Abstract
We have analyzed proteome dynamics during light-induced development of rice (Oryza sativa) chloroplasts from etioplasts using quantitative two-dimensional gel electrophoresis and tandem mass spectrometry protein identification. In the dark, the etioplast allocates the main proportion of total protein mass to carbohydrate and amino acid metabolism and a surprisingly high number of proteins to the regulation and expression of plastid genes. Chaperones, proteins for photosynthetic energy metabolism, and enzymes of the tetrapyrrole pathway were identified among the most abundant etioplast proteins. The detection of 13 N-terminal acetylated peptides allowed us to map the exact localization of the transit peptide cleavage site, demonstrating good agreement with the prediction for most proteins. Based on the quantitative etioplast proteome map, we examined early light-induced changes during chloroplast development. The transition from heterotrophic metabolism to photosynthesis-supported autotrophic metabolism was already detectable 2 h after illumination and affected most essential metabolic modules. Enzymes in carbohydrate metabolism, photosynthesis, and gene expression were up-regulated, whereas enzymes in amino acid and fatty acid metabolism were significantly decreased in relative abundance. Enzymes involved in nucleotide metabolism, tetrapyrrole biosynthesis, and redox regulation remained unchanged. Phosphoprotein-specific staining at different time points during chloroplast development revealed light-induced phosphorylation of a nuclear-encoded plastid RNA-binding protein, consistent with changes in plastid RNA metabolism. Quantitative information about all identified proteins and their regulation by light is available in plprot, the plastid proteome database (http://www.plprot.ethz.ch).
Plastids perform essential biosynthetic and metabolic functions in plants, including photosynthetic carbon fixation and synthesis of amino acids, fatty acids, starch, and secondary metabolites (Neuhaus and Emes, 2000; Lopez-Juez and Pyke, 2005). In response to tissue-specific and environmental signals, they differentiate into specialized plastid types that can be distinguished by their structure, pigment composition (color), and function. Examples of such different plastid types are elaioplasts in seed endosperm, chromoplasts in fruits and petals, amyloplasts in roots, etioplasts in dark-grown leaves, and chloroplasts in photosynthetically active leaf tissues (Neuhaus and Emes, 2000; Lopez-Juez and Pyke, 2005). Depending on their specific biosynthetic activity and energy metabolism, plastids are broadly classified as photosynthetic and nonphotosynthetic plant organelles. Photosynthetic chloroplasts synthesize sugar phosphates that are catabolized by oxidative metabolism to produce NADPH and ATP. Nonphotosynthetic plastid types import cytosolic sugar phosphates and ATP, which are necessary to sustain their anabolic metabolism. This difference in energy metabolism is often used to distinguish the autotrophic chloroplast from heterotrophic plastids.
Illumination of plant leaves that have developed in the dark triggers the conversion of etioplasts into chloroplasts. This plastid differentiation process is paralleled by the transition from heterotrophic to autotrophic energy metabolism, which involves massive reorganization of the etioplast proteome to support photosynthesis-dependent autotrophic growth. General aspects of this transition have been investigated using different plants and were mainly focused on the accumulation of individual proteins and synthesis of chlorophyll and other pigments (for review, see Lopez-Juez and Pyke, 2005). A more comprehensive analysis of nonphotosynthetic plastid metabolism revealed considerable metabolite fluxes between different metabolic pathways, such as carbon flux to starch, amino acids, lipids, and protein biosynthesis (Neuhaus and Emes, 2000; Tetlow et al., 2004). It is therefore conceivable that the transition from heterotrophic to autotrophic plastid metabolism involves most essential metabolic functions to support the availability of sufficient energy and reduction equivalents for different metabolic pathways.
Differentiation of chloroplasts from etioplasts in response to light requires coordination and integration of plastid and nuclear gene expression. For example, the nucleus controls plastid gene expression through import of specific nuclear-encoded sigma factors, protein kinases that phosphorylate transcription factors, or nucleases and RNA-binding proteins (RNPs) that control plastid RNA stability (for review, see Link, 2003; Bollenbach et al., 2004). Plastids, in turn, regulate the expression of nuclear genes for photosynthetic proteins by feedback controls that are not fully understood, but most likely involve specific tetrapyrrole pathway intermediates (for review, see Surpin et al., 2002; Strand et al., 2003; Beck, 2005). More recent data further suggest a signaling function of plastid gene expression and a role of photosynthetic redox signals in plastid-to-nucleus signaling (Fey et al., 2005; Pesaresi et al., 2006). Although global adaptation of plant gene expression to light requires integrated control of plastid and nuclear gene expression, very early events must involve plastid gene expression and the plastid protein complement. This is important because light-induced synthesis of chlorophyll generates potentially harmful molecules that are photoreactive and must be rapidly scavenged at the site where they are synthesized (i.e. by the plastid's intrinsic protection system; Apel and Hirt, 2004).
To better understand the response of etioplasts to light-induced signal transduction pathways that initiate chloroplast differentiation processes, it is possible to analyze rearrangements in the etioplast proteome in response to light. Several proteomics studies using different plant cell organelles have already been reported (for review, see Peck, 2005; Agrawal and Rakwal, 2006; Baginsky and Gruissem, 2006). Although a few studies analyzed chloroplast proteome dynamics (Lonosky et al., 2004; Giacomelli et al., 2006), most were focused on a detailed, but static, analysis of the chloroplast proteome or suborganellar compartments (for review, see van Wijk, 2004; Baginsky and Gruissem, 2004, 2006). This approach has certain limitations because chloroplasts have highly abundant photosynthetic proteins that impact the dynamic range and detection of low-abundance proteins (Baginsky et al., 2005). This becomes apparent when the identical (or nearly identical) set of chloroplast proteins was repeatedly identified in different studies, whereas the reported detection rate of new proteins is small (Kleffmann et al., 2006; Peltier et al., 2006). This is not unexpected because most abundant chloroplast proteins are involved in photosynthesis. Moreover, profiling of static proteomes limits information on proteome dynamics, such as the ordered rearrangement of the proteome during plastid differentiation. Transcriptional profiling can only be indicative of quantitative changes at the transcript level and does not provide information about protein levels or posttranslational modifications (e.g. Belostotsky and Rose, 2005). Insight into the regulation of proteome dynamics is essential, however, to understand how proteome changes affect metabolic activities and regulatory processes during plastid differentiation (Neuhaus and Emes, 2000; Weber et al., 2005). Plastid protein dynamics most likely also relate to different protein-targeting routes that exist in plastids, but comprehensive information on the regulation of protein targeting during plastid differentiation is currently not available (Jarvis and Robinson, 2004).
Here we report a targeted proteomics approach to explore protein dynamics during the differentiation of rice (Oryza sativa) etioplasts into chloroplasts. Analysis of the etioplast proteome is not constrained by abundant photosynthetic proteins, therefore shifting the dynamic range to facilitate the detection of low-abundance proteins that have regulatory functions (e.g. transcription, RNA metabolism; Zychlinski et al., 2005). Using two-dimensional (2-D) gel electrophoresis coupled with tandem mass spectrometry (MS/MS) analysis, we have developed a detailed 2-D map of the etioplast proteome. The etioplast proteome 2-D map provides insight into protein abundance and the prevalence of different metabolic pathways that constitute etioplast metabolism. Based on proteome dynamics during the first 8 h after light induction, we present a comprehensive model of global reorganization of heterotrophic etioplast metabolism to autotrophic chloroplast metabolism. All protein data can be accessed via an interactive 2-D proteome map at plprot, the plastid proteome database (http://www.plprot.ethz.ch; Kleffmann et al., 2006).
RESULTS AND DISCUSSION
The Rice Etioplast Proteome
We recently reported a new method to isolate intact and pure etioplasts from etiolated rice leaves (Zychlinski et al., 2005), which is based on several differential centrifugation steps followed by Nycodenz density gradient centrifugation. Semiquantitative protein profiling along the Nycodenz gradient established that isolated etioplasts were free of protein contamination from other cell organelles (Zychlinski et al., 2005). For the proteome analysis reported here, we isolated etioplasts from 7-d-old rice seedlings, solubilized the protein in detergent-containing buffer, and established a quantitative proteome map by 2-D gel electrophoresis. Proteins from stained spots were identified by matrix-assisted laser-desorption ionization (MALDI)-tandem time-of-flight (TOF/TOF) analysis using the MASCOT database search algorithm for unambiguous protein identification (see “Materials and Methods”). For assignment of protein quantities, we integrated data from four independent experiments (biological replicates) and averaged the protein-staining intensities (Sypro Ruby) for each protein spot. Using this approach, we identified 369 proteins with high confidence. We compared these data with our shotgun proteome analysis of the etioplast proteome (Zychlinski et al., 2005) and found that 237 etioplast proteins were exclusively identified using the 2-D approach and 118 proteins exclusively by the shotgun approach.
This difference in protein detection can be attributed to the different physicochemical properties of proteins that influence their identification in the two different proteomics strategies. Analysis of membrane proteins by 2-D gel electrophoresis is seriously limited by solubility constraints that have a minor impact on shotgun proteomics. As expected, membrane proteins, such as transporters and several plastid-encoded proteins, are significantly underrepresented on the 2-D gel, whereas they were readily detected with the shotgun approach. On the other hand, we reached greater overall proteome coverage with the 2-D PAGE approach reported here because we could detect low-abundance proteins, such as 3-dehydroquinate and chorismate synthase. These enzymes are active in the synthesis of aromatic amino acids and represent only two examples of proteins that were detected on the 2-D map, but not with the shotgun approach. In general, proteome analyses reach their greatest depth when they combine different strategies that exploit the diverse physicochemical properties of proteins. Combining both studies, we have identified 477 unique proteins from rice etioplasts, which makes the etioplast proteome the best-characterized proteome of all heterotrophic plastid types analyzed to date.
BLAST searches of etioplast proteins against all plastid proteins that were already identified in large-scale proteome analyses (Altschul et al., 1997; Kleffmann et al., 2006) revealed that 88 etioplast proteins were not previously detected in proteome studies of chloroplasts or undifferentiated Bright-Yellow 2 plastids. When compared to other plastid types, these proteins are more prevalent in etioplasts and therefore point to etioplast-specific metabolic and regulatory functions. Most of these proteins have no reported function and therefore may represent new metabolic activities or regulatory mechanisms (Supplemental Table S1). Additionally, several of the proteins previously unidentified in large-scale proteome analyses are involved in plastid gene expression comprising ribosomal proteins, an RNA helicase, and the α-subunit of the plastid-encoded RNA polymerase. This supports our earlier conclusion that the etioplast gene expression apparatus appears to be highly tuned to support a rapid response of plastid gene expression to light (Zychlinski et al., 2005). The prevalence of proteins involved in gene expression distinguishes etioplasts from other fully developed and differentiated plastid types. Together, our results demonstrate that proteome analysis of different plastid types can be used as an efficient strategy to increase the number of newly identified proteins on the way to establish the complete proteome of higher plant plastids.
Plastid Protein Targeting
Most of the nuclear-encoded plastid proteins contain N-terminal transit peptides (TPs). On the basis of TP predictions from genome sequences, the approximate number of expected plastid proteins has been calculated (Abdallah et al., 2000). The robustness of this approach depends on the sensitivity and specificity value of the target prediction algorithm. The specificity value indicates how many proteins predicted to localize to plastids are true plastid proteins (Emanuelsson et al., 2000). The proteomics approach we have used does not allow conclusions about specificity because lack of protein detection does not necessarily suggest a false-positive prediction. However, proteomics can provide information about the sensitivity of target prediction algorithms. Provided that the set of etioplast proteins reported here comprises only true plastid proteins, the sensitivity of correct target prediction is 68% (Table I). This value is lower than reported (Emanuelsson et al., 2000), but consistent with previously reported plastid proteome and bioinformatics analyses (Kleffmann et al., 2004; Richly and Leister, 2004; Nair and Rost, 2005; Zychlinski et al., 2005; Peltier et al., 2006). Of all etioplast proteins we have identified, TargetP predicts that 68% are localized to plastids, 14% to mitochondria, 14% to any other location, and 3% to the secretory pathway (Table I). Although we identified a small number of putative contaminants, most of the proteins not predicted by TargetP are true plastid proteins. Examples include the putative shikimate/quinate 5-dehydrogenase and stroma ascorbate peroxidase, both well-known plastid proteins that are predicted by TargetP for the secretory pathway and a plastid diaminopimelate decarboxylase that is predicted for any other location.
Table I.
Identified proteins and prediction of subcellular localization
| TargetP Prediction | Shotguna | 2-D PAGE | In Both | Nonredundant |
|---|---|---|---|---|
| Plastid | 168 | 245 | 97 | 316 |
| Mitochondria | 22 | 55 | 11 | 66 |
| Secretory pathway | 10 | 11 | 5 | 16 |
| Any other location | 24 | 50 | 11 | 63 |
| Plastid genome | 16 | 8 | 8 | 16 |
| Total | 240 | 369 | 132 | 477 |
As reported by Zychlinski et al. (2005).
False-negative prediction of true plastid proteins can have several reasons. TPs differ in amino acid composition, which makes reliable prediction per se difficult (Bruce, 2001). To identify differences in TP composition between identified rice etioplast proteins (this study) and Arabidopsis (Arabidopsis thaliana) chloroplast proteins (Kleffmann et al., 2004), we aligned 39 N-terminal sequences (50 amino acids) of those rice proteins without a predictable plastid TP that have a homolog in Arabidopsis with a predictable plastid TP. The alignment supports the view that the difference in targeting prediction of evolutionary related proteins stems from different TP compositions because the 39 N termini from rice are significantly enriched in the unpolar/aliphatic amino acids Ala and Leu (Supplemental Fig. S1A). A more general alignment of 100 randomly chosen Arabidopsis and rice proteins with a predicted TP supports the view that the occurrence of Ala and Leu distinguishes rice TPs from those of Arabidopsis. The latter are enriched in the polar amino acid Ser (Supplemental Fig. S1, B and C).
Furthermore, different TP structures could have evolved to maintain efficient import of low-abundance proteins in the presence of highly abundant photosynthetic proteins (Kubis et al., 2003, 2004; Ivanova et al., 2004; Baldwin et al., 2005). We have recently shown that TargetP prediction sensitivity decreases for nuclear-encoded plastid proteins with lower transcript levels (Baginsky et al., 2005). This could suggest that low-abundance proteins use TPs with modified amino acid composition that cannot be recognized by TargetP (Jarvis and Robinson, 2004). Another reason for the false-negative plastid-targeting prediction is the existence of alternative import routes that differ from the common inner translocation chloroplast/outer translocation chloroplast complex protein import pathway. Recent reports on chloroplast protein import via the secretory pathway (Chen et al., 2004; Villarejo et al., 2005) and the existence of internal targeting information (Miras et al., 2002) support this view. Furthermore, dual targeting has already been reported for a substantial number of plastid proteins that likely contribute significantly to intracellular protein trafficking (Silva-Filho, 2003).
Despite the exceptions of plastid protein import discussed above, primary import of plastid proteins occurs via translocation by the outer translocation chloroplast and inner translocation chloroplast complexes (for review, see Soll and Schleiff, 2004; Bedard and Jarvis, 2005; Kessler and Schnell, 2006). Following protein translocation, the N-terminal TP is recognized and cleaved by a stromal-processing peptidase (SPP; Richter and Lamppa, 2003). TPs have no significant sequence or structure conservation, suggesting that the substrate specificity of SPP may be rather low. It was suggested, however, that cysteins in the TP are involved in the cleavage reaction by coordinating the zinc ion in the SPP catalytic site (Richter and Lamppa, 2003). We analyzed the MS spectra of the rice etioplast proteins for acetylated N termini to obtain new insights into TP length and exact localization of the cleavage site. N-terminal acetylation is a common posttranslational modification of proteins in eukaryotes and has also been reported for a number of chloroplast proteins now (Gomez et al., 2002; Ferro et al., 2003). Chloroplasts contain at least one putative N-acetyltransferase that was recently identified in Arabidopsis chloroplasts (Kleffmann et al., 2004) supporting enzyme-catalyzed N-terminal acetylation. We detected 13 acetylated N termini (Table II), which closely match the cleavage site predicted by TargetP and suggest an average TP length of 50 amino acids (Table II). Although this number of acetylated N termini is still small, the data are nevertheless useful to obtain additional information about the chemical properties of the actual cleavage site. Statistical analysis of multiple sequence alignments around the cleavage site points to several Args located at various distances upstream of the cleavage site and an enrichment of aliphatic amino acids immediately proximal to the cleavage site (Supplemental Fig. S2).
Table II.
Acetylated N-terminal peptides that were identified by MALDI-TOF/TOF analyses
Some peptides were identified several times; in these cases, only the highest Mascot Score is provided.
| Protein | Identifier | Identified Acetylated Peptide | Peptide Start-End | TP-Length (Cleavage Site)a | Mascot Scoreb |
|---|---|---|---|---|---|
| Expressed protein | LOC_Os03g21370 | Ac-AVAVDSDQQGSPEPPDQEAKPK | 48-69 | 47 (C/RC4) | 112 |
| Expressed protein | LOC_Os03g01030 | Ac-ADLLGDFGAR | 44-53 | 43 (C/RC3) | 41 |
| 29-kD ribonucleoprotein A, chloroplast precursor | LOC_Os07g43810 | Ac-VAVSSEVEEEEGGAESEGEFAEDLK | 57-81 | 38 (C/RC2) | 210 |
| Plastid-specific ribosomal protein 2 | LOC_Os09g10760 | Ac-SSSVLEAPEEVAAR | 57-70 | 53 (C/RC1) | 127 |
| Expressed protein | LOC_Os05g01970 | Ac-AAAGGPLPTVLVTGAGGR | 38-55 | 37 (C/RC5) | 129 |
| RNA recognition motif family protein | LOC_Os03g25960 | Ac-VAVSEEVETEEDEEEEEEGSGGEEF SDDLR | 58-87 | 56 (C/RC1) | 101 |
| Chloroplast inositol phosphatase | LOC_Os07g37250 | Ac-VATAGDVPPTVAETK | 51-65 | 49 (C/RC5) | 27 |
| Glucose-1-P adenylyltransferase large subunit 1 | LOC_Os05g50380 | Ac-VLTSDAGPDTLHVR | 61-74 | 74 (C/RC5) | 106 |
| 3-Oxoacyl-(acyl-carrier-protein) synthase III | LOC_Os04g55060 | Ac-ASTVDDGVVSAAAAPKPR | 52-69 | 51 (M/RC4) | 142 |
| Expressed protein | LOC_Os08g15500 | Ac-(A)AAASAPAPATPVQAQQR | 46/47-62 | 67 (C/RC2) | 80/97 |
| Isocitrate dehydrogenase, NADP dependent | LOC_Os04g42920 | Ac-AAAAAAVAEQHR | 48-59 | 47 (C/RC4) | 61 |
| Putative enoyl-ACP reductase | LOC_Os08g23810 | Ac-AM(ox)SSESGPQGLPIDLR | 138-153 | – (AO/RC2) | 84 |
| Expressed protein | LOC_Os10g30870 | Ac-VILGPDGRPIGGGPR | 48-62 | 46 (C/RC2) | 49 |
Prediction of localization and cleavage site was performed with TargetP; in parentheses, prediction of localization (C, chloroplast; M, mitochondria; S, secretory pathway; AO, any other) and reliability class of prediction (1–5).
MASCOT scores above 34 (P < 0.05) were considered significant; all spectra were manually examined for correct assignment.
Etioplast Pathways and Metabolic Activities
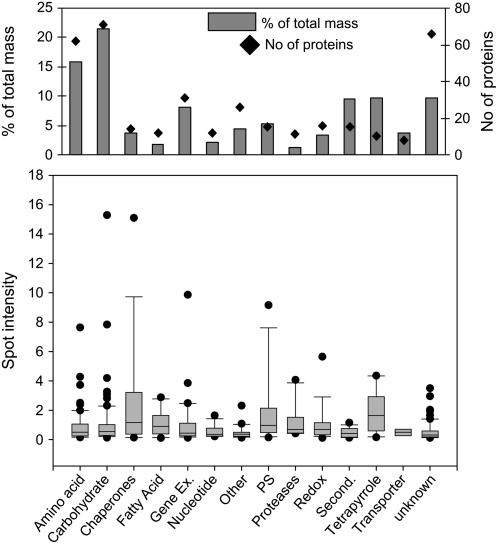
The rice etioplast proteome map provides quantitative information about the identified proteins that can be used to estimate relative pathway prevalence. This is feasible because Sypro Ruby is a proportional stain that provides direct correlation between staining intensity and protein abundance. Because membrane proteins are not completely soluble in mild detergent, they are not included in the quantitative analysis. Figure 1 shows a comprehensive presentation of total protein mass distribution relative to specific metabolic functions. The largest group of identified proteins (diamonds, top graph) and 38% of the total assigned protein masses (gray bars, top graph) represent amino acid (16%) and carbohydrate (22%) metabolism pathways. In addition, approximately 28% of the total assigned protein masses represent proteins involved in gene expression, secondary metabolism, and tetrapyrrole biosynthesis (Fig. 1). Proteins that participate in nucleotide and fatty acid metabolism are represented only by a small number of total assigned protein masses.
Figure 1.
Quantitation of etioplast proteins based on 2-D gel electrophoresis and Sypro Ruby staining. Identified proteins (in total 369) were classified by their molecular function. The top graph shows the number of identified proteins in each functional category (black diamonds) and the sum of all individual staining intensities of the proteins in each functional category (gray bars). Percentage of total mass was calculated by dividing the summed staining intensities of the proteins in each category by the total staining intensity of all identified proteins on the gel. The bottom graph shows the distribution of staining intensities of the proteins in each functional category as box plots. Median values (line in the box), the 25% and 75% percentile (bottom and top end of the box), the 5% and 95% percentile (bottom and top bars), and the outlier (single dots) are indicated.
Figure 1 also shows individual protein abundance in each functional category as the median value of spot intensity. Interestingly, based on this analysis, chaperones are among the most abundant etioplast proteins, especially the 60-kD chaperonin (CPN60), the heat shock 70-kD protein (HSP70), and a 10-kD chaperonin (Supplemental Table S2). Proteins that function in photosynthesis and the subunits of the ATP synthase complex are already moderately abundant in the dark. An important biosynthetic function of etioplasts is the synthesis of chlorophyll via the tetrapyrrole pathway. In the dark, this pathway terminates at protochlorophyllide because subsequent reactions depend on the presence of light (for review, see Grimm, 1998). As expected, Figure 2 shows that enzymes involved in chlorophyll biosynthesis belong to the most abundant proteins in etioplasts, consistent with rapid production of chlorophyll after illumination. All other metabolic pathways are represented by individual proteins of average abundance.
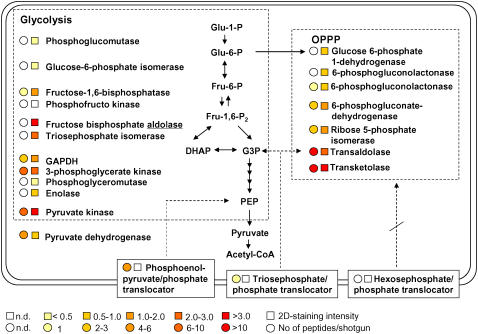
Figure 2.
Sugar phosphate import, glycolysis, and OPPP. Depicted are the identified proteins and their abundance (staining intensity in the 2-D map [squares] and number of peptides in shotgun proteomics [circles]) together with potential interconnections between these two pathways (glycolysis and OPPP).
Etioplasts rely on the import of cytosolic sugar phosphates and ATP for their energy metabolism (Neuhaus and Emes, 2000; Fischer and Weber, 2002; Weber et al., 2005). Our recent proteomics results confirm biochemical data that etioplasts import triose phosphates via a triose phosphate translocator, but not hexose phosphates (Neuhaus and Emes, 2000; Tetlow et al., 2004; Zychlinski et al., 2005). The conversion of triose phosphates into hexose phosphates (e.g. to feed the oxidative pentose phosphate pathway [OPPP]) requires, among other enzymes, Fru 1,6-bisphosphatase, which is activated by reduced thioredoxin (Scheibe, 1994; Scheibe et al., 2005). This type of redox regulation was thought to be restricted to photosynthetic chloroplasts, which reduce ferredoxin and thioredoxin via their photosynthetic electron transport. We identified low concentrations of Fru 1,6-bisphosphatase (0.162 staining intensity), suggesting that etioplasts may convert imported triose phosphates into hexose phosphates. Similar to nonphotosynthetic amyloplasts (Balmer et al., 2006), etioplasts also contain thioredoxin (m type, 0.3 staining intensity), ferredoxin-NADP reductase (0.717 staining intensity), ferredoxin (Zychlinski et al., 2005), and a putative thioredoxin reductase (0.257 staining intensity), suggesting that thioredoxin reduction could also occur in nonphotosynthetic etioplasts (Schürmann, 2003; Balmer et al., 2006; Fig. 2). Detection of a set of enzymes that enable etioplasts to catalyze the conversion of triose phosphates into hexose phosphates suggests that etioplasts feed Glc 6-P into the OPPP (Fig. 2).
Early Events in Light-Induced Differentiation of Etioplasts to Chloroplasts
Single quantitative measurements of plastid proteomes as discussed above and reported in the literature to date do not capture proteome dynamics and therefore do not reveal regulatory aspects of plastid differentiation. Taking advantage of the 2-D map, we explored the response of the etioplast proteome to illumination to address two questions. First, what are early regulatory processes that integrate the light signal with control of plastid gene expression; and second, what is the sequence of events in the transition of heterotrophic etioplast metabolism to autotrophic chloroplast metabolism? To answer these questions, we analyzed changes in the etioplast proteome at 2, 4, and 8 h after illumination. Figure 3A documents the light-induced changes in plastid morphology and chlorophyll accumulation. The etioplast-specific prolamellar body, which contains a ternary complex of protochlorophyllide, protochlorophyllide oxidoreductase, and NADPH, is disassembled during the first 2 h in the light as the thylakoid membrane system develops (Apel et al., 1980; Vothknecht and Westhoff, 2001; Fig. 3A). In parallel, protochlorophyllide is photoreduced to chlorophyllide and further converted into chlorophyll as indicated by red chlorophyll fluorescence (Fig. 3A).
Figure 3.
Light-induced development of chloroplasts from etioplasts. A, Plastids that were isolated from etiolated rice plants after the indicated illumination times. The left image shows light microscopy images of the plastids and the right image shows the corresponding red chlorophyll fluorescence. B, Regulation of chlorophyll-binding proteins by light. We followed the spot intensity of chlorophyll-binding proteins on the different 2-D gels after the indicated illumination times. All chlorophyll-binding proteins were up-regulated by light. Protein spots are: 1 and 2, LHC II type I (CAB21); 3 to 5, LHC II type I (CAB 26); 6, LHC II type III.
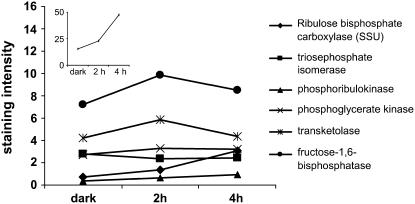
We evaluated the suitability of our experimental approach to reveal light-dependent regulation of proteins by following the accumulation of known light-induced proteins during the early phase of chloroplast development. As expected, thylakoid membrane proteins, such as the chlorophyll-binding proteins, accumulated rapidly after illumination (Fig. 3B). In parallel, enzymes of the Calvin cycle accumulated (Fig. 4), including Fru 1,6-bisphosphatase and Rubisco (Fig. 4, inset). Interestingly, Rubisco is one of the most abundant etioplast proteins and its light induction was strongest among the Calvin cycle enzymes. Together, the results confirmed that 2-D PAGE was suitable to reveal significant increases in protein accumulation as early as hours after illumination. We therefore chose 2-D PAGE analysis to identify molecular processes that may be involved in the regulation of plastid and nuclear gene expression during the early illumination phase.
Figure 4.
Quantification of Calvin cycle enzymes. We followed the spot intensity of Calvin cycle enzymes after illumination and plotted the staining intensity against the illumination time. Large subunit of Rubisco, whose light induction is the strongest among all Calvin cycle enzymes, is shown in the inset.
Regulatory Modules That Adjust Gene Expression in Response to Illumination
Developmental adaptations to light depend on retrograde communication between the plastid and nucleus that may involve intermediates of the tetrapyrrole pathway (for review, see Nott et al., 2006). We therefore analyzed whether the abundance of enzymes active in this pathway was regulated by illumination. With the exception of glutamyl-tRNA reductase (GluTR), all known enzymes of the tetrapyrrole pathway were detected, confirming that they accumulate to moderately high levels in the dark (Fig. 5; Supplemental Table S2). No significant changes of enzyme abundance were visible during the first 4 h of illumination (Fig. 5), although the flux through this pathway is significantly increased by light (Grimm, 1998). Tetrapyrrole pathway flux is regulated by feedback inhibition of GluTR by aminolaevulinic acid (Goslings et al., 2004). It is therefore possible that the increase in tetrapyrrole pathway flux is regulated by the abundance of GluTR or modification of its catalytic activity. Because we have not detected GluTR in our proteomics study, it is difficult to distinguish between these two possibilities. We therefore compared our results with light induction of the tetrapyrrole pathway at the transcriptional level. To this end, we used data obtained from Arabidopsis because transcript data of the early light induction phase are not available for rice (Zimmermann et al., 2004). Such a comparison is valid because recent data confirmed that transcriptional regulation is very similar for Arabidopsis and rice at later phases of light induction (Jiao et al., 2005). The transcript data from Arabidopsis support our finding that enzyme abundance is not significantly changed during the first 2 h of illumination (Supplemental Fig. S3). After 5 h of illumination, expression of all genes of known enzymes involved in the tetrapyrrol pathway was up-regulated at the transcriptional level, except for the GluTR genes. These were expressed at very low levels throughout the illumination period, which is most likely the reason why GluTR was not detected at the protein level. The proteomics and transcriptomics data together suggest that the accumulation of enzymes of the tetrapyrrole pathway in the dark is sufficient to support a rapid increase in pathway flux in response to light (Fig. 5) and that tetrapyrrole pathway flux is likely increased by modification of the GluTR catalytic activity rather than by increased GluTR abundance.
Figure 5.
Regulation of the tetrapyrrole biosynthesis pathway by illumination. Spot intensities of individual enzymes of the tetrapyrrole biosynthesis pathway after 0-, 2-, and 4-h illumination are presented.
Light also controls changes in plastid gene expression. For example, control of plastid mRNA stability is an important mechanism in the regulation of plastid gene expression (for review, see Hayes et al., 1999; Bollenbach et al., 2004). Transcription rates of many chloroplast DNA-encoded genes do not change significantly during chloroplast development, whereas the relative half-lives of plastid mRNAs increase (Klaff and Gruissem, 1991; Stern et al., 1997). These observations suggest that genes encoding proteins for photosynthetic functions are transcribed, but their mRNAs are rapidly degraded in etioplasts. This is consistent with the presence of enzymes for the efficient degradation of structured mRNAs in etioplasts, including helicases, endonucleases, and exonucleases (Zychlinski et al., 2005). Thus, RNA degradation enzymes must be controlled by light to increase the half-lives of mRNAs from photosynthetic genes.
It was previously proposed that plastid RNPs are involved in the regulation of mRNA stability (Hayes et al., 1999). We have identified RNP29 in two protein spots on the 2-D map, one of which is shifted toward a higher molecular mass and a more acidic pI (Fig. 6), which is characteristic of phosphorylation. Pro-Q-Diamond specifically stains the protein with the higher molecular mass and more acidic pI, suggesting that this protein represents the phosphorylated form of RNP29 (data not shown). Full staining of both protein spots with Sypro Ruby showed that the ratio of phosphorylated and unphosphorylated RNP29 increased from 0.41 at 0 h to 0.88 at 2 h of illumination (Fig. 6). Phosphorylation of RNPs alters their affinity for RNA and has been proposed to increase the stability of plastid mRNAs in response to external signals (Lisitsky and Schuster, 1995; Bollenbach et al., 2004; Loza-Tavera et al., 2006). Light-induced RNP phosphorylation shown here could therefore protect mRNAs for photosynthetic proteins from degradation and could be the primary regulatory event during light-dependent chloroplast development. Other RNA degradation enzymes, such as polyribonucleotide phosphorylase (Hayes et al., 1996; Baginsky et al., 2001), CSP41 (Yang et al., 1996), and an RNA helicase (Zychlinski et al., 2005) remain unchanged after 2 and 4 h of illumination (Supplemental Table S2).
Figure 6.
Light-induced phosphorylation of a plastid RNP. The graph shows the ratio of phosphorylated to unphosphorylated RNP29 (5850.m00157) on the basis of the Sypro Ruby staining intensity (shown as inset). The top-left spot was identified as the phosphorylated version of RNP29 by a positive phosphoprotein-specific Pro-Q-Diamond stain (data not shown).
Global Rearrangement of Metabolic Modules toward Autotrophy
We next addressed the transition of heterotrophic etioplast metabolism to the autotrophic metabolism of developing chloroplasts. One adjustment to light within the etioplast proteome is the accumulation of proteins with a function in translation (Table III; Fig. 7; Supplemental Table S2). These proteins comprise ribosomal proteins and the translation elongation factors P and Tu. Both elongation factors are of pivotal functional importance for the translation process. Elongation factor P stimulates the peptidyltransferase function of ribosomes and is essential for viability in Escherichia coli (Aoki et al., 1997). Elongation factor Tu delivers aminoacyl tRNAs to the acceptor site of ribosomes and is thought to be rate limiting for the speed of translation elongation (Pape et al., 1998). It is therefore conceivable that the availability of these translation factors is crucial for rapid and efficient translation in plastids and their molecular function suggests that higher in vivo concentrations of these proteins allow for higher translation rates. Efficient translation machinery is fundamental for reorganization of proteomes during developmental processes and necessary for fast accumulation of enzymes that are needed in photosynthetic chloroplasts. An additional reason for the need for an efficient translation system in developing chloroplasts is the damage of proteins by photooxidative processes that accompany the onset of photosynthetic electron transport. Damaged proteins must be rapidly degraded and replaced by newly synthesized proteins (Aro et al., 1993).
Table III.
Light-regulated reorganization of the etioplast's nonphotosynthetic metabolism toward photosynthesis-dependent autotrophy
Total protein mass is shown as the sum of staining intensities of proteins in each functional category after 0-, 2-, and 4-h illumination. PS, Photosynthesis.
| Dark | 2-h Light | 4-h Light | |
|---|---|---|---|
| Amino acid | 57.5 | 44.7 | 37.3 |
| Carbohydrate | 76.8 | 86.8 | 112.3 |
| PS and energy | 30.0 | 32.6 | 37.8 |
| Gene expression | 34.8 | 39.7 | 42.7 |
| Fatty acid | 13.7 | 10.6 | 9.4 |
| Nucleotide | 6.3 | 4.5 | 5.3 |
| Redox | 16.3 | 17.0 | 16.0 |
| Chaperones | 34.1 | 36.2 | 38.1 |
| Hypothetical/unknown | 31.4 | 29.0 | 27.6 |
| Secondary | 6.7 | 5.3 | 6.2 |
| Tetrapyrrole | 20.0 | 20.7 | 17.1 |
| Proteases | 13.5 | 15.6 | 17.5 |
| Other | 15.1 | 13.5 | 13.5 |
Figure 7.
Reorganization of etioplast metabolism after illumination. The total protein-staining intensity for proteins in each functional category as identified from etioplasts (dark) was set to 100%. The absolute changes of protein mass in each functional category after 2- and 4-h illumination are provided as the difference from the dark value (2/4-h light value minus 100%). PS, Photosynthesis.
The reorganization of plastid proteomes and the removal of damaged proteins not only require protein synthesis, but also machinery for their degradation. We detected strong light-induced accumulation of plastid proteases, especially of those proteases that belong to the Clp protease system (Fig. 7; Supplemental Table S2). Clp proteases are important for plastid viability and constitute an efficient protein degradation system (Fig. 7; for review, see Sakamoto, 2006). It can be speculated that degradation of etioplast proteins fills the plastid amino acid reservoir, which makes de novo synthesis of amino acids during a phase of plastid-type transition unnecessary. This could explain why the relative abundance of amino acid-synthesizing enzymes decreases in the early illumination phase (Fig. 7), although most of their genes are induced at the transcriptional level in Arabidopsis (data acquired from Genevestigator; Zimmermann et al., 2004; Supplemental Fig. S3). In contrast to the enzymes of amino acid metabolism, Calvin cycle enzymes and thylakoid membrane proteins accumulate to higher levels in the light because they are immediately needed for construction of photosynthetic machinery. Their accumulation at the protein level correlates with induction at the transcript level and suggests a positive correlation between transcript and protein levels for these proteins during the deetiolation process (Supplemental Fig. S3).
The examples reported above illustrate that data at different levels of gene expression are necessary and must be integrated for full comprehension of the numerous molecular processes that constitute the development of chloroplasts from etioplasts. Quantitative information about proteins as reported here is necessary to infer regulatory events that take place between the expression of a gene and the metabolite that is synthesized by the gene product. Recent analyses with Arabidopsis confirmed that, in many cases, no apparent correlations between transcript levels, protein activity, and metabolite accumulation exist, suggesting that regulation occurs at different nodes in the network (Gibon et al., 2004, 2006). Knowledge about the concentration of an enzyme, however, may not be sufficient to infer metabolite turnover rates because it is often unfeasible to correlate enzyme abundance with enzyme activity. This can be attributed to posttranslational regulation of enzyme activity. Global analysis of all measurable posttranslational modifications, paralleled by detailed analysis of their effect on enzyme activity and kinetics, are additional sources of information that, when integrated with transcript- and protein-profiling data, provide deeper insights into the functioning of a cell.
CONCLUSION
Our comprehensive proteome analysis of chloroplast development from etioplasts shows that this transition is associated with a significant increase of proteins for photosynthesis and enzymes involved in the Calvin cycle. At the gene regulatory level, proteins that control translation of plastid mRNAs are up-regulated and mRNAs are stabilized by light-induced phosphorylation of a plastid RNP. Because most plastid genes encode proteins that function in photosynthesis, the increased translation rates of their mRNAs support the rapid assembly of a functional thylakoid membrane system. We suggest that most of the energy required for the early phase of chloroplast development is generated by the OPPP and a partial glycolysis module. Further experiments, especially analysis of metabolite and pathway fluxes, will be necessary to expand this model.
MATERIALS AND METHODS
Isolation of Rice Plastids
For plastid isolation, 200 g of rice (Oryza sativa cv japonica) seeds were washed in 5% sodium hydrochloride solution for 10 min, rinsed four times with deionized water, and swollen overnight at 29°C. Seeds were transferred to wet Vermiculite supplemented with one-half-strength concentrated Murashige and Skoog medium (3:2 [v/v] vermiculite:medium). Seedlings were grown in the dark at constant 29°C for 10 d. Prior to isolation of plastids, the plants were illuminated for 0, 2, 4, and 8 h at 29°C. Plastid isolation was performed as described before (Zychlinski et al., 2005). In brief, plastids were purified by a combination of two consecutive density gradient centrifugation steps using Nycodenz as the density gradient medium and several differential centrifugation steps. Each step of the isolation procedure was carried out at 4°C. Batches of 50-g plant material, excluding the roots, were homogenized in 500 mL of etioplast isolation solution (EIS; 10 mm HEPES/KOH, pH 7.8, 2 mm EDTA, 2 mm MgCl2, 1 mm tetrasodiumpyrophosphate, 600 mm sorbitol) supplemented with 0.2% (w/v) bovine serum albumin using a blender. The homogenate was filtered through two layers of Miracloth. The homogenization step and filtration were repeated once. The two filtrates were pooled and refiltered through four layers of Miracloth. The filtrate was subsequently centrifuged for 4 min at 200g to remove cellular debris. The supernatant was recentrifuged for 10 min at 8,000g. Pellets containing plastids were carefully resuspended in EIS supplemented with 0.2% (w/v) bovine serum albumin and subjected to Nycodenz density gradient centrifugation. To this end, the plastid suspension was adjusted with 50% Nycodenz stock solution (50% [w/v] Nycodenz, 10 mm HEPES/KOH, pH 7.8, 2 mm EDTA, 2 mm MgCl2, 1 mm tetrasodiumpyrophosphate, 5 mm dithiothreitol [DTT]) to a final Nycodenz concentration of 30% and a maximal volume of 20 mL. Five milliliters of plastid suspension in 30% Nycodenz were loaded into a 30-mL Corex tube (no. 8445) and finally overlaid with a Nycodenz step gradient of 6 mL 25%, 8 mL 20%, 6 mL 15%, and 3 mL 10% Nycodenz in ESI. The gradient was centrifuged for 45 min at 4°C and 8,000g. The two yellowish bands (bands 2 and 3) at the interface of 20% to 15% and 25% to 20% Nycodenz were harvested from the gradient, pooled, and diluted 3-fold (v/v) with EIS plus 5 mm DTT. The organelle suspension was than centrifuged for 5 min at 8,000g to remove the remaining Nycodenz. The pellet was resuspended in maximal 20-mL EIS per centrifugal tube and used for the second centrifugation step at 500g for 10 min. The pellet was resuspended as before and centrifuged again for 15 min at 500g. The resulting first plastid pellet was subjected to a second Nycodenz gradient centrifugation using only one gradient. From the second gradient, the two yellowish bands were harvested separately, subjected to differential centrifugation, and further processed as described before (Zychlinski et al., 2005). Although both bands contain intact etioplasts that do not differ in their protein pattern as judged by 2-D gel electrophoresis (Zychlinski et al., 2005) they were further analyzed separately as time point 0 h A (top band 2) and 0 h B (bottom band 3). In the gradients of time points 2, 4, and 8 h, only band 2 plastids were visible. Pellets containing the isolated plastids were stored at −80°C.
2-D PAGE
Plastids isolated from plant material grown from 200g seeds were resuspended in approximately 100-μL solubilization buffer (40 mm Tris-base, 7 m urea, 2 m thiourea, 2% CHAPS, 0.5% Brij 35, 0.4% carrier ampholites, 2 mm tributyl phosphine, 20 mm DTT, protease inhibitor cocktail EDTA-free [Roche]) and adjusted to a protein concentration of at least 1 μg/μL. Insoluble material was pelleted for 30 min at 40,000g. For the first dimension, 100 μg of protein were loaded in a total volume of 420-μL solubilization buffer without Tris-base by in-gel rehydration onto 24-cm-long strips with an immobilized linear pH gradient from 4 to 7 (Bio-Rad). Rehydration was performed overnight. Proteins were separated using the IPGphor (GE Healthcare, formerly Amersham Biosciences) for a total of approximately 80 kVh by the following voltage gradient: 3 h from 500 to 2,000 V, 1.5 h from 2,000 to 4,000 V, 15 h at 4,000 V, 0.5 h from 4,000 to 8,000 V, and up to 80 kVh at 8,000 V. Prior to the second dimension, the immobilized pH gradient strips were equilibrated for the reduction/alkylation steps, first for 10 min in equilibration buffer (6 m urea, 2% SDS, 50 mm Tris/HCl, pH 8.8, 20% glycerol) containing 2% DTT and next for 10 min in equilibration buffer containing 2.5% iodacetamide. The second dimension was performed in laboratory-made homogeneous 12% polyacrylamide gels using the Ettan Dalt II unit (GE Healthcare). From time point 0 h A, four different plastid isolations were analyzed in four different electrophoretic separations to include four biological and technical replicates in this experiment. For time points 0 h B, 2 and 4 h of illumination, two biological and technical replicates were analyzed. Altogether, this adds up to six biological replicates for the dark control (0 h A and 0 h B) and four biological replicates for illumination (2 and 4 h light). Only one gel was prepared for the time point of 8 h of illumination, which was not integrated into the quantitative time course experiment.
Protein Detection and Gel Image Analysis
Proteins were detected by in-gel staining with Sypro Ruby (Steinberg et al., 2000) according to the manufacturer's instructions. For detection of phosphoproteins, some gels were stained with the phosphoproptein-specific Pro-Q-Diamond stain (Invitrogen, formerly Molecular Probes) prior to the total protein stain according to the manufacturer's protocol. All gels were scanned at a resolution of 100 μm/pixel using a Typhoon 9400 scanner (GE Healthcare). The electropherograms were analyzed with ProteomWeaver software (Bio-Rad). Therefore, gels of each time point (0 h A, 0 h B, 2 and 4 h) were assigned to different groups. After automated spot detection and spot matching, replicates of one group (one time point) were averaged into an average gel. All gels were included in the statistics. The time point 0 h A was used as the reference group. We considered a relative staining intensity of 0.1 for statistics and protein identification. Proteins that are absent from gels of time point 0 h and those that are strongly up-regulated at time points 2, 4, and 8 h were compared in an additional experiment. In the latter case, spot detection and matching were manually verified.
Spot Excision and In-Gel Protein Digest
Spots were excised from gels of time point 0 h B. Only spots that were also present in the average gel of group 0 h A and having a relative staining intensity of at least 0.1 in the average gel of group 0 h B were considered for the spot-picking process. Proteins that were highly up-regulated during the time course were picked from gels of time points 4 and 8 h. Gel spots were excised using the GelPix (Genetix) robot. Because no carryover was detected in preliminary experiments, we excised the spots by their staining intensity in descending order. Altogether 945 different protein spots were excised from gel 1 of group 0 h B and further processed. Protein spots that were not identified were picked again from gel 2 of group 0 h B. Digestion, Zip tipping, and MALDI target spotting were carried out with a Tecan liquid-handling robot (Genesis ProTeam 150; Tecan AG) using standard protocols according to the manufacturer's instructions. In brief, gel slices were washed in 30% acetonitrile in 50 mm ammonium bicarbonate for 5 min at 37°C and shrunken in 80% acetonitrile for 10 min at room temperature prior to the addition of 25 to 50 ng trypsin in 4 μL 5 mm Tris/HCl, pH 8.2, per spot (Shevchenko et al., 1996). After 5 min, the reswollen gel pieces were overlaid with an additional 5 μL 5 mm Tris/HCl, pH 8, and incubated for 3 h at 37°C. Digestion was stopped with 12 μL of 1% trifluoroacetic acid (TFA). Subsequently, tryptic peptides were extracted and desalted with prewetted (with 80% acetonitril in 0.1% TFA) and washed (with 0.1% TFA) μ-C18 ZipTips (Millipore Corporation) by five binding cycles and two washing steps in 0.1% TFA. Bound peptides were eluted with 0.8 μL MALDI matrix solution (3 mg α-cyano-4-hydroxycinnamic acids in 65% acetonitrile containing 0.1% TFA) and directly spotted onto the MALDI target.
MALDI-TOF/TOF Analysis and Protein Identification
Samples were analyzed on a 4700 proteomics analyzer MALDI-TOF/TOF system (Applied Biosystems), which is equipped with a Nd:YAG laser operating at 200 Hz. All mass spectra were recorded in positive reflector mode and they were generated by accumulating data from 5,000 laser pulses. First, MS spectra were recorded from the standard peptides on each of the six calibration spots and the default calibration parameters of the instrument were updated. Subsequently, MS spectra were recorded for all sample spots on the plate and internally calibrated using signals from autoproteolytic fragments of trypsin if these signals were detectable. Up to five spectral peaks per spot that met the threshold criteria were included in the acquisition list for MS/MS spectra. Peptide fragmentation was performed at a collision energy of 1 kV and a collision gas pressure of approximately 2 × 10−7 Torr. Data from 6,000 laser pulses were summed up for each fragment ion spectrum. Global Proteome Server explorer software (Applied Biosystems) was used for submitting data acquired with the mass spectrometer for database searching. MS and MS/MS data were searched using Mascot version 1.9.05 (Matrix Science) as the search engine (www.matrixscience.com) against the The Institute for Genomic Research rice protein database (downloaded January, 2006). Typically, the following search settings were used: maximal number of missed cleavages, 1; peptide tolerance, 25 to 50 ppm; MS/MS tolerance, 0.2 amu. Carboxyamidomethylation of Cys was set as fixed modification and oxidation of Met was selected as variable modification. In additional searches, acetylation of the N terminus and oxidation of Met were set as variable modifications and semitrypsin was selected as protease specificity.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison of the amino acid composition of rice and Arabidopsis plastid transit peptides.
Supplemental Figure S2. Amino acid composition around the transit peptide cleavage site.
Supplemental Figure S3. Light induction for selected proteins at the transcriptional level.
Supplemental Table S1. Proteins only found in etioplasts.
Supplemental Table S2. List of all identified proteins with staining intensity following illumination.
Supplementary Material
Acknowledgments
We would like to thank the staff of the Functional Genomics Center Zurich for their support and Dr. Johannes Fütterer for critical reading of the manuscript.
This work was supported by funds from the Eidgenössische Technische Hochschule Zurich and Strategic Excellence Project Life Sciences (to W.G. and S.B.) and generous fellowships from the VELUX foundation (to A.Z.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sacha Baginsky (sbaginsky@ethz.ch).
The online version of this article contains Web-only data.
References
- Abdallah F, Salamini F, Leister D (2000) A prediction of the size and evolutionary origin of the proteome of chloroplasts of Arabidopsis. Trends Plant Sci 5 141–142 [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Rakwal R (2006) Rice proteomics: a cornerstone for cereal food crop proteomes. Mass Spectrom Rev 25 1–53 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Dekany K, Adams SL, Ganoza MC (1997) The gene encoding the elongation factor P protein is essential for viability and is required for protein synthesis. J Biol Chem 272 32254–32259 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Apel K, Santel HJ, Redlinger TE, Falk H (1980) The protochlorophyllide holochrome of barley (Hordeum vulgare L.): isolation and characterization of the NADPH:protochlorophyllide oxidoreductase. Eur J Biochem 111 251–258 [DOI] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143 113–134 [DOI] [PubMed] [Google Scholar]
- Baginsky S, Gruissem W (2004) Chloroplast proteomics: potentials and challenges. J Exp Bot 55 1213–1220 [DOI] [PubMed] [Google Scholar]
- Baginsky S, Gruissem W (2006) Arabidopsis thaliana proteomics: from proteome to genome. J Exp Bot 57 1485–1491 [DOI] [PubMed] [Google Scholar]
- Baginsky S, Kleffmann T, von Zychlinski A, Gruissem W (2005) Analysis of shotgun proteomics and RNA profiling data from Arabidopsis thaliana chloroplasts. J Proteome Res 4 637–640 [DOI] [PubMed] [Google Scholar]
- Baginsky S, Shteiman-Kotler A, Liveanu V, Yehudai-Resheff S, Bellaoui M, Settlage RE, Shabanowitz J, Hunt DF, Schuster G, Gruissem W (2001) Chloroplast PNPase exists as a homo-multimer enzyme complex that is distinct from the Escherichia coli degradosome. RNA 7 1464–1475 [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Wardle A, Patel R, Dudley P, Park SK, Twell D, Inoue K, Jarvis P (2005) A molecular-genetic study of the Arabidopsis Toc75 gene family. Plant Physiol 138 715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer Y, Vensel WH, Cai N, Manieri W, Schurmann P, Hurkman WJ, Buchanan BB (2006) A complete ferredoxin/thioredoxin system regulates fundamental processes in amyloplasts. Proc Natl Acad Sci USA 103 2988–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CF (2005) Signaling pathways from the chloroplast to the nucleus. Planta 222 743–756 [DOI] [PubMed] [Google Scholar]
- Bedard J, Jarvis P (2005) Recognition and envelope translocation of chloroplast preproteins. J Exp Bot 56 2287–2320 [DOI] [PubMed] [Google Scholar]
- Belostotsky DA, Rose AB (2005) Plant gene expression in the age of systems biology: integrating transcriptional and post-transcriptional events. Trends Plant Sci 10 347–353 [DOI] [PubMed] [Google Scholar]
- Bollenbach TJ, Schuster G, Stern DB (2004) Cooperation of endo- and exoribonucleases in chloroplast mRNA turnover. Prog Nucleic Acid Res Mol Biol 78 305–337 [DOI] [PubMed] [Google Scholar]
- Bruce BD (2001) The paradox of plastid transit peptides: conservation of function despite divergence in primary structure. Biochim Biophys Acta 1541 2–21 [DOI] [PubMed] [Google Scholar]
- Chen MH, Huang LF, Li HM, Chen YR, Yu SM (2004) Signal peptide-dependent targeting of a rice alpha-amylase and cargo proteins to plastids and extracellular compartments of plant cells. Plant Physiol 135 1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- Ferro M, Salvi D, Brugiere S, Miras S, Kowalski S, Louwagie M, Garin J, Joyard J, Rolland N (2003) Proteomics of the chloroplast envelope membranes from Arabidopsis thaliana. Mol Cell Proteomics 2 325–345 [DOI] [PubMed] [Google Scholar]
- Fey V, Wagner R, Brautigam K, Pfannschmidt T (2005) Photosynthetic redox control of nuclear gene expression. J Exp Bot 56 1491–1498 [DOI] [PubMed] [Google Scholar]
- Fischer K, Weber A (2002) Transport of carbon in non-green plastids. Trends Plant Sci 7 345–351 [DOI] [PubMed] [Google Scholar]
- Giacomelli L, Rudella A, van Wijk KJ (2006) High light response of the thylakoid proteome in Arabidopsis wild type and the ascorbate-deficient mutant vtc2-2: a comparative proteomics study. Plant Physiol 141 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Hohne M, Hendriks JH, Palacios N, Cross J, Selbig J, Stitt M (2004) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Usadel B, Blaesing OE, Kamlage B, Hoehne M, Trethewey R, Stitt M (2006) Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol 7 R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez SM, Nishio JN, Faull KF, Whitelegge JP (2002) The chloroplast grana proteome defined by intact mass measurements from liquid chromatography mass spectrometry. Mol Cell Proteomics 1 46–59 [DOI] [PubMed] [Google Scholar]
- Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K (2004) Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J 40 957–967 [DOI] [PubMed] [Google Scholar]
- Grimm B (1998) Novel insights in the control of tetrapyrrole metabolism of higher plants. Curr Opin Plant Biol 1 245–250 [DOI] [PubMed] [Google Scholar]
- Hayes R, Kudla J, Gruissem W (1999) Degrading chloroplast mRNA: the role of polyadenylation. Trends Biochem Sci 24 199–202 [DOI] [PubMed] [Google Scholar]
- Hayes R, Kudla J, Schuster G, Gabay L, Maliga P, Gruissem W (1996) Chloroplast mRNA 3′-end processing by a high molecular weight protein complex is regulated by nuclear encoded RNA binding proteins. EMBO J 15 1132–1141 [PMC free article] [PubMed] [Google Scholar]
- Ivanova Y, Smith MD, Chen K, Schnell DJ (2004) Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol Biol Cell 15 3379–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P, Robinson C (2004) Mechanisms of protein import and routing in chloroplasts. Curr Biol 14 R1064–R1077 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Ma L, Strickland E, Deng XW (2005) Conservation and divergence of light-regulated genome expression patterns during seedling development in rice and Arabidopsis. Plant Cell 17 3239–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F, Schnell DJ (2006) The function and diversity of plastid protein import pathways: a multilane GTPase highway into plastids. Traffic 7 248–257 [DOI] [PubMed] [Google Scholar]
- K laff P, Gruissem W (1991) Changes in chloroplast mRNA stability during leaf development. Plant Cell 3 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffmann T, Hirsch-Hoffmann M, Gruissem W, Baginsky S (2006) plprot: a comprehensive proteome database for different plastid types. Plant Cell Physiol 47 432–436 [DOI] [PubMed] [Google Scholar]
- Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjolander K, Gruissem W, Baginsky S (2004) The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol 14 354–362 [DOI] [PubMed] [Google Scholar]
- Kubis S, Baldwin A, Patel R, Razzaq A, Dupree P, Lilley K, Kurth J, Leister D, Jarvis P (2003) The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell 15 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis S, Patel R, Combe J, Bedard J, Kovacheva S, Lilley K, Biehl A, Leister D, Rios G, Koncz C, et al (2004) Functional specialization amongst the Arabidopsis Toc159 family of chloroplast protein import receptors. Plant Cell 16 2059–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G (2003) Redox regulation of chloroplast transcription. Antioxid Redox Signal 5 79–87 [DOI] [PubMed] [Google Scholar]
- Lisitsky I, Schuster G (1995) Phosphorylation of a chloroplast RNA-binding protein changes its affinity to RNA. Nucleic Acids Res 23 2506–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonosky PM, Zhang X, Honavar VG, Dobbs DL, Fu A, Rodermel SR (2004) A proteomic analysis of maize chloroplast biogenesis. Plant Physiol 134 560–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juez E, Pyke KA (2005) Plastids unleashed: their development and their integration in plant development. Int J Dev Biol 49 557–577 [DOI] [PubMed] [Google Scholar]
- Loza-Tavera H, Vargas-Suarez M, Diaz-Mireles E, Torres-Marquez ME Gonzalez de la Vara LE, Moreno-Sanchez R, Gruissem W (2006) Phosphorylation of the spinach chloroplast 24 kDa RNA-binding protein (24RNP) increases its binding to petD and psbA 3′ untranslated regions. Biochimie 88 1217–1228 [DOI] [PubMed] [Google Scholar]
- Miras S, Salvi D, Ferro M, Grunwald D, Garin J, Joyard J, Rolland N (2002) Non-canonical transit peptide for import into the chloroplast. J Biol Chem 277 47770–47778 [DOI] [PubMed] [Google Scholar]
- Nair R, Rost B (2005) Mimicking cellular sorting improves prediction of subcellular localization. J Mol Biol 348 85–100 [DOI] [PubMed] [Google Scholar]
- Neuhaus HE, Emes MJ (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51 111–140 [DOI] [PubMed] [Google Scholar]
- Nott A, Jung HS, Koussevitzky S, Chory J (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57 739–759 [DOI] [PubMed] [Google Scholar]
- Pape T, Wintermeyer W, Rodnina MV (1998) Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J 17 7490–7497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SC (2005) Update on proteomics in Arabidopsis: where do we go from here? Plant Physiol 138 591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier JB, Cai Y, Sun Q, Zabrouskov V, Giacomelli L, Rudella A, Ytterberg AJ, Rutschow H, van Wijk KJ (2006) The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Mol Cell Proteomics 5 114–133 [DOI] [PubMed] [Google Scholar]
- Pesaresi P, Masiero S, Eubel H, Braun HP, Bhushan S, Glaser E, Salamini F, Leister D (2006) Nuclear photosynthetic gene expression is synergistically modulated by rates of protein synthesis in chloroplasts and mitochondria. Plant Cell 18 970–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richly E, Leister D (2004) An improved prediction of chloroplast proteins reveals diversities and commonalities in the chloroplast proteomes of Arabidopsis and rice. Gene 329 11–16 [DOI] [PubMed] [Google Scholar]
- Richter S, Lamppa GK (2003) Structural properties of the chloroplast stromal processing peptidase required for its function in transit peptide removal. J Biol Chem 278 39497–39502 [DOI] [PubMed] [Google Scholar]
- Sakamoto W (2006) Protein degradation machineries in plastids. Annu Rev Plant Biol 57 599–621 [DOI] [PubMed] [Google Scholar]
- Scheibe R (1994) Light regulation of chloroplast enzymes. Naturwissenschaften 81 443–448 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56 1481–1489 [DOI] [PubMed] [Google Scholar]
- Schürmann P (2003) Redox signaling in the chloroplast: the ferredoxin/thioredoxin system. Antioxid Redox Signal 5 69–78 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68 850–858 [DOI] [PubMed] [Google Scholar]
- Silva-Filho MC (2003) One ticket for multiple destinations: dual targeting of proteins to distinct subcellular locations. Curr Opin Plant Biol 6 589–595 [DOI] [PubMed] [Google Scholar]
- Soll J, Schleiff E (2004) Protein import into chloroplasts. Nat Rev Mol Cell Biol 5 198–208 [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Chernokalskaya E, Berggren K, Lopez MF, Diwu Z, Haugland RP, Patton WF (2000) Ultrasensitive fluorescence protein detection in isoelectric focusing gels using a ruthenium metal chelate stain. Electrophoresis 21 486–496 [DOI] [PubMed] [Google Scholar]
- Stern DB, Higgs DC, Yang J (1997) Transcription and translation in chloroplasts. Trends Plant Sci 2 308–315 [Google Scholar]
- Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421 79–83 [DOI] [PubMed] [Google Scholar]
- Surpin M, Larkin RM, Chory J (2002) Signal transduction between the chloroplast and the nucleus. Plant Cell 14 S327–S338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow IJ, Rawsthorne S, Raines C, Emes MJ (2004) Plastid metabolic pathways. Annual Plant Reviews 13 60–125 [Google Scholar]
- van Wijk KJ (2004) Plastid proteomics. Plant Physiol Biochem 42 963–977 [DOI] [PubMed] [Google Scholar]
- Villarejo A, Buren S, Larsson S, Dejardin A, Monne M, Rudhe C, Karlsson J, Jansson S, Lerouge P, Rolland N, et al (2005) Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat Cell Biol 7 1224–1231 [DOI] [PubMed] [Google Scholar]
- Vothknecht UC, Westhoff P (2001) Biogenesis and origin of thylakoid membranes. Biochim Biophys Acta 1541 91–101 [DOI] [PubMed] [Google Scholar]
- Weber AP, Schwacke R, Flugge UI (2005) Solute transporters of the plastid envelope membrane. Annu Rev Plant Biol 56 133–164 [DOI] [PubMed] [Google Scholar]
- Yang J, Schuster G, Stern DB (1996) CSP41, a sequence-specific chloroplast mRNA binding protein, is an endoribonuclease. Plant Cell 8 1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinski A, Kleffmann T, Krishnamurthy N, Sjolander K, Baginsky S, Gruissem W (2005) Proteome analysis of the rice etioplast: metabolic and regulatory networks and novel protein functions. Mol Cell Proteomics 4 1072–1084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.