Abstract
Thiamine confers systemic acquired resistance (SAR) on susceptible plants through priming, leading to rapid counterattack against pathogen invasion and perturbation of disease progress. Priming reduces the metabolic cost required for constitutive expression of acquired resistance. To investigate the effects of priming by thiamine on defense-related responses, Arabidopsis (Arabidopsis thaliana) was treated with thiamine and effects of pathogen challenge on the production of active oxygen species, callose deposition, hypersensitive cell death, and pathogenesis-related 1 (PR1)/Phe ammonia-lyase 1 (PAL1) gene expression was analyzed. Thiamine did not induce cellular and molecular defense responses except for transient expression of PR1 per se; however, subsequent Pseudomonas syringae pv tomato challenge triggered pronounced cellular defense responses and advanced activation of PR1/PAL1 gene transcription. Thiamine treatment and subsequent pathogen invasion triggered hydrogen peroxide accumulation, callose induction, and PR1/PAL1 transcription activation in Arabidopsis mutants insensitive to jasmonic acid (jar1), ethylene (etr1), or abscisic acid (abi3-3), but not in plants expressing bacterial NahG and lacking regulation of SAR (npr1 [nonexpressor of PR genes 1]). Moreover, removal of hydrogen peroxide by catalase almost completely nullified cellular and molecular defense responses as well as SAR abolishing bacterial propagation within plants. Our results indicated that priming is an important cellular mechanism in SAR by thiamine and requires hydrogen peroxide and intact NPR1.
Plants have developed an effective immanent surveillance mechanism and pathogen invasions often induce ubiquitous plant defense responses that activate biochemical and structural changes within plant cells. A specific plant's resistance (R) gene product functions as a signaling receptor for the corresponding avirulence (Avr) gene product from the pathogen. The key differences between the compatible (susceptible) and incompatible (resistant) interactions are the early recognition of pathogen attack and the timely expression of defense responses (Yang et al., 1997; McDowell and Dangl, 2000; Lu et al., 2004; Bennett et al., 2005). One of the most noticeable features in the incompatible interaction is abrupt cell death, also termed hypersensitive response (HR), a restricted cell death defining further pathogen progress, which is initiated by the interaction between the R gene product and the Avr gene product. R gene-dependent resistance has been known to share several defense-related responses with systemic resistance induced by plant defense activators.
Systemic resistance is induced by exogenous application of salicylic acid (SA; Delaney, 1997), bacterial elicitors (Desikan et al., 2001), and plant defense activators, such as benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH; Friedrich et al., 1996; Gorlach et al., 1996; Bokshi et al., 2003), and β-aminobutyric acid (BABA; Zimmerli et al., 2000; Jakab et al., 2001; Ton and Mauch-Mani, 2004; Hamiduzzaman et al., 2005; Ton et al., 2005). These defense activators confer broad-spectrum resistance by stimulating common defense mechanisms; for example, oxidative burst (Iriti and Faoro, 2003), accumulation of defense-related materials (Benhamou and Belanger, 1998; Jeun et al., 2000), secondary metabolite production (Kauss et al., 1993), and pathogen-specific defense structure (Brown et al., 1998; Huckelhoven et al., 1999).
Defense activators and specific rhizobacteria confer enhanced disease protection capacity to various host species against multiple pathogens. However, some of these agents do not always trigger cellular and molecular defense responses per se (Graham and Graham, 1994; Jeun et al., 2000; Lyngkjaer and Carver, 2000; Conrath et al., 2001; Ton and Mauch-Mani, 2004; Ton et al., 2005). Subsequent pathogen challenge on plants pretreated with the above agents entailed augmented defense-related responses, such as fortified defense-related gene expression, accumulation of active oxygen species (AOS), callose deposition, and papillae formation singly or in combination. This facet brings up the rapid and efficient activation of defense systems in immunized animals and humans infected by a pathogen (Ludewig et al., 1998; Cho et al., 2002). The enhanced capacity to express pertinent defense mechanisms is termed elicitation competency (Graham and Graham, 1994) or priming (Conrath et al., 2001, 2002). Agents inducing priming do not activate plant defense responses, but rather mobilize plants in a state of enhanced ability to suppress future pathogen attacks (Zimmerli et al., 2000; Jakab et al., 2001, 2005; Conrath et al., 2002; Pozo et al., 2004; Ton et al., 2005). In this respect, primed plants share many characteristics with resistant plants harboring R genes. There is ample evidence that priming is not an uncommon phenomenon in induced resistance. Low doses of plant defense activators, such as BTH, BABA, 2,6-dichloroisonicotinic acid (DCINA), SA, and methyl jasmonate are capable of priming susceptible hosts (Conrath et al., 2002; Si-Ammour et al., 2003; Faize et al., 2004). Priming is one of the most efficient types of induced resistance because the metabolic investment of the plant for the constitutive activation of the defense system is reduced or prevented. Currently, molecular research on priming has been focused on identifying plant genes through transcriptome analyses, screening of gene-trap lines, and map-based cloning.
Defense activators and subsequent pathogen infection trigger cellular defense responses involving oxidative burst and callose induction (Conrath et al., 2001; Park et al., 2002; Tanaka et al., 2003; Faize et al., 2004; Pare et al., 2005). Besides arresting pathogen proliferation in planta, AOS are involved in cell wall reinforcement (Olivain et al., 2003) and act as signaling molecules stimulating systemic resistance (Bolwell et al., 1995, 1998; Tenhaken et al., 1995; Wojtaszek, 1997; Alvarez et al., 1998; Chamnongpol et al., 1998). Callose is a fluorescent β-1,3-glucan complex and rapid deposition of callose is an indicator of defense-related responses (Devadas et al., 2002; Ton and Mauch-Mani, 2004; Flors et al., 2005). In the absence of elicitors or subsequent stresses, methyl jasmonate, SA, or DCINA alone did not induce AOS elevation and/or callose accumulation in cultured parsley (Petroselinum crispum) cells and cucumber (Cucumis sativus) hypocotyls (Kauss et al., 1999; Conrath et al., 2001). Oxidative burst and subsequent defense responses within primed plants mimics R gene-dependent plant defense responses in rice (Oryza sativa; Jia et al., 2000; Ahn et al., 2005a) and Arabidopsis (Ton et al., 2005).
Expression of a set of pathogenesis-related (PR) and defense-related genes has been assumed as one of the reliable molecular markers whether systemic resistance is conditioned or not (Friedrich et al., 1996; Guo et al., 1998; Reuber et al., 1998). In contrast, some agents enhanced plant resistance without direct stimulation of PR gene expression and successful disease protection was achieved (Midoh and Iwata, 1997; Abbasi and Graham, 2001; Graham et al., 2003). To dissect priming phenomena at the molecular level, expression profiling of defense-related genes (Kohler et al., 2002), analyses of transcriptomes altered by priming agents (Verhagen et al., 2004; Pare et al., 2005; Wang et al., 2005), and functional analyses of priming-impaired Arabidopsis mutants have been conducted (Ton et al., 2005). More detailed investigations have revealed that expression patterns of defense genes are largely dependent on the gene that is being monitored. For example, BTH exhibits a dual role at the level of defense gene expression in suspension-cultured parsley cells (Katz et al., 1998) and intact Arabidopsis (Kohler et al., 2002).
In previous research, we presented the alternative role of thiamine as a plant defense activator (Ahn et al., 2005b). The effects of thiamine on disease resistance are prevented in Arabidopsis mutants impaired in SA accumulation. Although expression of PR genes was very transient, rapid and robust transcription was initiated by pathogen challenge and successful blast disease protection was achieved in rice. These phenomena indicate that activated defense status was conditioned in thiamine-treated rice plants.
The objectives of this research were to investigate the defense-associated cellular and molecular responses of plants treated with thiamine and, further, to dissect the strict correlation between defense responses and priming. To achieve these goals, the expression pattern of PR1 and Phe ammonia-lyase1 (PAL1) and the induction of cellular defense responses like AOS accumulation and callose deposition were analyzed in Arabidopsis and its several defense-defective mutants. Results here demonstrate that thiamine-induced priming is in dwelling in plants without physiological alterations and is dependent on hydrogen peroxide accumulation, SA, and NPR1 (nonexpressor of PR genes 1).
RESULTS
Thiamine Protects Arabidopsis from Pseudomonas syringae pv tomato Infection
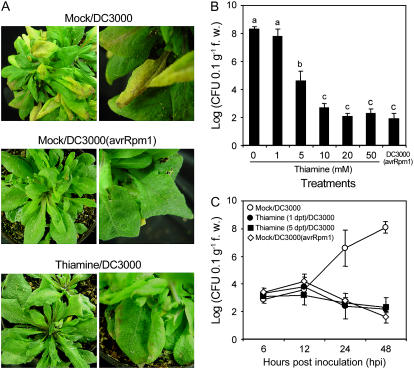
Arabidopsis disease, caused by Pseudomonas syringae pv tomato strain DC3000 (DC3000), is significantly abrogated by systemic acquired resistance (SAR) conditioned by thiamine spray (Ahn et al., 2005b). In this experiment, Arabidopsis was inoculated with DC3000 5 d after 10 mm thiamine spray to determine thiamine-induced priming. Most leaves without thiamine exhibited water-soaked symptoms at 24 to 36 h postinoculation (hpi), turned light yellow at 48 hpi, and finally wilted and died 5 d postinoculation (dpi; Fig. 1A). In contrast, Arabidopsis treated with 10 mm thiamine showed no visible symptoms by DC3000 at 24 hpi. Minute, dark-brown spots were observed at 24 to 36 hpi on the same leaves. These were similar to the symptoms of leaves infected with incompatible DC3000 (avrRpm1). No further symptoms of disease progression were seen thereafter.
Figure 1.
Effect of thiamine application on disease progression in Arabidopsis. A, Arabidopsis ecotype Col-0 plants were inoculated with virulent P. syringae pv tomato strain DC3000 (1 × 108 CFU mL−1 in 250 μg mL−1 Tween 20 [mock]) at 4 h after spraying mock or thiamine (10 mm in mock) solutions. Col-0 was also inoculated with DC3000 expressing avrRpm1 [DC3000 (avrRpm1)] as a resistance control. Samples were collected from 25 plants at 4 dpi. The necrotic lesion on Arabidopsis ecotype Col-0 caused by DC3000 was suppressed in thiamine-treated plants. B, DC3000 growth in the leaves of Arabidopsis ecotype Col-0 treated with increasing concentrations of thiamine prior to DC3000 inoculation. Samples (±1 g fresh weight) were collected from five plants 3 dpi. Each bar represents the mean ± se. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test; P < 0.05). C, Inhibitory effects of thiamine on bacterial growth in Arabidopsis. Arabidopsis ecotype Col-0 was inoculated with DC3000 1 and 5 d after 10 mm thiamine treatment. Samples (±1 g fresh weight) were collected from five plants at 6, 12, 24, and 48 h after inoculation. Each point represents the mean ± se of bacterial count. dpt, Days posttreatment.
Different concentrations of thiamine were applied and disease progression was assessed to determine the dose dependency of SAR. Thiamine spray did not cause any visible alterations in the plants. The 1 mm concentration had no reducing effect on the bacterial titer (Fig. 1B), but bacterial growth was reduced significantly with 5 mm and further by 10 mm. The effect on bacterial growth of higher concentrations of thiamine was not significantly different from that of 10 mm. Moreover, 10 mm thiamine inhibited bacterial growth similar to that of incompatible interaction, indicating that 10 mm is sufficient for subsequent experiments about the defense-related responses conditioned by thiamine.
In addition, Figure 1C showed that 10 mm thiamine-induced SAR was retained for more than 5 d. Leaves challenged with DC3000 1 and 5 d after 10 mm thiamine treatment showed significant reduction of pathogen growth, similar to that of the incompatible interaction. On the other hand, DC3000 propagated robustly 12 to 24 hpi in the mock-treated leaves.
Thiamine Primed Augmented Expression of PR1 and PAL1
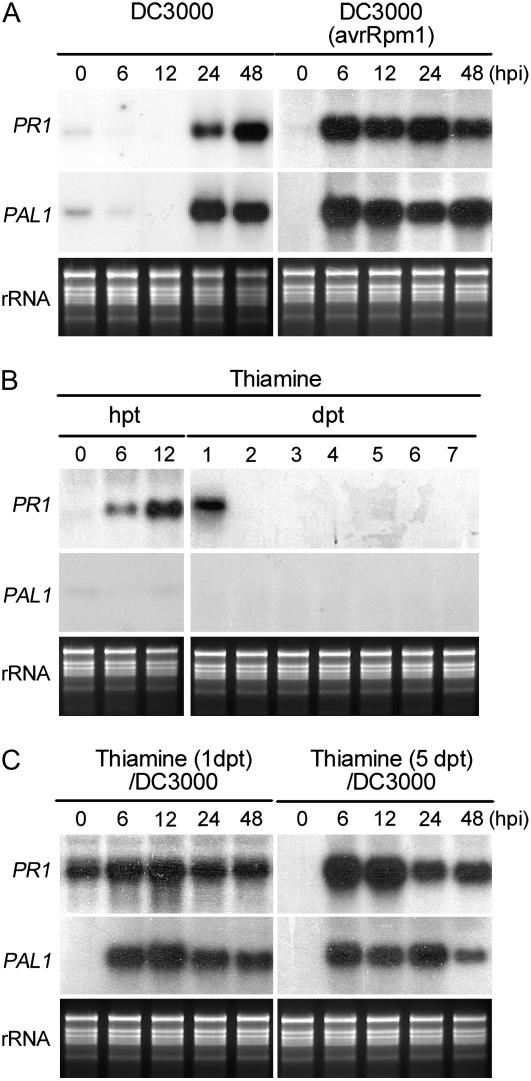
Rapid accumulation of PR gene transcripts has been recognized as one of the molecular indicators for the expression of plant defense responses (Friedrich et al., 1996; van Loon, 1997; van Loon and van Strein, 1999; Kim et al., 2001). To investigate the kinetics of thiamine action, expression patterns of PR1 and PAL1 genes were analyzed. Transcription of both genes was observed 24 hpi in ecotype Columbia (Col-0) leaves inoculated with virulent DC3000 (Fig. 2A). On the other hand, induction of both genes peaked at 6 hpi and was retained thereafter in Col-0 leaves infected with avirulent DC3000 (avrRpm1). Thiamine (10 mm in 250 μg mL−1 Tween 20) treatment transiently induced PR1 expression from 6 to 24 h after treatment (Fig. 2B). However, PAL1 mRNA was not accumulated by thiamine treatment. Figure 2C also shows the expression patterns of PR1 and PAL1 in the plants treated with thiamine and challenged 1 and 5 d later with DC3000. Transcripts of PR1 and PAL1 were highly accumulated at 6 hpi in both treatments.
Figure 2.
PR1 and PAL1 gene expression induced by thiamine treatment and DC3000 inoculation at varying hours posttreatment (hpt), days posttreatment (dpt), hpi, and dpi. Bacterial inoculation and thiamine treatment were performed as described in Figure 1C. A, PR1 and PAL1 gene expression induced by virulent DC3000 or avirulent DC3000 (avrRpm1) infection at 0, 6, 12, 24, and 48 hpi. B, Expression of PR1 and PAL1 genes in Arabidopsis sprayed with 10 mm thiamine at 0, 6, and 12 hpt and 1, 2, 3, 4, 5, 6, and 7 dpt. C, PR1 and PAL1 gene expression in Arabidopsis challenged with virulent DC3000 1 and 5 d after 10 mm thiamine treatment. Total RNA was extracted from the leaves of five Arabidopsis plants recovered 0, 6, 12, 24, and 48 hpi with DC3000.
Cellular Defense-Related Responses
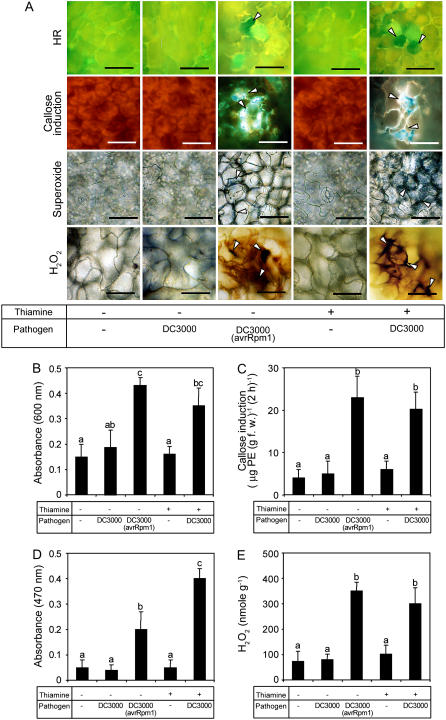
Cellular defense responses induced by thiamine and pathogen inoculation were analyzed. DC3000 (avrRpm1) rapidly induced HR and callose deposition within 12 and 6 hpi, respectively (Fig. 3A). On the other hand, virulent DC3000 infection did not trigger HR and callose deposition within the same time. Thiamine alone did not trigger both responses in Arabidopsis leaves, but thiamine and virulent pathogen challenge induced both responses within 6 and 12 hpi. Quantitative analyses further confirmed the results. Spectrophotometric estimation of Evans blue remained within the dead cells, indicating that virulent DC3000 infection triggered an outbreak of HR within 12 hpi in the thiamine-pretreated leaves (Fig. 3B). The amount of cell death was comparable with that in the leaves challenged with avirulent DC3000 (avrRpm1; Fig. 3B). Callose deposition was also primed by thiamine treatment. Thiamine or DC3000 alone did not induce callose deposition, but DC3000 challenge on the thiamine-treated leaves induced rapid deposition of callose. The amount of callose accumulated within the thiamine and DC3000-treated leaves was 5 times higher than that detected in thiamine-treated or DC3000-inoculated leaves (Fig. 3C).
Figure 3.
Effects of priming by thiamine and pathogen challenge on the cellular defense responses in Arabidopsis. Arabidopsis ecotype Col-0 was sprayed with 10 mm thiamine (+) in 250 μg mL−1 Tween 20 (mock) or mock only (−). Five days after thiamine treatment, Arabidopsis was inoculated with virulent DC3000 (+). Mock (−) was inoculated with avirulent DC3000 (avrRpm1; +). A, Microscopic observation and quantification of hydrogen peroxide and callose deposition were performed on leaves recovered at 6 hpi. Analyses of superoxide accumulation and HR were conducted on leaves harvested 3 and 12 hpi. Blue formazan precipitate or deep-brown color indicates  or H2O2 production. The presence of fluorescence indicates callose deposition. Cell death was determined by the presence (live) or absence (dead) of luminescence. Arrowheads indicate cell death, callose deposition, and superoxide and hydrogen peroxide production. Bars = 50 μm. B, Effects of thiamine and/or DC3000 inoculation on the HR examined by staining with Evans blue. C, Effects of thiamine and/or DC3000 inoculation on callose deposition. D, Effects of thiamine and/or DC3000 inoculation on
or H2O2 production. The presence of fluorescence indicates callose deposition. Cell death was determined by the presence (live) or absence (dead) of luminescence. Arrowheads indicate cell death, callose deposition, and superoxide and hydrogen peroxide production. Bars = 50 μm. B, Effects of thiamine and/or DC3000 inoculation on the HR examined by staining with Evans blue. C, Effects of thiamine and/or DC3000 inoculation on callose deposition. D, Effects of thiamine and/or DC3000 inoculation on  accumulation. E, Effects of thiamine and/or DC3000 inoculation on hydrogen peroxide accumulation. Data presented in B to E were taken in experiments conducted three times. Each bar represents the mean ± se. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test; P < 0.05).
accumulation. E, Effects of thiamine and/or DC3000 inoculation on hydrogen peroxide accumulation. Data presented in B to E were taken in experiments conducted three times. Each bar represents the mean ± se. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test; P < 0.05).
Oxidative burst has often been implicated in hypersensitive cell death (Levine et al., 1994); hence, the effect of thiamine on the accumulation of reactive oxygen species was analyzed. Thiamine (10 mm) treatment did not induce superoxide and hydrogen peroxide accumulation; however, DC3000 challenge provoked augmented accumulation of AOS in thiamine-treated leaves (Fig. 3, A, D, and E). Moreover, the level of hydrogen peroxide production at 6 hpi in leaves pretreated with thiamine was similar to that challenged with avirulent pathogens. Superoxide induction in primed leaves was higher than that induced by DC3000 (avrRpm1) infection.
Catalase Nullifies Priming Induced by Thiamine
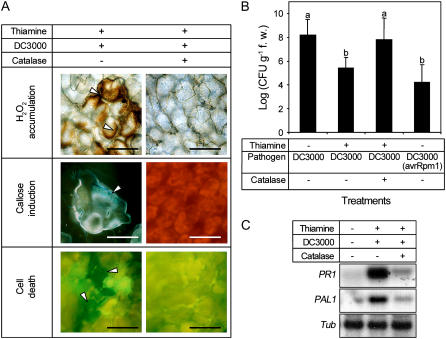
Catalase was infiltrated into thiamine-treated leaves with virulent pathogens to investigate the role of hydrogen peroxide accumulation on cellular defense responses and thiamine-induced priming. Callose deposition, cell death, bacterial growth in planta, and PR1/PAL1 gene expression were investigated. Thiamine-induced priming of cellular and molecular defense responses was nullified by exogenous catalase that scavenges hydrogen peroxide. Hydrogen peroxide accumulation, callose deposition, and HR in thiamine/DC3000-treated leaves were also abolished (Fig. 4A). Priming by thiamine inhibited bacterial growth in Arabidopsis; however, catalase treatment also interdicted this effect (Fig. 4B). The augmented transcription of PR1 and PAL1 was triggered by the virulent pathogen in thiamine-primed leaves; however, this effect was prohibited by the addition of catalase (Fig. 4C).
Figure 4.
Effects of catalase on cellular defense responses, bacterial growth, and PR1/PAL1 gene expression in Arabidopsis treated with thiamine and challenged with DC3000. Virulent DC3000 (5 × 106 CFU mL−1) and/or 5,000 units mL−1 catalase (+) were infiltrated with needleless syringes into Arabidopsis (ecotype Col-0) leaves 5 d after spraying with 10 mm thiamine in 250 μg mL−1 Tween 20 (+) or 250 μg mL−1 Tween 20 only (mock; −). A, Effects of exogenous application of catalase on cellular defense responses induced by thiamine. Samples for determination of hydrogen peroxide accumulation, callose deposition, and cell death (HR) were harvested 6, 6, and 12 hpi, respectively. Arrowheads indicate each response. Bars = 50 μm. B, Titers of DC3000 in Arabidopsis Col-0 plants leaves sprayed with thiamine and/or infiltrated with catalase. DC3000 (avrRpm1) plants are infiltrated with avirulent DC3000 (avrRpm1). Each bar represents the mean ± se. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test; P < 0.05). C, Analysis of PR1 and PAL1 gene expression in the Col-0 leaves sprayed with thiamine and infiltrated with virulent DC3000 and/or catalase. Total RNA was extracted from five plants 6 h after infiltration, separated using denaturing gel electrophoresis, and transferred to nylon membrane. The blots were hybridized with Arabidopsis PR1 and PAL1 probes labeled with [32P]dCTP. All experiments were done at least three times and similar results were obtained.
Priming Is Dependent on SA and the NPR1 Gene in Arabidopsis
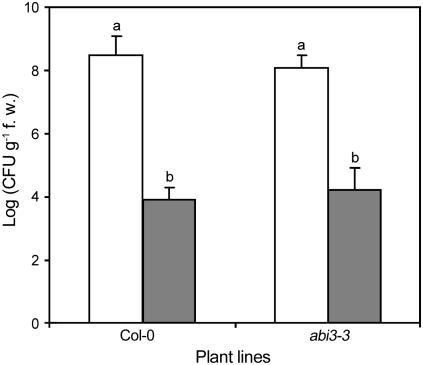
Disease inhibition and PR1 mRNA accumulation by thiamine were nullified by the expression of bacterial NahG and NPR1 mutation (Ahn et al., 2005b). Recently, an abscisic acid (ABA)-related signaling pathway was implicated in BABA-induced priming of defense responses in Arabidopsis (Ton and Mauch-Mani, 2004). To test whether priming by thiamine acts through an ABA-dependent signaling pathway, the effects of thiamine on DC3000 proliferation within the Col-0 and abi3-3 plants were evaluated. The level of pathogen growth in abi3-3 plants was similar to Col-0, and thiamine-induced SAR was not affected by this mutation (Fig. 5). This result suggests that thiamine-induced disease resistance is not related to an ABA-dependent defense-signaling pathway.
Figure 5.
Effects of thiamine on pathogen growth in Col-0 and abi3-3. Bacterial growth is shown on Col-0 and abi3-3 challenged with DC3000 5 d after thiamine treatment. Data were from experiments conducted independently three times. Each bar represents the mean ± se. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test; P < 0.05).
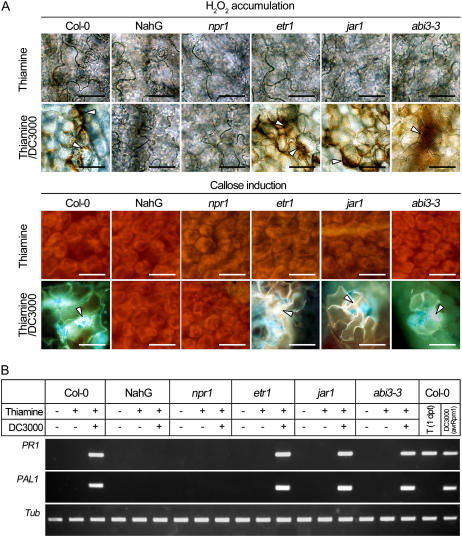
Hydrogen peroxide accumulation and callose deposition were analyzed to confirm whether thiamine-induced priming is absent in NahG and npr1 plants. As shown in Figure 6A, thiamine spray did not affect the production and accumulation of hydrogen peroxide and callose in all plant lines tested. Fortified induction of these defense-related materials was evident in the Col-0, etr1 (an altered perception of ethylene mutant), jar1 (a mutant that displays reduced sensitivity to methyl jasmonate), and abi3-3 (a mutant insensitive to ABA) plants treated with thiamine and challenged 5 d later with DC3000. However, NahG and npr1 plants failed to accumulate hydrogen peroxide and did not show callose deposition when challenged with DC3000. Apparently, both lines are insensitive to priming by thiamine.
Figure 6.
Effects of priming by thiamine on the accumulation of hydrogen peroxide, callose deposition, and PR1/PAL1 transcription in Arabidopsis Col-0 and its mutants. DC3000 was inoculated 5 d after thiamine spray and samples were recovered 6 hpi. A, Hydrogen peroxide accumulation and callose deposition in Arabidopsis Col-0, NahG, npr1, etr1, jar1, and abi3-3. B, Transcription of PR1 and PAL1 in Arabidopsis Col-0, NahG, npr1, etr1, jar1, and abi3-3. Data were from Arabidopsis sprayed with 10 mm thiamine and 250 μg mL−1 Tween 20 (+) or 250 μg mL−1 Tween 20 only (mock; −). Samples were harvested 6 hpi. In addition, leaves of Col-0 were harvested 1 d after thiamine spray (T [1 dpt]) and avirulent DC3000 (avrRpm1) inoculation.
The effects of priming by thiamine at the molecular level were assessed. Expression of PR1 and PAL1 was assayed in thiamine- and/or DC3000-treated leaves by reverse transcription-PCR. PR1 and PAL1 transcripts were not accumulated in all plant leaves harvested 5 d after thiamine spray or 6 h after challenge with DC3000 (Fig. 6B). mRNA of both genes was transcribed in the Col-0 plant inoculated 6 h later with DC3000 (avrRpm1). Expression of PR1 and PAL1 was augmented in thiamine- and DC3000-treated Col-0, etr1, jar1, and abi3-3 plants. In contrast, these treatments did not provoke PR1 and PAL1 transcriptions in the NahG and npr1 plants.
DISCUSSION
This study further supports our previous research on the novel function of thiamine as a plant defense activator (Ahn et al., 2005b). In spite of the lack of PR gene transcription, rice plants treated with thiamine showed blast resistance up to 15 d. This result implies that priming might play a key role in thiamine-induced blast resistance. To further explain this phenomenon, the effects of thiamine on cellular and molecular defense responses were analyzed using Arabidopsis and P. syringae pv tomato.
Thiamine ranging from 5 to 50 mm protects Arabidopsis from bacterial infection (Fig. 1, A and B). This effect was evident in Arabidopsis challenged with DC3000 1 and 5 d after thiamine treatment (Fig. 1C). Results of the in vitro experiment also showed that thiamine did not arrest growth of bacterial pathogens (data not shown). Enhanced disease perturbation in the absence of a direct effect on the causal pathogen confirms the alternative role of thiamine as a plant defense activator. PR1 gene expression has been used as one of the molecular markers determining whether a plant is ready to counteract against pathogen attack. Although PR1 expression was induced by thiamine, this was very transient and disappeared 2 d after treatment. Thiamine did not affect PAL1 transcription per se (Fig. 2B). Interestingly, transcription of both genes was fortified and advanced in Arabidopsis 5 d prior to pathogen challenge (Fig. 2C). Similar expression patterns of PR1 and PAL1 were evident in Arabidopsis inoculated with avirulent pathogens (Fig. 2A). Distinctive disease protection and augmented expression of defense-related genes after subsequent infection of virulent pathogens were clear molecular evidence for priming Arabidopsis by thiamine. SAR accompanied by priming was observed in Arabidopsis and grapevine (Vitis vinifera) treated with BABA (Hamiduzzaman et al., 2005; Ton et al., 2005) and BTH (Kohler et al., 2002). Results of this study further indicated that strong and long-lasting PR1 transcription by chemical treatment is not crucial evidence for the induction of SAR. Disease protection in the absence of PR1 expression was observed in wheat (Triticum aestivum; Gorlach et al., 1996; Stadnik and Buchenauer, 2000), barley (Hordeum vulgare; Jarosch et al., 2003) treated with BTH and DCA, and rice and tobacco (Nicotiana tabacum) treated with brassinolide (Nakashita et al., 2003).
Pathogen challenge triggered fortified AOS accumulation within leaf tissue of thiamine-treated Arabidopsis (Fig. 3). Thiamine and virulent pathogen challenge was required for potentiated AOS production. On the contrary, thiamine spray or virulent pathogen inoculation did not induce AOS burst at the same time (Fig. 3A). These results indicate that rapid AOS production should be one of the defense mechanisms of priming by thiamine. Accordingly, pronounced AOS accumulation followed by pathogen challenge is one of the typical responses of primed plants (Neuenschwander et al., 1995; Desikan et al., 1998; Huckelhoven et al., 1999; Able et al., 2000; Orozco-Cardenas et al., 2001; Pellinen et al., 2002; Shinogi et al., 2003). Thiamine did not affect callose deposition and hypersensitive cell death per se. However, pathogen challenge also provoked augmented callose induction within leaf tissues of Arabidopsis treated with thiamine. Similar results were obtained from microscopic observation of hypersensitive cell death. Our findings indicated that primed Arabidopsis by thiamine was in a surveillance state extremely sensitive to pathogen challenge and, in addition, priming and R gene-dependent resistance shared several features, including AOS burst, callose deposition, hypersensitive cell death, and expression patterns of defense-related genes. Similar results were previously described (Kohler et al., 2002; Graham et al., 2003; Faize et al., 2004; Ton et al., 2005). In particular, the expression pattern of the PAL1 gene is almost completely identical to potentiated cellular defense responses because pathogen challenge triggered strong and advanced augmentation of both responses within plants primed by thiamine application. Apparently, PAL1 and AOS are involved in defense-related metabolism, SA accumulation (Mauch-Mani and Slusarenko, 1996; Smith-Becker et al., 1998), and callose induction (Lyngkjaer and Carver, 2000).
Hydrogen peroxide is also involved in hypersensitive cell death (Lyngkjaer and Carver, 2000; Houot et al., 2001; Kachroo et al., 2003) and acted as a signaling molecule in cellular defense responses (Alvarez et al., 1998; Hu et al., 2003; Fitzgerald et al., 2004). Catalase infiltration almost completely perturbed hydrogen peroxide accumulation by pathogen challenge in thiamine-treated Arabidopsis. This was accompanied by abolition of disease protection, callose induction, hypersensitive cell death, and PR1/PAL1 expression (Fig. 4A). These results strongly suggest that hydrogen peroxide is required for priming by thiamine. Moreover, callose induction and hypersensitive cell death were under the control of hydrogen peroxide in priming by thiamine. Dependence of cell wall fortification on AOS was also reported in Arabidopsis (Razem and Bernards, 2003; Al-Daoude et al., 2005). Hydrogen peroxide also played a substantial role in DCINA-induced SAR (Huckelhoven et al., 1999) and could have contributed to effective papillae (comprised with callose) formation (Bestwick et al., 1997). Catalase did not inhibit pathogen proliferation (van Wees and Glazebrook, 2003); hence, results here clearly indicate that inhibition of AOS prevented priming by thiamine and resulted in SAR impairment.
No discrete hydrogen peroxide accumulation and callose induction were observed in wild-type Col-0 and mutants (Fig. 6A). Challenge of virulent pathogens at 5 d postthiamine spray strongly induced both cellular and molecular defense-related responses in wild-type and etr1, jar1, and abi3-3 plants. In contrast, the same treatment and inoculation did not trigger both events in the NahG and npr1 lines. In parallel, pathogen inoculation triggered fortified transcription PR1 and PAL1 genes in Col-0, etr1, jar1, and abi3-3 lines and this augmented transcription was nullified by bacterial NahG expression and NPR1 mutation (Fig. 6B). It could be concluded from our findings that priming by thiamine exerts its effects through the SA-dependent signaling pathway and might be dependent on hydrogen peroxide accumulation. Recently, priming effects by BABA were investigated in tobacco (Siegrist et al., 2000) and Arabidopsis (Ton and Mauch-Mani, 2004). Interestingly, the mode of action of priming by BABA in tobacco and Arabidopsis was distinct. Protection of tobacco mosaic virus infection by BABA-induced priming was nullified by the expression of bacterial NahG, whereas abolition of Alternaria brassicicola and Plectosphaerella cucumerina infections was not influenced by SA accumulation, but was dependent on ABA. This indicated that acting sites of thiamine and BABA are different in the Arabidopsis defense-signaling cascades and imply that the mode of action of a priming agent is highly influenced by the kind of hosts and pathogens.
The results further demonstrated that priming and its associated cellular and molecular defense mechanisms were induced by thiamine. Thiamine altered the plant into a highly competent state for a long time in the absence of detectable variations. Subsequent pathogen challenge triggered augmented molecular and cellular defense-related responses in thiamine-applied Arabidopsis. Besides its physiological and genetic importance, priming by thiamine could be one of the most economical and effective resistances because expression of defense-related mechanisms in the absence of pathogen requires the plant's metabolic investment necessary for growth or other fitness-related processes (Purrington, 2000; Heil, 2001; van Hulten et al., 2006). Along with conventional antibiotics, previously developed plant defense activators, biocontrol organisms, and improved seed varieties, thiamine could provide novel disease control strategies that satisfy environmental regulations.
MATERIALS AND METHODS
Plant Materials, Chemical Treatment, and Pathogen Challenge
Seeds of the Arabidopsis (Arabidopsis thaliana) ecotype Col-0, the Col-0 expressing bacterial NahG gene, and mutants (npr1, etr1, jar1, and abi3-3) from this line were obtained from The Arabidopsis Information Resource. Arabidopsis was grown in a growth chamber at 22°C, 65% to 70% relative humidity, and 16 h of illumination daily. Four-week-old Arabidopsis was used for chemical treatment and pathogen inoculation.
Thiamine (10 mm, unless otherwise indicated) in 250 μg mL−1 Tween 20 (mock) was hand sprayed onto Arabidopsis until all the plants were covered with fine droplets. The DC3000 and DC3000 (avrRpm1) strains of Pseudomonas syringae pv tomato, each containing pVSP61 and pVSP61 carrying avrRpm1, were cultivated on King's medium B with 50 μg mL−1 kanamycin for 48 h at 28°C. To inoculate Arabidopsis with DC3000 and DC3000 (avrRpm1), bacterial cells were retrieved from the medium with 0.85% NaCl and mock, and the concentration was adjusted to 1 × 108 or 5 × 106 colony-forming units (CFU) mL−1. At least 25 plants of Arabidopsis ecotype Col-0 were inoculated per treatment. Bacterial suspension (1 × 108 CFU mL−1) was sprayed until all leaves were covered with fine droplets 1 or 5 d after thiamine or mock treatment. To investigate the effect of catalase on priming by thiamine, bacterial suspension (5 × 106 CFU mL−1) and catalase (5,000 units mL−1) were also infiltrated into the parenchyma tissue of rosette leaves with a 1-mL needleless plastic syringe. The inoculated plants were kept in a dew chamber for 16 h at 25°C and 100% relative humidity and then transferred to a growth chamber with a 16-h light/8-h dark regime at 25°C and 80% relative humidity. Bacterial growth was assessed by determining the CFU of 1 g (fresh weight) of leaves of five Arabidopsis plants through plating appropriate dilutions on King's B medium containing 50 μg mL−1 kanamycin.
Histochemistry: Superoxide, Hydrogen Peroxide, and Callose
To investigate the effect of thiamine on oxidative burst and callose deposition, more than 10 plants applied with 10 mm thiamine or mock were challenged 5 d later with virulent DC3000. Histochemical detection of superoxide and hydrogen peroxide were performed as described previously (Wohlgemuth et al., 2002). To observe superoxide production, leaves were harvested at 3 hpi and infiltrated immediately with 10 mm sodium azide (NaN3; Sigma) and 0.1% (w/v) nitroblue tetrazolium solution. To determine the accumulation of hydrogen peroxide, leaves were recovered at 6 hpi and stained with 0.1% (w/v) diaminobenzidine (Sigma). Then leaves were cleared with 96% (v/v) ethanol, preserved in 50% (v/v) ethanol, and observed under a light microscope. Superoxide and hydrogen peroxide were indicated as blue formazan formation and red-brown precipitate under the light microscope. To determine callose deposition, leaves were recovered at 6 hpi, fixed with lactophenol, and stained with 0.1% (w/v) aniline blue. Fluorescence of callose was detected with an epifluorescence microscope (E800; Nikon) using a V-2A filter (Reuber et al., 1998). More than 15 leaves from five randomly selected plants were observed in each experiment. These experiments were done at least three times.
Quantitative Determination of Superoxide Radical, Hydrogen Peroxide, and Callose
Superoxide and hydrogen peroxide were extracted from thiamine-treated and/or pathogen-inoculated Arabidopsis leaves and quantified as described (Neuenschwander et al., 1995; Ukeda et al., 1997; Willekens et al., 1997; Frahry and Schopfer, 2001) with some modifications. Arabidopsis leaves were harvested, pulverized with Geno/Grinder (SPEX CertiPrep), extracted with perchloric acid, and debris was removed by centrifugation at 5,000g, 4°C for 15 min. The recovered supernatants were purified using AG 1-X8 resin (Bio-Rad Laboratories). Superoxide level within purified plant extract was determined spectrophotometrically in the presence of 500 μm Na, 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) bezenesulfonic acid hydrate (Sigma), and NADH. Hydrogen peroxide levels were determined using Autolumat LB953 luminometer (EG & G Derthold) as described (Baker et al., 2002). Each reaction mixture contained 0.72 units of peroxidase and 77.6 mm luminol. Extraction and measurement of callose from Arabidopsis leaves were done as described (Kohler and Conrath, 2000). Arabidopsis leaves were harvested and pulverized after complete removal of chlorophyll with ethanol. Callose was extracted from the remaining tissues through boiling in dimethyl sulfoxide and the debris was removed by centrifugation at 5,000g, 4°C for 15 min. After centrifugation, 1 m NaOH and loading mixture containing aniline blue were added to the supernatant. Total fluorescence at 479 nm was determined and fluorescence of callose was calculated by subtracting the autofluorescence in the parallel assay performed without aniline blue. Callose within 1 g fresh weight was quantified based on comparison with the epifluorescence of known amounts of the commercial β-1,3-glucan pachyman (Calbiochem). Samples were harvested from 20 plants in each experiment and these experiments were repeated more than three times.
Detection and Quantification of Cell Death
The effects of thiamine and DC3000 inoculation on cell death were determined. Rosette leaves were recovered 5 d after 10 mm thiamine spray, inoculated with DC3000, and harvested 12 h later. Dead cells or tissues were detected by dye-staining methods. Recovered Arabidopsis leaves were stained with 100 μg mL−1 fluorescein diacetate (Sigma). After 30 min, leaf tissues were observed under a fluorescence microscope using an excitation filter at 450 nm. To quantify dead cells, leaf discs (0.5 mm in diameter) were stained for 30 min with 0.25% Evans blue (Sigma) and washed to remove excess stain (Mino et al., 2002). Dye bound to dead cells was extracted with 1 mL of 50% methanol supplemented with 1% SDS for 1 h at 50°C. Absorbance at 600 nm was estimated with 10-fold dilution of the above dye extract.
RNA Isolation and Expression Analyses
Total RNA was extracted using the lithium chloride precipitation method (Davis and Ausubel, 1989). Hybridization analysis was performed as described (Ahn et al., 2005b). Analyses of PR1 and PAL1 gene expression were performed using reverse transcription-PCR as described (Pieterse et al., 1998). Leaves of Col-0, NahG, npr1, etr1, jar1, and abi3-3 were recovered from five plants of each treatment and total RNA was prepared. First-strand cDNA was synthesized from 50 ng total RNA using a Reverse-iT first-strand synthesis kit and anchored oligo(dT) as indicated by the manufacturer's instructions (AB Gene). Independent PCR using equal aliquots (0.5 μL) of cDNA samples was performed using the gene-specific primers described (Vieira Dos Santos et al., 2003). The tubulin gene was amplified as a quantitative control (Lee et al., 2000). The experiments were repeated at least twice and similar results were obtained.
Acknowledgments
We deeply thank Dr. Maria Excelsis M. Orden for editing this article.
This work was supported by the National Institute of Agricultural Biology (grant to I.-P.A.) and the Crop Functional Genomics Center of the 21st Century Frontier Research Program (grant no. CG2211 to S.-C.S.) with funds from the Ministry of Science and Technology and Rural Development Administration of the Korean government.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Il-Pyung Ahn (jinhyung@rda.go.kr).
References
- Abbasi PA, Graham TL (2001) Age-related regulation of induced isoflavonoid responses in soybean lines differing in inherent elicitation competency. Physiol Mol Plant Pathol 59 143–152 [Google Scholar]
- Able AJ, Guest DI, Sutherland MW (2000) Hydrogen peroxide yields during the incompatible interaction of tobacco suspension cells inoculated with Phytophthora nicotianae. Plant Physiol 124 899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn I-P, Kim S, Kang S, Suh S-C, Lee Y-H (2005. a) Rice defense mechanisms against Cochliobolus miyabeanus and Magnaporthe grisea are distinct. Phytopathology 95 1248–1255 [DOI] [PubMed] [Google Scholar]
- Ahn I-P, Kim S, Lee Y-H (2005. b) Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol 138 1505–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daoude A, de Torres Zabala M, Ko J-H, Grant M (2005) RIN13 is a positive regulator of the plant disease resistance protein RPM1. Plant Cell 17 1016–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer P, Ishikawa A, Dixon RA, Lamb CJ (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92 773–784 [DOI] [PubMed] [Google Scholar]
- Baker JC, O'Neill NR, Deahl K, Lydon J (2002) Continuous production of extracellular antioxidants in suspension cells attenuates the oxidative burst detected in plant microbe interactions. Plant Physiol Biochem 40 641–644 [Google Scholar]
- Benhamou N, Belanger RR (1998) Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato. Plant Physiol 118 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, Mehta M, Grant M (2005) Biophoton imaging: a nondestructive method for assaying R gene responses. Mol Plant Microbe Interact 18 95–102 [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokshi AI, Morris SC, Deverall BJ (2003) Effects of benzothiadiazole and acetylsalicylic acid on -1,3-glucanase activity and disease resistance in potato. Plant Pathol 52 22–27 [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23 517–532 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh C-K, Murphy TM (1998) Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol 116 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I, Trethowan J, Kerry M, Mansfield J, Bolwell GP (1998) Localization of components of the oxidative cross-linking of glycoproteins and of callose synthesis in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestris and French bean mesophyll cells. Plant J 15 333–343 [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H Jr, Van Montagu M, Inze D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 95 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Hayashi T, Datta SK, Takabayashi K, Van Uden JH, Horner A, Corr M, Raz E (2002) IFN-αβ promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J Immunol 168 4907–4913 [DOI] [PubMed] [Google Scholar]
- Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7 210–216 [DOI] [PubMed] [Google Scholar]
- Conrath U, Thulke O, Katz V, Schwindling S, Kohler A (2001) Priming as a mechanism in induced systemic resistance of plants. Eur J Plant Pathol 107 113–119 [Google Scholar]
- Davis KR, Ausubel FM (1989) Characterization of elicitor-induced defense responses in suspension-cultured cells of Arabidopsis. Mol Plant Microbe Interact 2 363–368 [Google Scholar]
- Delaney TP (1997) Genetic dissection of acquired resistance to disease. Plant Physiol 113 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ (2001) Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol 126 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signaling in cell death and defense against pathogens. Plant J 30 467–480 [DOI] [PubMed] [Google Scholar]
- Faize M, Faize L, Koike N, Ishizaka M, Ishii H (2004) Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology 94 604–612 [DOI] [PubMed] [Google Scholar]
- Fitzgerald HA, Chern MS, Navarre R, Ronald PC (2004) Overexpression of (At)NPR1 in rice leads to a BTH- and environment-induced lesion-mimic/cell death phenotype. Mol Plant Microbe Interact 17 140–151 [DOI] [PubMed] [Google Scholar]
- Flors V, Ton J, Jakab G, Mauch-Mani B (2005) Abscisic acid and callose: team players in defence against pathogens? J Phytopathol 153 377–383 [Google Scholar]
- Frahry G, Schopfer P (2001) NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta 212 175–183 [DOI] [PubMed] [Google Scholar]
- Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Rella MG, Meier B, Dincher SS, Staub T, Uknes S, et al (1996) A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J 10 61–70 [Google Scholar]
- Gorlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorp M, Staub T, Ward E, Kessmann H, et al (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8 629–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MY, Graham TL (1994) Wound-associated competency factors are required for the proximal cell responses of soybean to the Phytophthora sojae wall glucan elicitor. Plant Physiol 105 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MY, Weidner J, Wheeler K, Pelow MJ, Graham TL (2003) Induced expression of pathogenesis-related protein genes in soybean by wounding and the Phytophthora sojae cell wall glucan elicitor. Physiol Mol Plant Pathol 63 141–149 [Google Scholar]
- Guo A, Durner J, Klessig DF (1998) Characterization of a tobacco epoxide hydrolase gene induced during the resistance response to TMV. Plant J 15 647–656 [DOI] [PubMed] [Google Scholar]
- Hamiduzzaman MM, Jakab G, Barnavon L, Neuhaus J-M, Mauch-Mani B (2005) β-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Mol Plant Microbe Interact 18 819–829 [DOI] [PubMed] [Google Scholar]
- Heil M (2001) The ecological concept of costs of induced systemic resistance (ISR). Eur J Plant Pathol 107 137–146 [Google Scholar]
- Houot V, Etienne P, Petitot A-S, Barbier S, Blein J-P, Suty L (2001) Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J Exp Bot 52 1721–1730 [PubMed] [Google Scholar]
- Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol 133 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckelhoven R, Fodor J, Preis C, Kogel KH (1999) Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol 119 1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriti M, Faoro F (2003) Benzothiadiazole (BTH) induces cell-death independent resistance in Phaseolus vulgaris against Uromyces appendiculatus. J Phytopathol 151 171–180 [Google Scholar]
- Jakab G, Cottier V, Toquin V, Rigoli G, Zimmerli L, Metraux J-P, Mauch-Mani B (2001) β-Aminobutyric acid-induced resistance in plants. Eur J Plant Pathol 107 29–37 [Google Scholar]
- Jakab G, Ton J, Flors V, Zimmerli L, Metraux J-P, Mauch-Mani B (2005) Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch B, Jansen M, Schaffrath U (2003) Acquired resistance functions in mlo barley, which is hypersusceptible to Magnaporthe grisea. Mol Plant Microbe Interact 16 107–114 [DOI] [PubMed] [Google Scholar]
- Jeun YC, Siegrist J, Buchenauer H (2000) Biochemical and cytological studies on mechanisms of systemically induced resistance to Phytophthora infestans in tomato plants. J Phytopathol 148 129–140 [Google Scholar]
- Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19 4004–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, He Z, Patkar R, Zhu Q, Zhong J, Li D, Ronald P, Lamb C, Chattoo BB (2003) Induction of H2O2 in transgenic rice leads to cell death and enhanced resistance to both bacterial and fungal pathogens. Transgenic Res 12 577–586 [DOI] [PubMed] [Google Scholar]
- Katz VA, Thulke OU, Conrath U (1998) A benzothiadiazole primes parsley cells for augmented elicitation of defense responses. Plant Physiol 117 1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Fauth M, Merten A, Jeblick W (1999) Cucumber hypocotyls respond to cutin monomers via both an inducible and a constitutive H2O2-generating system. Plant Physiol 120 1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Franke R, Krause K, Conrath U, Jeblick W, Grimmig B, Matern U (1993) Conditioning of parsley (Petroselinum crispum) suspension cells increases elicitor-induced incorporation of cell wall phenolics. Plant Physiol 102 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ahn IP, Park C, Park SG, Park SY, Jwa NS, Lee YH (2001) Molecular characterization of the cDNA encoding an acidic isoform of PR-1 protein in rice. Mol Cells 11 115–121 [PubMed] [Google Scholar]
- Kohler A, Conrath U (2000) Extraction and quantitative determination of callose from Arabidopsis leaves. Biotechniques 28 1084–1086 [DOI] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U (2002) Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol 128 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Suh S-S, Park E, Cho E, Ahn JH, Kim S-G, Lee JS, Kwon YM, Lee I (2000) The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 14 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb CJ (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593 [DOI] [PubMed] [Google Scholar]
- Lu G, Jantasuriyarat C, Zhou B, Wang GL (2004) Isolation and characterization of novel defense response genes involved in compatible and incompatible interactions between rice and Magnaporthe grisea. Theor Appl Genet 108 525–534 [DOI] [PubMed] [Google Scholar]
- Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel RM (1998) Dendritic cells efficiently induce protective antiviral immunity. J Virol 72 3812–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngkjaer MF, Carver TLW (2000) Conditioning of cellular defence responses to powdery mildew in cereal leaves by prior attack. Mol Plant Pathol 1 41–49 [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B, Slusarenko AJ (1996) Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 8 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25 79–82 [DOI] [PubMed] [Google Scholar]
- Midoh N, Iwata M (1997) Expression of defense-related genes by probenazole or 1,2-benzisothiazole-3(2H)-one 1,1-dioxide. J Pesticide Sci 22 45–47 [Google Scholar]
- Mino M, Maekawa K, Ogawa K, Yamagishi H, Inoue M (2002) Cell death processes during expression of hybrid lethality in interspecific F1 hybrid between Nicotiana gossei domin and Nicotiana tabacum. Plant Physiol 130 1776–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33 887–898 [DOI] [PubMed] [Google Scholar]
- Neuenschwander U, Vernooij B, Friedrich L, Uknes S, Kessmann H, Ryals J (1995) Is hydrogen peroxide a second messenger of salicylic acid in systemic acquired resistance? Plant J 8 227–233 [Google Scholar]
- Olivain C, Trouvelot S, Binet M-N, Cordier C, Pugin A, Alabouvette C (2003) Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and nonpathogenic strains of Fusarium oxysporum. Appl Environ Microbiol 69 5453–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13 179–191 [PMC free article] [PubMed] [Google Scholar]
- Pare PW, Farag MA, Krishnamachari V, Zhang H, Ryu C-M, Kloepper JW (2005) Elicitors and priming agents initiate plant defense responses. Photosynth Res 85 149–159 [DOI] [PubMed] [Google Scholar]
- Park D-S, Landini S, Graham MY, Graham TL (2002) Induced distal defence potentiation against Phytophthora sojae in soybean. Physiol Mol Plant Pathol 60 293–310 [Google Scholar]
- Pellinen RI, Korhonen M-S, Tauriainen AA, Palva ET, Kangasjarvi J (2002) Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiol 130 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, Van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo MJ, Van Loon LC, Pieterse CMJ (2004) Jasmonates—signals in plant-microbe interactions. J Plant Growth Regul 23 211–222 [Google Scholar]
- Purrington CB (2000) Costs of resistance. Curr Opin Plant Biol 3 305–308 [DOI] [PubMed] [Google Scholar]
- Razem FA, Bernards MA (2003) Reactive oxygen species production in association with suberization: evidence for an NADPH-dependent oxidase. J Exp Bot 54 935–941 [DOI] [PubMed] [Google Scholar]
- Reuber TL, Plotnikova JM, Dewdney J, Rogers EE, Wood W, Ausubel FM (1998) Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J 16 473–485 [DOI] [PubMed] [Google Scholar]
- Shinogi T, Suzuki T, Kurihara T, Narusaka Y, Park P (2003) Microscopic detection of reactive oxygen species generation in the compatible and incompatible interactions of Alternaria alternata Japanese pear pathotype and host plants. J Gen Plant Pathol 69 7–16 [Google Scholar]
- Si-Ammour A, Mauch-Mani B, Mauch F (2003) Quantification of induced resistance against Phytophthora species expressing GFP as a vital marker: β-aminobutyric acid but not BTH protects potato and Arabidopsis from infection. Mol Plant Pathol 4 237–248 [DOI] [PubMed] [Google Scholar]
- Siegrist J, Orober M, Buchenauer H (2000) β-Aminobutyric acid-mediated enhancement of resistance in tobacco to tobacco mosaic virus depends on the accumulation of salicylic acid. Physiol Mol Plant Pathol 56 95–106 [Google Scholar]
- Smith-Becker J, Marois E, Huguet EJ, Midland SL, Sims JJ, Keen NT (1998) Accumulation of salicylic acid and 4-hydroxybenzoic acid in phloem fluids of cucumber during systemic acquired resistance is preceded by a transient increase in phenylalanine ammonia-lyase activity in petioles and stems. Plant Physiol 116 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnik MJ, Buchenauer H (2000) Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f. sp. tritici. Physiol Mol Plant Pathol 57 25–34 [Google Scholar]
- Tanaka N, Che FS, Watanabe N, Fujiwara S, Takayama S, Isogai A (2003) Flagellin from an incompatible strain of Acidovorax avenae mediates H2O2 generation accompanying hypersensitive cell death and expression of PAL, Cht-1, and PBZ1, but not of Lox in rice. Mol Plant Microbe Interact 16 422–428 [DOI] [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb CJ (1995) Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA 92 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Metraux J-P, Mauch-Mani B (2005) Dissecting the β-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 17 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B (2004) β-Amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38 119–130 [DOI] [PubMed] [Google Scholar]
- Ukeda H, Maeda S, Ishii T, Sawamura M (1997) Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal Biochem 251 206–209 [DOI] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103 753–765 [Google Scholar]
- van Loon LC, van Strein EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55 85–97 [Google Scholar]
- van Wees SCM, Glazebrook J (2003) Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv phaseolicola is due to degradation products of salicylic acid. Plant J 33 733–742 [DOI] [PubMed] [Google Scholar]
- Verhagen BWM, Glazebrook J, Zhu T, Chang H-S, van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact 17 895–908 [DOI] [PubMed] [Google Scholar]
- Vieira Dos Santos C, Letousey P, Delavault P, Thalouarn P (2003) Defense gene expression analysis of Arabidopsis thaliana parasitized by Orobanche ramosa. Phytopathology 93 451–457 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ohara Y, Nakayashiki H, Tosa Y, Mayama S (2005) Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol Plant Microbe Interact 18 385–396 [DOI] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inze D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16 4806–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel H-J, Overmyer K, Kangasjarvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25 717–726 [Google Scholar]
- Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shah J, Klessig DF (1997) Signal perception and transduction in plant defense responses. Genes Dev 11 1621–1639 [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Metraux JP, Mauch-Mani B (2000) Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc Natl Acad Sci USA 97 12920–12925 [DOI] [PMC free article] [PubMed] [Google Scholar]