Abstract
The steroid hormone aldosterone is secreted both in the setting of intravascular volume depletion and hyperkalemia, raising the question of how the kidney maximizes NaCl reabsorption in the former state while maximizing K+ secretion in the latter. Mutations in WNK4 cause pseudohypoaldosteronism type II (PHAII), a disease featuring increased renal NaCl reabsorption and impaired K+ secretion. PHAII-mutant WNK4 achieves these effects by increasing activity of the Na-Cl cotransporter (NCC) and the Na+ channel ENaC while concurrently inhibiting the renal outer medullary K+ channel (ROMK). We now describe a functional state for WNK4 that promotes increased, rather than decreased, K+ secretion. We show that WNK4 is phosphorylated by SGK1, a mediator of aldosterone signaling. Whereas wild-type WNK4 inhibits the activity of both ENaC and ROMK, a WNK4 mutation that mimics phosphorylation at the SGK1 site (WNK4S1169D) alleviates inhibition of both channels. The net result of these effects in the kidney would be increased K+ secretion, because of both increased electrogenic Na+ reabsorption and increased apical membrane K+ permeability. Thus, modification at the PHAII and SGK1 sites in WNK4 impart opposite effects on K+ secretion, decreasing or increasing ROMK activity and net K+ secretion, respectively. This functional state for WNK4 would thus promote the desired physiologic response to hyperkalemia, and the fact that it is induced downstream of aldosterone signaling implicates WNK4 in the physiologic response to aldosterone with hyperkalemia. Together, the different states of WNK4 allow the kidney to provide distinct and appropriate integrated responses to intravascular volume depletion and hyperkalemia.
Keywords: hypertension, pseudohypoaldosteronism type II, epithelial Na+ channel, renal outer medullary K+ channel
The precise control of fluid and electrolyte homeostasis is critical for survival and normal function of terrestrial animals. The kidneys' regulation of Na+, Cl−, K+, and intravascular volume balance constitutes a complex, interdependent system that nonetheless must be able to modulate the reabsorption of individual components in response to a complex and changing environment. The net balance of Na+ and Cl− is the major determinant of intravascular volume, allowing proper perfusion and delivery of oxygen and nutrients to tissues. K+ homeostasis is essential for the proper activity of electrically excitable cells, whose resting potential is largely determined by the K+ gradient across the plasma membrane.
The net balance of Na+, K+, and Cl− is determined under normal conditions by their handling in the distal nephron. Sodium and chloride are reabsorbed by either of two mechanisms: Electroneutral Na-Cl cotransport occurs via the thiazide-sensitive Na-Cl cotransporter (NCC) in the distal convoluted tubule and connecting tubule, and electrogenic Na+ reabsorption occurs via the epithelial Na+ channel ENaC in the connecting tubule and collecting duct (1). Electrogenic Na+ reabsorption provides the electrical driving force for either Cl− reabsorption via paracellular flux or, alternatively, for K+ secretion, which, under normal circumstances, occurs via the renal outer medullary K+ channel (ROMK) (2). The key roles of each of these mediators in normal volume, blood pressure, and electrolyte homeostasis is demonstrated by the consequences of mutations that result in increased or diminished activities of these gene products (3).
The activities of these mediators of electrolyte flux are regulated by levels of the steroid hormone aldosterone, which is released from the adrenal glomerulosa and acts via its cognate receptor in the distal nephron to increase activities of ENaC (4), NCC (5), and ROMK (6, 7). Aldosterone levels are increased in response to two distinct physiologic perturbations. Intravascular volume depletion results in increased secretion of the aspartyl protease renin, which increases formation of the peptide angiotensin II, a direct secretagogue for aldosterone (8). In this setting, the resulting increased renal NaCl reabsorption restores intravascular volume toward normal. Aldosterone release is also stimulated directly by high plasma K+ levels; in this setting, increased ENaC activity is used to drive increased K+ secretion, restoring K+ levels toward normal (8).
These different effects produced by high aldosterone levels raise the question of how the kidney distinguishes between hypovolemia and hyperkalemia to achieve the proper physiologic response: maximizing NaCl reabsorption without K+ secretion in the former state and maximizing K+ secretion in the latter. In the setting of such complex systems biology, rare mutations that disrupt overall system behavior can be enlightening. The human disease pseudohypoaldosteronism type II (PHAII), which features hypertension due to increased salt balance and hyperkalemia due to impaired K+ secretion, has provided new insight. Positional cloning demonstrated that this disease is caused by mutations in WNK1 and WNK4, serine–threonine kinases expressed in the distal nephron and other salt-transporting tissues (9). WNK4 has been shown to be a regulator of each of the major Na+, Cl−, and K+ flux pathways in the distal nephron; wild-type WNK4 inhibits activity of NCC, ENaC, and ROMK (refs. 10–12; see also ref. 13 in this issue of PNAS). Importantly, however, PHAII-causing mutations, which cluster within a short acidic stretch of the protein, have divergent effects on these flux pathways. Whereas PHAII-mutant WNK4 abrogates inhibition of NCC and ENaC and selectively increases epithelial permeability for Cl−, it increases inhibition of ROMK (10–15). These effects are expected to maximize renal NaCl reabsorption while impairing K+ secretion, accounting for the hypertension and hyperkalemia seen in PHAII patients.
We have proposed that the renal effects of PHAII-mutant WNK4 constitute the desired response to hypovolemia, and we have suggested that WNK4 is a molecular switch with at least two distinct states that can achieve different effects in response to physiologic perturbation (12). The clustered PHAII mutations define a site that can switch WNK4 to drive renal NaCl retention without K+ secretion.
Recognizing that WNK4 has a basal inhibitory state and another that promotes NaCl reabsorption while inhibiting K+ secretion, we have proposed that WNK4 might also have a third alternative state in which it would promote K+ secretion by alleviating inhibition of both electrogenic Na+ reabsorption and K+ secretion (12). This state would enable the proper response to aldosterone signaling in the setting of hyperkalemia. A major direct transcriptional target of aldosterone signaling is the serine–threonine kinase SGK1 (16). We now report the identification and characterization of a specific SGK1 phosphorylation site in WNK4 and demonstrate that mutations that mimic SGK1 phosphorylation of this site eliminate WNK4's inhibition of both ENaC and ROMK. In contrast to the effects of PHAII mutations, the effects at this SGK1 site would be expected to promote K+ secretion in response to aldosterone, the appropriate response to hyperkalemia. These findings implicate WNK4 in aldosterone-mediated K+ secretion and define a third, distinct state for the WNK4 switch. These findings have implications for the mechanisms of aldosterone signaling, as well as for salt, potassium, and blood pressure homeostasis.
Results
An SGK1 Phosphorylation Site in WNK4.
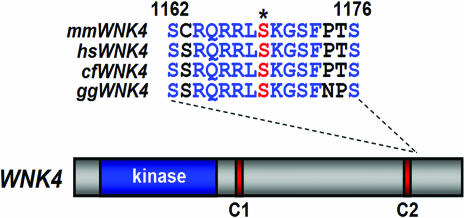
The amino acid sequence of WNK4 contains a strongly predicted SGK1 phosphorylation site at serine-1169, (SCRQRRLSKGSFPTS). This site is predicted to be phosphorylated by SGK1 by both the SCANSITE (17) and NetPhos (18) prediction servers. This putative SGK1 phosphorylation site is highly conserved among WNK4 orthologs, from chicken to human, suggesting its functional significance (Fig. 1).
Fig. 1.
Identification of a conserved SGK1 regulatory site within WNK4 kinase. The structure of WNK4 is shown, including the location of its kinase domain (blue) and two coil domains (red); the sequence of a highly conserved SGK/Akt consensus phosphorylation motif is shown for mouse (Mus musculus), human (Homo sapiens), dog (Canis familiaris), and chicken (Gallus gallus). WNK4 proteins were aligned with Clustal W. The predicted site of SGK1 phosphorylation (serine-1169 in mouse WNK4) is indicated with an asterisk and in red.
To determine whether SGK1 directly phosphorylates WNK4, we purified a GST-tagged fragment of WNK4 (WNK4 amino acid residues 1127–1179; see Methods) that contains the putative SGK1 phosphorylation site. The kinase activity of SGK1 requires either its phosphorylation at serine-422 or replacement of serine-422 with aspartic acid (19). We incubated WNK4 peptides with [γ-32P]ATP and either inactive or active SGK1 (SGK1S422D). Active, but not inactive, SGK1 robustly phosphorylated the WNK4 peptide (Fig. 2a). Mutation of the putative WNK4 phosphorylation site, serine 1169, to alanine completely abolished phosphorylation of the WNK4 peptide, consistent with this being the phosphorylation site (Fig. 2a). Akt (protein kinase B), the closest relative of SGK1 in the AGC family of protein kinases, phosphorylated WNK4 to a much lesser extent than SGK1 (data not shown).
Fig. 2.
SGK1 phosphorylates WNK4 at serine-1169 and complexes with WNK4 in mammalian kidney cells. (a) SGK1 phosphorylates WNK4 at serine-1169. The indicated (+) GST-tagged, C-terminal fragments of WNK4 (residues 1127–1179) containing either the wild-type serine or substitution of alanine at position 1169 were purified and incubated with active or inactive SGK1 in the presence of [γ-32P]ATP; labeling of WNK4 was detected after PAGE by autoradiography as described in Methods, and total protein was revealed by Coomassie staining. (b) WNK4 and SGK1 coimmunoprecipitate in HEK-293T cells. Indicated constructs were expressed in HEK-293 cells, and lysates were immunoprecipitated with anti-HA antibodies, fractionated, transferred to membranes, and probed with indicated antibodies as described in Methods. SGK1 was detected by anti-FLAG antibodies, and WNK4 was detected by anti-HA.
To determine whether a physical complex containing SGK1 and WNK4 can be detected in mammalian cells, we transfected and expressed FLAG-tagged SGK1 and HA-tagged WNK4 in human embryonic kidney cells (HEK-293T). Immunoprecipitation of wild-type WNK4 or PHAII-causing mutant WNK4 with anti-HA antibodies coimmunoprecipitates SGK1 to a similar extent (Fig. 2b). SGK1 also coimmunoprecipitates mutants of WNK4 in which serine-1169 is mutated to aspartic acid or alanine. Together, these results indicate that SGK1 can exist in a physical complex with WNK4 in mammalian kidney cells and that SGK1 phosphorylates WNK4 at serine-1169. These findings raise the question of whether this phosphorylation has functional effects on WNK4's activity.
WNK4S1169D Relieves Inhibition of ENaC.
To test whether mutations in WNK4 that mimic phosphorylation at serine-1169 alter WNK4's activity, we compared amiloride-sensitive Na+ currents in Xenopus oocytes expressing ENaC plus with either wild-type WNK4 or WNK4S1169D (mimicking phosphoserine at position 1169) by using a two-electrode voltage clamp. Whereas wild-type WNK4 inhibits ENaC activity, WNK4S1169D showed a highly significant difference, with nearly complete loss of inhibitory activity (P = 2.4 × 10−3 vs. ENaC + wild-type WNK4; see Fig. 3). Western blotting shows that WNK4S1169D is expressed like its wild-type counterpart, so the loss of inhibition cannot be explained by reduced WNK4 expression. This effect showed specificity for the S1169D mutation, because another mutation at the same site, WNK4S1169A, inhibited ENaC to an extent similar to wild-type WNK4 (P = 0.46 vs. ENaC + wild-type WNK4; see Fig. 3). Thus, mimicking phosphorylation at the SGK1 consensus phosphorylation site in WNK4 abrogates WNK4's inhibition of ENaC activity. This loss of ENaC inhibition with mutation at the WNK4 SGK1 phosphorylation site is similar to the effect of a PHAII-causing mutation at amino acid 562 in WNK4 (13).
Fig. 3.
WNK4S1169D mutation alleviates inhibition of ENaC. cRNAs encoding the indicated proteins were injected into Xenopus oocytes, and amiloride-sensitive, whole-cell Na+ currents were recorded as described in Methods. Cumulative results of amiloride-sensitive currents measured at −100 mV are shown as a proportion of the ENaC control; mean ± SE for each group is shown. A minimum of 29 oocytes were studied in each group. The ENaC alone and wild-type WNK4 data are the same data reported in ref. 13; data for all experimental conditions were collected from the same batches of oocytes concurrently. Indicated P values represent the results of two-tailed Student's t tests. Western blotting of oocyte lysates confirms the presence of WNK4-HA in the corresponding experimental groups.
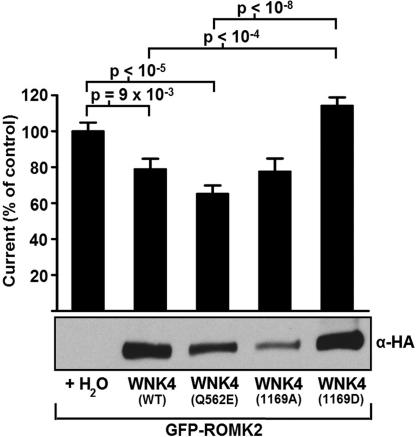
Opposing Effects of WNK4Q562E and WNK4S1169D on ROMK.
Like its inhibitory effect on ENaC and NCC, wild-type WNK4 inhibits the activity of the K+ channel ROMK (ref. 7 and Fig. 4). Whereas PHAII-mutant WNK4 alleviates WNK4's inhibition of ENaC and NCC, PHAII-mutant WNK4 has the opposite effect on ROMK, showing enhanced inhibition. In contrast to the inhibition of ROMK seen with PHAII–WNK4, WNK4S1169D has the opposite effect, completely alleviating inhibition of ROMK (Fig. 4). The difference in ROMK activity between oocytes expressing WNK4S1169D and either wild-type WNK4 or PHAII–WNK4 is highly significant (P = 2 × 10−5 and 4 × 10−9, respectively). This effect is not due to impaired production of WNK4, as shown by Western blotting (Fig. 4). The ROMK activity seen with WNK4S1169D is actually slightly higher than for ROMK in the absence of WNK4 (16% increase, P = 0.04). Thus, in contrast to the similar effects of PHAII and SGK1 site mutations on ENaC activity, these mutations impart opposite effects on ROMK activity and define distinct states of WNK4 function.
Fig. 4.
WNK4S1169D mutation alleviates inhibition of ROMK. cRNAs encoding the indicated proteins were injected into Xenopus oocytes, and Ba2+-sensitive whole-cell K+ currents were recorded. Cumulative results of Ba2+-sensitive whole-cell K+ currents measured at +40 mV are shown as a proportion of the ROMK control. A minimum of 19 oocytes were analyzed in each group. Indicated P values represent the results of two-tailed Student's t tests. Western blotting of oocyte lysates confirms the presence of WNK4-HA in the corresponding experimental groups.
Discussion
The application of genetics to the analysis of complex systems has great potential to identify key nodes that govern system behavior. WNK4 was discovered because its mutation in humans had the paradoxical effects of increasing renal NaCl reabsorption while inhibiting K+ secretion, effects that suggested the existence of a previously unrecognized element regulating these transport pathways (9). Subsequent work has demonstrated that wild-type WNK4 inhibits structurally unrelated channels and cotransporters, including NCC, ENaC, and ROMK (10–13). Single missense mutations that cause PHAII alleviate inhibition of the former two targets but increase inhibition of the latter and also selectively increase epithelial Cl− permeability. The net consequences of these effects are to orchestrate an increase in reabsorption of Na+ and Cl− while simultaneously inhibiting K+ secretion (10–15). These distinct yet concerted effects on diverse targets, resulting from single amino acid substitutions, indicate that WNK4 is a molecular switch that can exist in distinct functional states that promote specific coordinated responses to physiologic perturbations; in the kidney, these functions serve to regulate the balance between NaCl reabsorption and K+ secretion. In vivo evidence in support of this model comes from genetically modified mice expressing either wild-type or PHAII-mutant WNK4 (20). In accord with expectations of the switch model, these alternative forms of WNK4 have opposite gain-of-function effects on blood pressure and K+ homeostasis (20).
These effects are poised to serve a mechanism by which the kidney can provide a differential response to aldosterone signaling in two distinct physiologic states, intravascular volume depletion and hyperkalemia, ensuring maximal NaCl reabsorption without K+ secretion in the former state and maximal K+ secretion in the latter state (12). The two states of WNK4 that have heretofore been identified would correspond to a basal equilibrium state and a second state, exemplified by PHAII mutations, which would play a role in the response to hypovolemia (Fig. 5). We have proposed that the strongly clustered mutations in WNK4 that cause PHAII define a site that is normally bound by a ligand in the setting of volume depletion, which sets the WNK4 switch into the salt-retaining state. Although the identity of this putative regulator has not yet been determined, the binding of calcium ions to this negatively charged segment of WNK4 as a consequence of signaling by angiotensin II or other hormones presents one possible mechanism.
Fig. 5.
WNK4 orchestrates differential responses to hypovolemia and hyperkalemia. WNK4 has at least three distinct functional states defined by the effects of wild-type WNK4, PHAII-mutant WNK4, and WNK4S1169D on downstream targets. The effects at two of these targets, ROMK and ENaC, are shown. We propose that intravascular volume depletion (Low IV Volume) and hyperkalemia (High K+), which are associated with either aldosterone plus angiotensin II (aldo + AII) or aldosterone alone, respectively, have differential effects at downstream targets. At equilibrium, WNK4 inhibits both ENaC and ROMK. PHAII mutation defines a state that alleviates inhibition of ENaC while augmenting inhibition of ROMK, promoting a net increase in renal Na+ reabsorption without K+ loss. Hyperkalemia results in increased aldosterone signaling, phosphorylation of the SGK1 site, and loss of WNK4 inhibition of both ENaC and ROMK. This results in a net increase in K+ secretion via increases in both the electrical driving force for K+ secretion and K+ channel activity.
From these considerations, we have speculated that there might exist a third state for WNK4 that would promote increased K+ secretion. This state would be activated in response to hyperkalemia with high aldosterone levels without activation of the renin–angiotensin system (12). The addition of this third WNK4 state would permit diverse signals entering the kidney to be integrated by a single target and would allow WNK4's activity at downstream targets to direct a coordinated and appropriate response to the different physiological settings in which aldosterone is released. We now present evidence for the existence of this third state. Modification of a single amino acid of WNK4 alleviates its inhibition of ENaC, which would increase electrogenic Na+ reabsorption and provide an increased lumen-negative potential, which is required for normal K+ secretion. This same mutation also abrogates inhibition of ROMK, the key channel for normal net renal K+ secretion. These are precisely the effects required for maximal K+ secretion in response to hyperkalemia (Fig. 5). Most significantly, the WNK4 modification that produces this third state is a substitution that mimics phosphorylation at the site phosphorylated by SGK1, a major transcriptional target of the activated mineralocorticoid receptor (16). This finding provides compelling evidence that aldosterone signaling modulates WNK4 activity through SGK1 to promote a state that favors K+ secretion. Combined with prior knowledge of the effects of PHAII-mutant WNK4, these findings indicate that WNK4 is capable of directing alternative coordinated responses appropriate to distinct physiologic perturbations.
These findings also have implications for the function of the related protein WNK1. Increased WNK1 expression due to intronic deletions also cause PHAII (9). In vitro work has suggested that at least part of WNK1's effect is through inhibition of WNK4 activity at NCC. Interestingly, SGK1 has been shown to be activated by WNK1 through a PI3 kinase-dependent mechanism (21, 22). This finding suggests that the inhibition of WNK4 by WNK1, as reported in ref. 23, may be mediated by SGK1.
These findings have a number of interesting implications and raise a number of important questions for further investigation. First, the observed effects of WNK4S1169D on ENaC and ROMK raise the question of how WNK4S1169D might affect other WNK4 targets. Specifically, the effects on NCC and paracellular Cl− flux will be of interest to examine. Because an isolated increase in aldosterone secretion, as occurs with aldosterone-producing tumors and glucocorticoid-remediable aldosteronism (24) commonly produce hypertension, we speculate that WNK4S1169D will likely increase NCC activity. It will be of interest to see the effects on these pathways in vitro and to determine the consequences of the S1169D mutation in vivo.
Second, it is unclear whether the three states of WNK4 thus far identified are discrete and mutually incompatible or whether their effects from combined modification at the PHAII and SGK1 sites can function in the same molecule. For example, one can imagine that modification of one or both sites might have allosteric effects that lock the molecule into distinct conformations or that recruit other proteins to complex with WNK4, thereby presenting or hiding specific functional domains of the protein. In this case, only one of these states could exist in a single molecule at one time. In this setting, graded responses to physiologic perturbation could nonetheless be achieved by variation in the proportion of molecules with each modification, which would in turn be determined by the relative magnitudes of the upstream signals. Alternatively, there might be dominant or additive effects of one modification regardless of other modifications in the same molecule (e.g., the combination of PHAII mutation and SGK1 modification might be additive or favor only the effects of PHAII mutation). These alternatives can be tested in part in vitro by examination of the effects of both modifications in the same molecule. In vivo experiments may also help distinguish among these possibilities. For example, the results of administration of aldosterone to mice harboring only PHAII-mutant WNK4 would indicate whether an increased kaliuretic effect could be achieved in the presence of the PHAII mutation.
From a clinical perspective, isolated aldosterone secretion without hyperkalemia or hypovolemia is nonphysiologic and classically produces increased renal salt reabsorption with variable hypokalemia. WNK4 activity could promote these effects after modification at the SGK1 site if, in addition to the described effects at ENaC and ROMK, NCC activity and/or paracellular Cl− flux is also increased. This observation leaves open the question of whether further modulation of WNK4 function might enable maximal K+ secretion without NaCl reabsorption, as would be desired in the setting of hyperkalemia. In this regard, it is interesting that WNK4's action to increase NCC activity and paracellular Cl− flux is kinase-dependent, whereas activation of ENaC and ROMK are kinase-independent. These observations raise the possibility that an independent mechanism to modulate WNK4's kinase activity could alter the balance between K+ secretion and NaCl reabsorption. In addition, this state of WNK4 that we have identified lends further support to the notion that maximizing K+ secretion can play an intrinsic role in NaCl balance. We have speculated that the well described but poorly understood blood pressure-lowering effect of supplemental dietary K+ (25) may occur because increased renal K+ secretion results in obligatory decreases in renal NaCl reabsorption. The current findings lend a new element to the mechanisms of K+ homeostasis and its relationship to NaCl handling.
Finally, the emerging importance of WNK4 in the regulation of diverse targets by both kinase-dependent and -independent mechanisms highlights that little is yet known about the specific biochemical mechanisms that link WNK4 activity to its downstream functional targets (11–14). This layer of integrated physiology revealed by the diverse effects of WNK4 demonstrates previously unrecognized complexity in the regulation of the balance between NaCl and K+ balance and affords the possibility of defining new physiology that may be manipulated for therapeutic benefit.
Methods
Molecular Cloning.
Mouse WNK4 with an appended 3′ hemagglutinin A (HA) tag in the pcDNA3.1− into pGH19 vectors are as described in ref. 13. A fragment of WNK4 corresponding to bases 3379–3537 (amino acids 1127–1179) was amplified from a plasmid template with primers containing restriction sites for 5′ SalI and 3′ NotI and cloned into pGEX-4T-1. Full-length and truncated clones of the α- (pSD5), β- (pSPORT), and γ- (pSPORT) subunits of the epithelial Na+ channel (ENaC) were as described in ref. 26, and SGK1-FLAG was provided by Cecilia Canessa (Yale University). pSPORT-eGFP-ROMK2 was published in ref. 12. All point mutations were introduced using the QuikChange site-directed mutagenesis system (Stratagene, La Jolla, CA).
In Silico Peptide Analysis of WNK4.
The complete peptide sequence of WNK4 was analyzed using SCANSITE (17) and NetPhos 2.0 (18) online prediction severs.
Phosphorylation Assays.
pGEX-4T-1-WNK4(1127–1179) and GST-control pGEX-4T-1 were transformed into BL21(DE3) cells, and expression of the peptide was induced by the addition of isopropyl β-d-thiogalactoside. Cells were pelleted and lysed in B-PER bacterial protein extraction reagent (Pierce, Rockford, IL) with 1.7 mM PMSF. Lysates were cleared, and glutathione-Sepharose 4B (Amersham Pharmacia, Piscataway, NJ) was mixed with the supernatants. After incubation for 1 h, the slurry was washed with PBS followed by kinase buffer [20 mM Hepes (pH 7.5)/10 mM MgCl2/10 mM MnCl2/1 mM DTT].
Ten microliters of each slurry sample was incubated with 10 ng of constitutively active or inactivated SGK1 or AKT (Upstate Biotechnology, Charlottesville, VA) in kinase buffer containing 5 μCi [γ-32P]ATP (1 Ci = 37 GBq) at 30°C for 25 min. Samples were washed three times with kinase buffer and loaded onto a 13% Tris·HCL gel for SDS/PAGE electrophoresis. Gels were stained with Coomassie brilliant blue and exposed to x-ray film for autoradiography.
Transient Transfection and Immunoprecipitation in Mammalian Cells.
HEK-293T cells at 70% confluency were transfected with 10 μg of pcDNA3.1 WNK4-HA and pCR2.1 SGK1-FLAG plasmid DNA by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Forty-eight hours after transfection, cells were washed and lysed at 4°C in lysis buffer [10 mM Tris·HCl (pH 8.0)/2.5 mM/MgCl2/5 mM EGTA (pH 8.0)/1% Triton X-100/one tablet of protease inhibitor mixture (Roche Molecular Biochemicals, Mannheim, Germany) per 10 ml of buffer]. Lysates were cleared by centrifugation, and the supernatant was used for immunoprecipitation. Twenty-five microliters of agarose beads preconjugated with mouse anti-HA antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added to each lysate and incubated with rotation at 4°C for 16–24 h. Beads were washed with lysis buffer, and the immunoprecipitated proteins were separated by SDS/PAGE gradient gel electrophoresis. Proteins were transferred for Western blotting to a 0.2-μm PVDF membrane (Bio-Rad, Hercules, CA).
Membranes were blocked in 10% nonfat dried milk in PBS with 0.1% Tween 20 (PBS-T). Rabbit anti-HA antibodies (Santa Cruz) or mouse anti-FLAG antibodies (Sigma, St. Louis, MO) were diluted 1:5,000 in 5% milk in PBS-T and incubated with the membrane for 1 h at room temperature. Blots were washed in PBS-T and probed with HRP-conjugated antibodies (Zymed, San Francisco, CA) diluted 1:5,000 in 5% milk in PBS-T. Membranes were washed, and chemiluminescence was performed (ECL-Plus; Amersham Pharmacia) following standard protocols.
Functional Studies in Xenopus laevis Oocytes.
Plasmids used for functional studies encoded wild-type, PHAII-mutant Q562E, and mutant 1169D or 1169A versions of mouse WNK4 (pGH19-WNK4-HA); rat α, β, and γ ENaC (pSD5-α rENaC, pSPORT1-β rENaC, and pSPORT1-γ rENaC); and pSPORT-eGFP-ROMK2.
Plasmids were linearized by restriction digestion, cRNA was transcribed, and X. laevis oocytes were harvested and defolliculated in compliance with institutional regulations as described in ref. 12.
For experiments with ROMK, oocytes were injected with ROMK cRNA alone or together with wild-type or mutant WNK4 cRNAs and incubated for 2–3 days, and Ba2+-sensitive whole-cell K+ currents were measured by a two-electrode voltage clamp as described in ref. 12. All reported K+ currents refer to Ba2+-sensitive currents at +40 mV. Experiments were pooled by normalizing the currents to the average of the ROMK control group. Effects of wild-type and PHAII-mutant WNK4 on ROMK reported here are systematically smaller than we have previously reported, which may be attributable to the WNK4 constructs used. Nonetheless, the relative effects of wild-type and PHAII–WNK4 on ROMK activity are as we reported in ref. 12.
For experiments with ENaC, ocytes were injected with cRNA encoding the α-, β-, and γ-subunits of rat ENaC alone or together with wild-type or mutant WNK4 cRNAs, and whole-cell, amiloride-sensitive Na+ currents were determined by two-electrode voltage clamping as described in ref. 13. All Na+ currents reported refer to Na+ currents measured in the absence of amiloride. Oocyte membrane potentials were held at −40 mV, and currents were measured at 20-mV steps from −160 to +80 mV. Na+ currents and current–voltage plots were recorded using pCLAMP software (Axon Instruments, Union City, CA). Experiments were pooled by normalizing the currents to the average of the ENaC control group.
For Western blots with lysates from oocytes, five oocytes from each experimental group were lysed with RIPA buffer [150 mM NaCl/10 mM Tris (pH 7.2)/0.1% SDS/1.0% Triton X-100/1% deoxycholate/5 mM EDTA]. Lysates were cleared by centrifugation, and 20 μg of each protein sample was fractionated by SDS/PAGE. Proteins were transferred for Western blotting to a 0.2-μm PVDF membrane (Bio-Rad) at 25 V and 4°C for 16 h. Membranes were probed as above by using rabbit anti-HA antibodies (Sigma) diluted 1:5,000 in 5% milk in PBS-T.
Statistical Analysis.
For all statistical comparisons, results were compared by two-tailed unpaired t tests or by nonparametric Mann–Whitney U tests if the data violated a normal distribution.
Acknowledgments
We thank Peter Aronson, Cecilia Canessa, and Gerhard Giebisch for helpful discussions. This work was supported in part by a National Institutes of Health Specialized Center of Research in Hypertension grant (to R.P.L.) and a Beckman Scholar award (to A.M.R.).
Abbreviations
- ENaC
epithelial Na+ channel
- NCC
Na-Cl cotransporter
- PHAII
pseudohypoaldosteronism type II
- PBS-T
PBS with 0.1% Tween 20
- ROMK
renal outer medullary K+ channel.
Footnotes
The authors declare no conflict of interest.
References
- 1.Reilly RF, Ellison DH. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 2.Lee DB, Huang E, Ward HJ. Am J Physiol. 2006;290:F20–F34. doi: 10.1152/ajprenal.00052.2005. [DOI] [PubMed] [Google Scholar]
- 3.Lifton RP, Gharavi AG, Geller DS. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 4.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 5.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wald H, Garty H, Palmer LG, Popovtzer MM. Am J Physiol. 1998;275:F239–F245. doi: 10.1152/ajprenal.1998.275.2.F239. [DOI] [PubMed] [Google Scholar]
- 7.Beesley AH, Hornby D, White SJ. J Physiol (London) 1998;509:629–634. doi: 10.1111/j.1469-7793.1998.629bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn SJ, Williams GH. Annu Rev Physiol. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- 9.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, et al. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 10.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CL, Angell J, Mitchell R, Ellison DH. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, et al. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 13.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. Proc Natl Acad Sci USA. 2007;104:4020–4024. doi: 10.1073/pnas.0611727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Proc Natl Acad Sci USA. 2004;101:4690–4694. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, et al. Proc Natl Acad Sci USA. 2004;101:14877–14882. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Proc Natl Acad Sci USA. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obenauer JC, Cantley LC, Yaffe MB. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom N, Gammeltoft S, Brunak S. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 19.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, et al. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 21.Xu BE, Stippec S, Lazrak A, Huang CL, Cobb MH. J Biol Chem. 2005;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- 22.Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. Proc Natl Acad Sci USA. 2005;102:10315–10320. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH. J Clin Invest. 2005;115:1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 25.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. N Eng J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 26.Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. Proc Natl Acad Sci USA. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]