutants remain GA responsive (Silverstone et al., 1997). Several additional spy alleles (spy-9, -12, -13, -15, and -17) also accumulate a higher amount of the RGA protein than wild type (Fig. 6C), indicating that this effect is not specific for the spy-8 allele.
Figure 5.
spy-8 partially suppresses rga-Δ17 but does not restore GA responsiveness. Plants were grown on soil for 36 d with 100 μm GA3 (+) or water (−) treatment (starting at day 18). All plants are homozygous for the genotype(s) indicated, except rga-Δ17; homo, plants are homozygous for rga-Δ17; hemi, plants are hemizygous for rga-Δ17. Representative plants are shown. The inflorescence stems of the spy-8 plants grew slower than wild type, but the final height is similar to wild type (Table II). [See online article for color version of this figure.]
Table IV.
spy-8 partially suppresses all phenotypes of rga-Δ17
Values are means ± se. hemi, Hemizygous; homo, homozygous; n.d., not determined. Final height for rga-Δ17 homozygotes was not determined, because they did not bolt. Branch number for rga-Δ17 homozygotes was not determined, because they died shortly after flowering.
| Genotype | Flowering Time
|
Final Height | Rosette Diameter | Branch No. | Seeds/Silique
|
||
|---|---|---|---|---|---|---|---|
| Total Leavesa | Days | Allb | Fertilec | ||||
| cm | cm | ||||||
| Ler | 6.9 ± 0.2 | 14.9 ± 0.5 | 15.1 ± 0.4 | 18.3 ± 0.5 | 3.0 ± 0.2 | 36.5 ± 1.2 | 36.5 ± 1.2 |
| spy-8 | 5.4 ± 0.3 | 13.8 ± 0.3 | 16.5 ± 1.6 | 7.4 ± 0.3 | 2.8 ± 0.3 | 2.7 ± 0.3 | 4.8 ± 0.4 |
| rga-Δ17 hemi | 13.4 ± 0.5 | 23.5 ± 0.7 | 3.6 ± 0.2 | 19.6 ± 0.5 | 10.8 ± 0.6 | 3.4 ± 0.4 | 15.1 ± 1.0 |
| rga-Δ17 homo | 14.0 ± 0.9 | 26.9 ± 1.2 | No bolting | 9.0 ± 0.4 | n.d. | Sterile | Sterile |
| spy-8 rga-Δ17 | 8.5 ± 0.3 | 16.3 ± 0.5 | 2.7 ± 0.2 | 18.2 ± 0.5 | 6.0 ± 0.4 | 26.0 ± 0.8 | 26.0 ± 0.8 |
Total leaves includes rosette and cauline leaves.
Fertility was measured by the number of seeds per silique on the primary inflorescence stem for 10 plants per line. The number of seeds in all siliques were counted and then averaged.
As in b, except that seeds per silique were averaged using only siliques that contained at least one seed. spy-8 and rga-Δ17 hemizygotes contain many completely sterile siliques.
Figure 6.
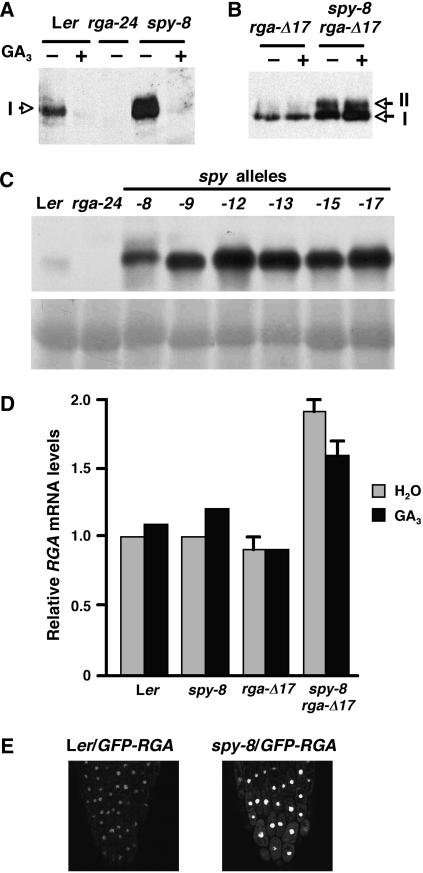
The levels of RGA and rga-Δ17 proteins, but not mRNA, are elevated in the spy mutant backgrounds. A to C, The blots contain 25 μg (in A and B) or 50 μg (in C) total protein from 8-d-old seedlings after 2-h treatment with water (−) or 100 μm GA3 (+) as labeled. Affinity-purified rabbit anti-RGA antibodies were used to detect the RGA (64 kD) and rga-Δ17 (62 kD) proteins (form I). The extra band (form II) may be a modified rga-Δ17. The images in B were developed after a shorter exposure on film than A and C. An image of the Ponceau-stained blot is shown below the immunoblot in C to confirm equal loading. D, Relative RGA mRNA levels determined by quantitative RT-PCR. Total RNA was isolated from wild type and various mutants after 100 μm GA3 (+) or water (−) treatment for 2 h. The relative RGA mRNA levels were determined by running three quantitative RT-PCR reactions for each sample and normalized using the housekeeping gene GAPC. Bars = means ± se. The value of water-treated wild type was arbitrarily set to 1.0. Proteins or mRNA in A to D were extracted from homozygous lines as labeled, except that the sample for the rga-Δ17 line was extracted from a mixture of hemi- and homozygous plants. E, GFP fluorescence in root tips of transgenic lines expressing GFP-RGA in wild-type or spy-8 backgrounds. The images were captured by confocal laser microscopy under an identical setting.
As shown previously (Dill et al., 2001), an extra faint protein band (form II) with a slower mobility than rga-Δ17 (form I) is present in protein extracts from rga-Δ17 plants (Fig. 6B). Both form I and form II of the rga-Δ17 protein (Fig. 6B) accumulated to a higher level in the spy-8 background, but the amount of form II increased more dramatically than form I. It is possible that the form II protein may be phosphorylated rga-Δ17, and, similar to O-GlcNAc modifications made by animal OGT, modification by SPY may block phosphorylation of RGA.
We also analyzed the impact of spy on the amounts of RGA and rga-Δ17 transcript accumulation by quantitative real-time reverse transcription (RT)-PCR using gene-specific primers (Fig. 6D). The RGA mRNA level in spy-8 is similar to wild type, indicating that the elevated amount of the RGA protein in spy-8 is not caused by an increased RGA transcript level. The RNA for rga-Δ17 was isolated from a mixed population of homozygous and hemizygous seedlings, because homozygous rga-Δ17 is sterile, whereas RNA for spy-8 rga-Δ17 was isolated from homozygous plants. Both rga-Δ17 and spy-8 rga-Δ17 plants contain two copies of the wild-type RGA locus and one or two copies of the rga-Δ17 transgene, and the amounts of the RGA transcript measured are the combination of both RGA and rga-Δ17 mRNAs. rga-Δ17 accumulates a wild-type level of the RGA mRNA, whereas spy-8 rga-Δ17 contains 2-fold higher amount of the RGA transcript. The slightly increased RGA mRNA level in spy-8 rga-Δ17 could be due to the extra copy of the rga-Δ17 transgene and/or altered feedback regulation of RGA expression resulting from the elevated activity in the GA response pathway that is caused by the spy mutation.
To examine the effect of spy on the subcellular localization of RGA, we generated a spy-8 line that contains the Prga:green fluorescent protein (GFP)-RGA transgene by crosses between spy-8 and a previously characterized transgenic Ler/Prga:GFP-RGA line (Silverstone et al., 2001). This Prga:GFP-RGA fusion gene was able to rescue the phenotype of a null rga allele, and the GFP-RGA fusion is localized to the nucleus in transgenic Arabidopsis (Silverstone et al., 2001; Fig. 6E). Confocal microscopy showed that loss of the SPY function does not alter the nuclear localization of the GFP-RGA protein in spy-8 GFP-RGA. Thus, SPY may modify and convert the RGA protein to a more active form, which may be tightly regulated by controlled proteolysis. In contrast, the less active form of RGA and rga-Δ17 in the spy-8 background may accumulate to a higher level.
DISCUSSION
To date, 20 loss-of-function spy alleles with defects in GA signaling have been identified (Fig. 1; Table I). The molecular defects of 19 of them have been characterized, and all of them except spy-4 contain mutations that are localized either in three of the TPRs (TPR6, TPR8, and TPR9) or in the catalytic C-terminal region of the OGT domain in SPY (Fig. 1). Phenotypic characterization of 12 spy alleles revealed that mutations in TPRs 6, 8, and 9 or the catalytic region affect GA signaling in floral induction and fertility. These sequences in SPY are also important for repressing GA-induced stem elongation, because all spy alleles partially suppress the dwarf phenotype of the GA-deficient mutant ga1, even though the spy single mutants are not always taller than wild-type Ler. Our results suggest that SPY regulates GA signaling through specific protein-protein interactions via TPRs 6, 8, and 9, and its OGT catalytic activity. The other TPRs may be important for SPY to function in other cellular pathways. We cannot rule out the possibility that the currently available spy alleles may affect SPY protein stability in general. Alternatively, the clustered mutations in these alleles may reflect the hot spots of mutagenesis, although they were generated by ethyl methanesulfonate (EMS), γ-radiation, or fast neutron (Table I).
The TPRs and the Catalytic Region Are Important for SPY's Function in GA Signaling
Six of the newly characterized spy mutations affect the TPR domain, and these, together with the previously characterized spy-1 and spy-2 mutations (Jacobsen et al., 1996), are clustered in TPRs 6, 8, and/or 9 (Fig. 1A). Four alleles represent missense mutations affecting Gly-268 of TPR 6. The spy-7 mutation deletes part of TPR9 and also changes an amino acid. spy-8 has the same mutation as spy-1; the last G of exon 8 is mutated to an A. The spy-1 mutation has been shown to cause an error in splicing that results in exon 8 being skipped. Interestingly, spy-2 affects the last G of intron 7 and also causes exon 8 to be skipped. The deletion of exon 8 from the SPY mRNA causes the in-frame deletion of portions of TPRs 8 and 9 from the SPY protein. While spy-2 has more severe phenotypes than spy-1 (data not shown) and spy-8, RT-PCR analysis indicates that in spy-1, but not spy-2, a small amount of wild-type-sized transcript is produced (S.E. Jacobsen and N.E. Olszewski, unpublished data). Therefore, spy-1 and spy-8 are probably weaker alleles than spy-2, because they encode a small amount of wild-type transcript. The clustering of mutations in TPRs 6, 8, and 9 suggested that only these TPRs are essential for GA signaling or that mutations affecting the other TPRs cause different phenotypes.
Gly-268, which is mutated to Glu in spy-6, spy-10, and spy-11, and to Arg in spy-9, is located at the position 8 in TPR 6 (Fig. 1B). A Gly residue is present at position 8 of all 10 SPY TPRs (Jacobsen et al., 1996). The amino acid residue at position 8 of TPRs is generally small and hydrophobic (Das et al., 1998; Blatch and Lässle, 1999). In the TPR crystal structure (Das et al., 1998; Scheufler et al., 2000; Jinek et al., 2004), position 8 is at the closest contact between a pair of antiparallel α helices, and a bulky residue at this position is predicted to affect the positioning of these helices (Das et al., 1998; Gounalaki et al., 2000; Scheufler et al., 2000). Mutations affecting position 8 disrupt the interaction with other proteins (de Boer et al., 1994; Ponting, 1996; Gounalaki et al., 2000). Therefore, the mutations affecting Gly-268 may be disrupting interactions between the enzyme subunits, interactions with a substrate, and/or interaction with a protein that regulates SPY activity.
Ten of the spy alleles are mutated in the C terminus of SPY, which is likely to be the catalytic domain. OGT catalytic domains contain two conserved regions, designated CDI and II, that are predicted to contribute to the formation of a UDP-GlcNAc-binding pocket that participates in transferring the sugar to the substrate (Lazarus et al., 2005). CDI is affected in spy-3, spy-12, spy-13, spy-14, and spy-15, while spy-5, spy-16, spy-17, spy-18, and spy-20 all are mutated near CDII (Figs. 1, A and C).
TPR8, TPR9, and the C Terminus of SPY Are Important for Regulating Trichome Branching and Inflorescence Phyllotaxy
Our studies suggested that TPRs 8 and 9 (partially deleted in spy-2) and the C-terminal region (deleted in spy-18) also play an essential role in regulating trichome development and inflorescence phyllotaxy. GA is important for trichome initiation (Chien and Sussex, 1996) and branching (Perazza et al., 1998). We found that spy alleles that affect TPRs 8 and 9 or the C terminus produced higher percentages of four-branch trichomes than wild type. Previously genetic studies revealed that mutations in four additional genes POLYCHOME (PYM), KAKTUS (KAK), RASTAFARI (RFI), and TRIPTYCHOME (TRY) also result in overbranched trichome development (Perazza et al., 1999). However, unlike the spy mutants, none of these mutants exhibit GA-related phenotypes in seed germination or floral induction. Epistasis analysis demonstrated that PYM, KAK, and RFI are likely to be downstream of SPY in the same pathway, whereas TRY is located in a separate pathway from SPY (Perazza et al., 1999). These findings suggest that SPY may affect trichome development through a branch of the GA signaling pathway.
Changes in leaf phyllotaxis have been observed in GA-treated Xanthium pennsylvanicum (Maksymowych and Erickson, 1977). However, in Arabidopsis, GA-deficient mutants and most of the GA-response mutants (except spy) do not exhibit aberrant phyllotaxy, suggesting that SPY may modulate phyllotaxis by interacting with components in another cellular pathway. It remains to be determined whether GA plays a role in regulating phyllotaxis in most species. Recent studies indicate that polar transport of auxin is involved in regulating the initiation and radial position of lateral organs in the Arabidopsis inflorescence meristem (Reinhardt et al., 2000, 2003). Cytokinin may also regulate phyllotaxis by inducing expansion of the shoot meristem, as suggested by the characterization of an altered phyllotaxy maize (Zea mays) mutant abph1 that is defective in a putative negative cytokinin response regulator (Giulini et al., 2004). Future analysis will be needed to determine whether SPY affects phyllotaxy by interacting with components in the cytokinin and/or auxin pathway.
SPY Has a Role in Embryo Development
Mutations in either the TPR domain or the catalytic domain caused a partially penetrant defect in cotyledon development (Table III). The primary defect in these mutants appears to be in the positioning and initiation of the cotyledons during embryogenesis. This is supported by the observations that mutant seedlings could have one, two fused, or three cotyledons. SPY has previously been shown to have a role in embryogenesis, because sec spy double mutant embryos fail to complete development (Hartweck et al., 2002). It is not clear whether the cotyledon phenotype in the spy mutants is due to altered GA signaling or defects in other developmental pathways, but GAs have been suggested to be essential for Arabidopsis seed development (Singh et al., 2002).
SPY Is Not Required for Nuclear Localization or Increased Stability of RGA
Based on the functions of animal OGTs, we previously hypothesized that SPY may activate RGA by GlcNAc modification (Silverstone et al., 1998). This idea is supported by a recent finding that the paralog of SPY, SEC, GlcNAc modifies RGA in a heterologous system (L. Hartweck and N.E. Olszewski, unpublished data). Here, we showed that GFP-RGA fusion protein is still localized to the nucleus in the spy mutant background. We further demonstrated that the spy mutation does not destabilize the RGA protein. These results eliminated the possibility that the nuclear localization and stability of RGA depend on SPY function. We therefore propose that the role of SPY is to directly increase RGA protein activity via O-GlcNAcylation. In rga-Δ17 plants, an extra protein band (form II) with a slower mobility than rga-Δ17 (form I) is present (Dill et al., 2001; Fig. 6B). Similarly, the gain-of-function DELLA mutant protein (sln1-d) in barley is also present in two forms (Gubler et al., 2002). The spy mutation caused an increased accumulation of both form I and form II of the rga-Δ17 protein, although the amount of form II appeared to increase more dramatically (Fig. 6B). This form II protein may be rga-Δ17 with posttranslational modification(s), although the nature of the modification has not been identified. One possibility is that form II may be phosphorylated rga-Δ17, and, similar to animal OGT, SPY may prevent phosphorylation of RGA. However, the form II of rga-Δ17 and sln1-d remains stable after GA treatment (Fig. 6B; Gubler et al., 2002), indicating that this specific modification is not sufficient to target these proteins for GA-induced degradation. This would be consistent with the results of two recent studies (although mutually conflicting); one shows that phosphorylation of the rice DELLA protein SLR1 is independent of its degradation in response to GA (Itoh et al., 2005), whereas the other suggests that dephosphorylation of RGL2 is required for its degradation (Hussain et al., 2005). Our model is mainly based on genetic data and on the effect of spy on RGA localization and stability. Alternatively, SPY and RGA may function independently in GA signaling. Additional biochemical studies are necessary to elucidate the nature of posttranslational modification in RGA and to investigate whether RGA is a substrate of SPY and whether GlcNAc modification of RGA increases RGA's activity in transcriptional regulation.
MATERIALS AND METHODS
Plant Lines and Mutant Phenotype Characterization
The original spy-2 and spy-4 mutants were isolated in the Col-0 and Wassilewskija ecotype backgrounds, respectively (Jacobsen and Olszewski, 1993; Jacobsen et al., 1996). To minimize the effect of different ecotype backgrounds, homozygous spy-2 and spy-4 lines that had been crossed with Ler (three times for spy-2 and four times for spy-4) were used in this study. The spy-6 (EMS) and spy-7 (γ-radiation; previously named gas1-1) alleles were obtained from S.E. Jacobsen and N. Harberd (Peng et al., 1997), respectively. spy-8 to spy-17 (EMS) and spy-18 to spy-20 (fast neutron) were obtained from screening suppressor mutations that rescued the ga1-3 phenotype (Silverstone et al., 1997, 1998). The double homozygous spy ga1-3 plants were crossed with Ler plants, and the spy single mutants were identified in the F2 generation by their spy phenotype. The spy-18 and spy-19 alleles were maintained as heterozygous lines, because the homozygotes in LD conditions are sterile. The spy-8 rga-Δ17 double mutant was generated by genetic crosses between spy-8 and a transgenic Ler line that carries the Prga:rga-Δ17 fusion gene (Dill et al., 2001). The double homozygous spy-8 rga-Δ17 plants were selected using the kanamycin-resistant marker (linked to the transgene) and by PCR using allele-specific cleaved amplified polymorphic sequence (CAPS) primers for spy-8 and SPY. Amplification of SPY and spy-8 genomic DNA using primer 400 (5′-AGGCTTGCAACAATTTGGGAG-3′) and primer 401 (5′-CCTTCTCAATCATGCTGGCA-3′) will generate a 344-bp product. ScrF1 digestion of the spy-8 PCR DNA will produce 220- and 124-bp fragments, whereas SPY DNA remains 344 bp. For complementation of the cotyledon defect, the gai-1 spy-2 2118 genotype was made by crossing gai-1 spy-2 (essentially in the Col-0 background; Swain et al., 2001) with No-0 plants containing a genomic SPY clone (2118) originally used for complementation studies (Jacobsen et al., 1996). Plants homozygous for gai-1 and spy-2 but hemizygous for the 2118 clone were identified and allowed to self pollinate. From these progeny plants homozygous for gai-1 and spy-2 without 2118 and plants homozygous for gai-1, spy-2, and 2118 were selected and progeny seed used for analyzing defects in cotyledon development.
For phenotypic characterization, the plants were grown under LD (16 h 140 μE light/8 h dark) and/or sd (8 h 160 μE light/16 h dark) conditions at 22°C. Ten plants of each line were used for the phenotypic measurements. The final height of the primary inflorescence stem of each plant was measured. The flowering time was scored as days to flower (when the flower bud was first visible without magnification) and as total number of leaves on the main stem. Fertility (average no. of seeds per silique) was measured by scoring the number of seeds in the first 15 siliques on the primary inflorescence stems of 10 plants.
Mapping Mutations by Detection of SSCP
spy plants, together with wild-type Col-0 and Ler, were grown in continuous light at 23°C for 3 weeks. Leaves were prepared for PCR as described by Klimyuk et al. (1993), and the SSCP analysis was performed as described previously (Sheffield et al., 1993; Bailey, 1995). Primers for SSCP are listed in Supplemental Table S1.
DNA Sequencing
DNA fragments with a SSCP between the wild-type and spy alleles were amplified by PCR from the alkaline-treated tissues in a 50-μL volume of amplification solution containing 250 μm of dNTPs (Pharmacia) without isotopes, 0.25 μm of each of the two primers, in 1× amplification buffer of 10 mm Tris-HCl, pH 8.3, 50 mm KCl, 2.5 mm MgCl2. Cycling conditions were: 94°C for 15 s; 52°C for 15 s; 72°C for 1 min; 35 cycles, followed by a 10-min extension at 72°C. PCR primers are listed in Supplemental Table S1. Amplified DNA fragments were gel purified for sequencing using the fmol sequencing kit (Promega) according to manufacturer's instructions.
Immunoblot Analysis of RGA and rga-Δ17 Proteins
Eight-day-old seedlings that were grown on Murashige and Skoog plates were treated with water or 100 μm GA3 for 2 h as described previously (Dill et al., 2004). The seeds of the rga-Δ17 line were produced from hemizygous parents, because homozygous plants are sterile. Wild-type seedlings were discarded, and a mixture of hemizygous and homozygous rga-Δ17 seedlings were treated and harvested for protein extraction. Total plant proteins were isolated and analyzed by immunoblot analysis using affinity-purified anti-RGA antibodies from a rabbit (DU176) as described (Silverstone et al., 2001). Ponceau staining was used to confirm equal loading.
Analysis of RGA mRNA Levels by Quantitative RT-PCR
Thirteen-day-old seedlings that were grown on Murashige and Skoog agar plates were treated with water or 100 μm GA3 for 2 h as described previously (Dill et al., 2004). For rga-Δ17, a mixture of hemizygous and homozygous rga-Δ17 seedlings were treated and harvested for RNA extraction (for the same reason described in the immunoblot analysis section). Total RNA was isolated, and the levels of RGA transcripts were quantified by real-time RT-PCR using gene-specific primers and a Roche Light Cycler as described (Dill et al., 2004). The housekeeping gene GAPC was used as a control to normalize all samples.
Confocal Laser Microscopy
The spy-8 GFP-RGA line was generated by genetic crosses between spy-8 and a transgenic Ler line that carries Prga:GFP-RGA (Silverstone et al., 2001). The double homozygous spy-8 GFP-RGA plants were selected using the kanamycin-resistant marker (linked to the transgene) and by PCR using allele-specific primers for spy-8 and SPY (see above). The root tips of 8-d-old Ler GFP-RGA and spy-8 GFP-RGA seedlings were excised with razor blades, and GFP fluorescence was detected by using a Zeiss LSM-410 confocal laser microscope as described (Silverstone et al., 2001).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Pairs of primers used for SSCP assays and DNA sequencing.
Supplementary Material
Acknowledgments
We thank N. Harberd for the spy-7 seed and S. Jacobsen for the spy-6 seed. We also thank Hou-Sung Jung for help with the generation of spy-8 GFP-RGA and confocal analysis and Lynn Hartweck for comments on the manuscript.
This work was supported by the National Science Foundation (grant nos. IBN–9723171, IBN–0078003, and IBN–0348814 to T.-p.S. and grant nos. MCB–9604126, MCB–9983583, and MCB–0112826 to N.E.O.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Tai-ping Sun (tps@duke.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bailey AL (1995) Single-stranded conformational polymorphisms. In MA Innis, DH Gelfand, JJ Sninsky, eds, PCR Strategies. Academic Press, San Diego, pp 121–129
- Blatch GL, Lässle M (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21 932–939 [DOI] [PubMed] [Google Scholar]
- Carol P, Peng J, Harberd NP (1995) Isolation and preliminary characterization of gas1-1, a mutation causing partial suppression of the phenotype conferred by the gibberellin-insensitive (gai) mutation in Arabidopsis thaliana (L.) Heynh. Planta 197 414–417 [DOI] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131 1055–1064 [DOI] [PubMed] [Google Scholar]
- Chien JC, Sussex IM (1996) Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol 111 1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer FI, Hart GW (2001) Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40 7845–7852 [DOI] [PubMed] [Google Scholar]
- Das AK, Cohen PTW, Barford D (1998) The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J 17 1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ, editor (1995) Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands
- de Boer M, Hilarius-Stokman PM, Hossle JP, Verhoeven AJ, Graf N, Kenney RT, Seger R, Roos D (1994) Autosomal recessive chronic granulomatous disease with absence of the 67-kD cytosolic NADPH oxidase component: identification of mutation and detection of carriers. Blood 83 531–536 [PubMed] [Google Scholar]
- Dill A, Jung H-S, Sun T-p (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Sun T-p (2001) Synergistic de-repression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Thomas SG, Hu J, Steber CM, Sun T-p (2004) The Arabidopsis F-box protein SLEEPY1 targets GA signaling repressors for GA-induced degradation. Plant Cell 16 1392–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian T, Lorbiecke R, Umeda M, Sauter M (2000) The cell cycle genes cycA1;1 and cdc2Os-3 are coordinately regulated by gibberellin in planta. Planta 211 376–383 [DOI] [PubMed] [Google Scholar]
- Filardo F (2004) SPY, a negative regulator of GA response in Arabidopsis thaliana. An investigation of the TPR domain. PhD thesis. La Trobe University, Melbourne, Australia
- Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8 77–85 [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Ait-ali T, Hynes LW, Ougham H, Peng J, Harberd NP (2002) Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14 3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Richards DE, Fleck B, Xie D, Burton N, Harberd NP (2004) The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulini A, Wang J, Jackson D (2004) Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430 1031–1034 [DOI] [PubMed] [Google Scholar]
- Gounalaki N, Tzamarias D, Vlassi M (2000) Identification of residues in the TPR domain of Ssn6 responsible for interaction with the Tup1 protein. FEBS Lett 473 37–41 [DOI] [PubMed] [Google Scholar]
- Greenboim-Wainberg Y, Maymon I, Borochov R, Alvarez J, Olszewski N, Ori N, Eshed Y, Weiss D (2005) Cross talk between gibberellin and cytokinin: the Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell 17 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Chandler P, White R, Llewellyn D, Jacobsen J (2002) GA signaling in barley aleurone cells: control of SLN1 and GAMYB expression. Plant Physiol 129 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M (2005) A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA 102 11266–11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartweck LM, Scott CL, Olszewski NE (2002) Two O-linked N-acetylglucosamine transferase genes of Arabidopsis thaliana L. Heynh. have overlapping functions necessary for gamete and seed development. Genetics 161 1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K (1992) PCR-SSCP: a method for detection of mutations. Genet Anal Tech Appl 9 73–79 [DOI] [PubMed] [Google Scholar]
- Hensel LL, Nelson MA, Richmond TA, Bleecker AB (1994) The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol 106 863–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Cao D, Cheng H, Wen Z, Peng J (2005) Identification of the conserved serine/threonine residues important for gibberellin-sensitivity of Arabidopsis RGL2 protein. Plant J 44 88–99 [DOI] [PubMed] [Google Scholar]
- Itoh H, Sasaki A, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Hasegawa Y, Minami E, Ashikari M, Matsuoka M (2005) Dissection of the phosphorylation of rice DELLA protein, SLENDER RICE1. Plant Cell Physiol 46 1392–1399 [DOI] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE (1996) SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA 93 9292–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E (2004) The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol 11 1001–1007 [DOI] [PubMed] [Google Scholar]
- King K, Moritz T, Harberd N (2001) Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimyuk VI, Carroll BJ, Thomas CM, Jones JD (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J 3 493–494 [DOI] [PubMed] [Google Scholar]
- Koornneef M, van der Veen JH (1980) Induction and analysis of gibberellin-sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 58 257–263 [DOI] [PubMed] [Google Scholar]
- Kreppel L, Blomberg MA, Hart GW (1997) Dynamic glycosylation of nuclear and cytosolic proteins. J Biol Chem 272 9308–9315 [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Hart GW (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase: role of the tetratricopeptide repeats. J Biol Chem 274 32015–32022 [DOI] [PubMed] [Google Scholar]
- Lazarus BD, Roos MD, Hanover JA (2005) Mutational analysis of the catalytic domain of O-linked N-acetylglucosaminyl transferase. J Biol Chem 280 35537–35544 [DOI] [PubMed] [Google Scholar]
- Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love DC, Hanover JA (2005) The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE 2005 re13. [DOI] [PubMed] [Google Scholar]
- Lubas WA, Frank DW, Krause M, Hanover JA (1997) O-linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 272 9316–9324 [DOI] [PubMed] [Google Scholar]
- Lubas WA, Hanover JA (2000) Functional expression of O-linked GlcNAc transferase: domain structure and substrate specificity. J Biol Chem 275 10983–10988 [DOI] [PubMed] [Google Scholar]
- Maksymowych R, Erickson RO (1977) Phyllotactic change induced by gibberellic acid in Xanthium shoot spices. Am J Bot 64 33–44 [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun T-p, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15 1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir RD, Sethy-Coraci I, Puglia K, Librizzi MD, Willis IM (1997) A tetratricopeptide repeat mutation in yeast transcription factor IIIC131 (TFIIIC131) facilitates recruitment of TFIIB-related factor TFIIIB70. Mol Cell Biol 17 7119–7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Shimada A, Takashi Y, Kim YC, Park SH, Ueguchi-Tanaka M, Suzuki H, Katoh E, Iuchi S, Kobayashi M, et al (2006) Identification and characterization of Arabidopsis gibberellin receptors. Plant J 46 880–889 [DOI] [PubMed] [Google Scholar]
- Ollendorff V, Donoghue DJ (1997) The serine/threonine phosphatase PP5 interacts with CDC16 and CDC27, two tetratricopeptide repeat-containing subunits of the anaphase-promoting complex. J Biol Chem 272 32011–32018 [DOI] [PubMed] [Google Scholar]
- Olszewski N, Sun T-p, Gubler F (2002) Gibberellin signaling:biosynthesis, catabolism, and response pathways. Plant Cell (Suppl) 14 S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signalling pathway that negatively regulates gibberellin responses. Genes Dev 11 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP (2002) The role of GA-mediated signalling in the control of seed germination. Curr Opin Plant Biol 5 376–381 [DOI] [PubMed] [Google Scholar]
- Perazza D, Herzog M, Hulskamp M, Brown S, Dorne AM, Bonneville JM (1999) Trichome cell growth in Arabidopsis thaliana can be derepressed by mutations in at least five genes. Genetics 152 461–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazza D, Vachon G, Herzog M (1998) Gibberellins promote trichome formation by up-regulating GLABROUS1 in Arabidopsis. Plant Physiol 117 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP (1996) Novel domains in NADPH oxidase subunits, sorting nexins, and PtdIns 3-kinases: binding partners of SH3 domains? Protein Sci 5 2353–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426 255–260 [DOI] [PubMed] [Google Scholar]
- Roos MD, Hanover JA (2000) Structure of O-linked GlcNAc transferase: mediator of glycan-dependent signaling. Biochem Biophys Res Commun 271 275–280 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong D-H, An G, Kitano J, Ashikari M, et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299 1896–1898 [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hatl FU, Moarefi I (2000) Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101 199–210 [DOI] [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97 5735–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield VC, Beck JS, Kwitek AE, Sandstrom DW, Stone EM (1993) The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics 16 325–332 [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T-p (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Jung H-S, Dill A, Kawaide H, Kamiya Y, Sun T-p (2001) Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Mak PYA, Casamitjana Martínez E, Sun T-p (1997) The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics 146 1087–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DP, Jermakow AM, Swain SM (2002) Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14 3133–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson C, Hart GW (2003) Dynamic interplay between O-GlcNAc and O-phosphate: the sweet side of protein regulation. Curr Opin Struct Biol 13 631–636 [DOI] [PubMed] [Google Scholar]
- Sun T-p, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55 197–223 [DOI] [PubMed] [Google Scholar]
- Swain SM, Singh DP (2005) Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends Plant Sci 10 123–129 [DOI] [PubMed] [Google Scholar]
- Swain SM, Tseng T-s, Olszewski NE (2001) Altered expression of SPINDLY affects gibberellin response and plant development. Plant Physiol 126 1174–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Tseng T-s, Thornton TM, Gopalraj M, Olszewski N (2002) SPINDLY is a nuclear-localized repressor of gibberellin signal transduction expressed throughout the plant. Plant Physiol 129 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Sun T-p (2004) Update on gibberellin signaling: a tale of the tall and the short. Plant Physiol 135 668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton TM, Swain SM, Olszewski NE (1999) Gibberellin signal transduction presents…the SPY who O-GlcNAc'd me. Trends Plant Sci 4 424–428 [DOI] [PubMed] [Google Scholar]
- Tseng TS, Salome PA, McClung CR, Olszewski NE (2004) SPINDLY and GIGANTEA interact and act in Arabidopsis thaliana pathways involved in light responses, flowering, and rhythms in cotyledon movements. Plant Cell 16 1550–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T-s, Swain SM, Olszewski NE (2001) Ectopic expression of the tetratricopeptide repeat domain of SPINDLY causes defects in gibberellin response. Plant Physiol 126 1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun T-p (2004) DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol 135 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D, Struhl K (1995) Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8-Tup1 corepressor complex to differentially regulated promoters. Genes Dev 9 821–831 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, et al (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437 693–698 [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291 2376–2378 [DOI] [PubMed] [Google Scholar]
- Wen C-K, Chang C (2002) Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Somerville CR (1995) Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol 108 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Obermann WM, Hartl FU (1998) Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J Biol Chem 273 18007–18010 [DOI] [PubMed] [Google Scholar]
- Yu H, Ito T, Zhao Y, Peng J, Kumar P, Meyerowitz EM (2004) Floral homeotic genes are targets of gibberellin signaling in flower development. Proc Natl Acad Sci USA 101 7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.