Abstract
We previously have described the cAMP-mediated induction of cholesterol and phospholipid efflux from the murine macrophage RAW264 cell line to lipid-free apolipoprotein acceptors. This induction of cholesterol efflux is associated with increased binding and association of apolipoprotein to the cells. In the present study, using primarily apolipoprotein AI (apoAI) as the acceptor, cAMP-dependent cholesterol efflux to apolipoprotein acceptors was associated with apoAI binding to coated pits, cellular uptake, and resecretion. After cell association and washing, 58% of the apoAI was resecreted during a 90-min chase period. In addition, after apoAI uptake and washing, cholesterol efflux was observed during a chase period without additional acceptors. Cholesterol efflux was partially blocked by chlorpromazine and hypertonic media, two inhibitors of coated pit endocytosis. Cholesterol efflux to apoAI was found to depend on extracellular calcium. By temporally separating the cAMP induction phase from the apoAI chase phase, calcium was found to be required during the apoAI chase phase rather than during the cAMP induction period. In the absence of calcium the 8-Br-cAMP-mediated induction of apoAI binding was maintained, but the specific apoAI cellular association was inhibited. The data are consistent with a model for cholesterol efflux to apolipoproteins that involves a calcium-dependent endocytic pathway, followed by recycling and the subsequent release of the nascent lipoprotein particle from the cell.
Foam cells, derived from monocyte-macrophage, play a pivotal role in the early fatty streak stage of atherosclerosis (1). The mass of cholesterol in a macrophage is a function of the amount of cholesterol it takes up and synthesizes endogenously, and the amount of cholesterol that it can dispose of. The unregulated uptake of cholesterol via macrophage scavenger receptors is considered the main mechanism of cholesterol accumulation in these cells. There are only two known pathways for macrophages to dispose of cholesterol: (i) via cholesterol efflux to cholesterol acceptors (2), and (ii) via cholesterol 27-hydroxylase, which converts cholesterol to a more water-soluble form (3). These methods of cholesterol disposal are the first steps of the reverse cholesterol transport pathway (4), a process in which cholesterol is removed from the periphery for delivery to the liver for disposal. However, despite much effort, the mechanism of cholesterol efflux from peripheral cells to extracellular acceptors is still not fully understood. Two major pathways are considered to be involved in the transfer of cholesterol from the cell membrane to acceptors. The first pathway, defined by Rothblat, Phillips, and their colleagues (5), involves high density lipoprotein (HDL)-like acceptors and has the properties of passive diffusion of cholesterol from cell membrane zones with a high cholesterol-to-phospholipid ratio down a concentration gradient to acceptor particles with lower cholesterol to phospholipid ratios. This pathway appears to be mediated by the membrane protein SR-B1, as the activity of this pathway is correlated with SR-B1 levels in various cell lines (6). The second pathway for cholesterol efflux, originally identified by Yokoyama (7, 8), uses lipid-free apolipoprotein acceptors, such as apolipoprotein E (apoE) and apolipoprotein AI (apoAI), as well as amphipathic peptides. Cholesterol efflux to these acceptors results in the creation of immature pre-β HDL-like particles, which can be further acted on by lecithin-cholesterol acyl transferase to lead to the formation of spherical HDL (9). The cellular mediators of this pathway are not known.
We previously reported that cholesterol efflux to apolipoproteins from the murine macrophage RAW264 cell line is absent in basal conditions, but inducible by treatment of the cells with cAMP analogues (10). Our data support the model that cAMP induces expression of a membrane receptor for apolipoproteins that transfers both cholesterol and phospholipids to the acceptor and results in the net efflux of cholesterol from cells (10). We now present ultrastructural, pharmacological, and pulse–chase studies that characterize a calcium-dependent pathway for cholesterol efflux to apolipoproteins involving receptor-mediated endocytosis in coated pits followed by the resecretion of a nascent lipoprotein particles.
METHODS
Materials.
Human apoAI and apoE2 were commercially obtained (PerImmune, Rockville, MD). Alternatively, apoAI was purified from human HDL as described (11), and its concentration was determined by an alkaline modification of the Lowry assay (12). Chlorpromazine, 8-Br-cAMP, and other fine chemicals were obtained from Sigma. Antibodies to annexins I, V, VI, and VII were from Santa Cruz Biotechnology.
Cholesterol Efflux Studies.
Cholesterol efflux experiments were performed as described (10), with minor alteration. Subconfluent RAW264 cells in 24-well dishes were cholesterol loaded and labeled in 0.5 ml of DGGB (DMEM supplemented with 50 mM glucose, 2 mM glutamine, and 0.2% BSA) containing 50 μg/ml [3H]cholesterol-labeled acetylated low density lipoprotein (LDL). On the next day, the cells were washed twice with PBS containing 0.2% BSA and chased with 1 ml of DGGB containing apoAI or human HDL with or without 0.3 mM 8-Br-cAMP. At the specified times, 100 μl of medium was removed for determination of radioactivity after centrifugation to remove cell debris. At the end of the chase period, the cells were dissolved in 0.5 ml of 0.2 M sodium hydroxide, and the radioactivity in an aliquot was measured. The percentage of cholesterol efflux was calculated by dividing the medium-derived radioactivity/dish by the sum of the radioactivity in the medium and the cells. Other variations of the basic time course of these experiments are detailed in the figure legends. For calcium-free conditions, calcium-free DMEM was substituted for DMEM in preparing DGGB, and cells were washed with PBS/0.2% BSA containing 1 mM EGTA. Cytotoxicity was assessed by measuring lactate dehydrogenase (LDH) activity (Cytotox96 kit, Promega) in the medium divided by total LDH activity released from the cells after cell lysis with 0.9% Triton X-100.
Apolipoprotein Binding, Association, and Degradation.
ApoAI (125 μg) in 0.1 M phosphate buffer, 3 M urea was iodinated with Iodo-beads (Pierce), according to the manufacturer’s instructions. The radiolabeled apoAI was purified by Sephadex G-25M column chromatography (Amersham Pharmacia) and purity of the labeled apoAI was verified by SDS/PAGE and autoradiography. Binding at 4°C was performed for 1 hr on nearly confluent cells in 24-well dishes, with 500–3,000 ng/ml of [125I]apoAI, as described (10). For calcium-free binding, cells were washed with PBS/0.2% BSA/1 mM EGTA, and binding was performed in calcium-free DGGB containing 20 μM EGTA. After the incubation, the cells were washed once with cold PBS/0.2% BSA and twice with cold PBS, and dissolved in 0.5 ml of 0.2 M sodium hydroxide. 125I radioactivity and cell protein in the lysate were determined. Specific binding was calculated as total binding minus nonspecific binding, which was determined in wells incubated with 40-fold excess nonlabeled apoAI, and normalized per mg of cell protein. Specific cellular association and degradation of [125I]apoAI were determined by incubation at 37°C for 3 hr by using 3,000 ng/ml apoAI, as previously reported. Specific uptake and degradation were calculated by subtracting the nonspecific uptake and degradation measured in the presence of 40-fold excess unlabeled apoAI. [125I]apoE2 (1,000 ng/ml), labeled with Bolton-Hunter reagent (New England Nuclear) as described (10), was used for a cell association study performed as described above.
Apolipoprotein Resecretion.
RAW264 cells were cholesterol-loaded with or without 0.3 mM 8-Br-cAMP for 16 hr. On the next day, after washing twice with prewarmed PBS, the cells were incubated for 1 hr at 37°C with either [125I]apoAI (1,200 ng/ml, 1712 cpm/ng) or [125I]apoE2 (1,000 ng/ml, 2,455 cpm/ng) in DGGB, with or without 0.3 mM 8-Br-cAMP. The cells then were washed five times with cold PBS containing 1 mM calcium chloride, 0.2% BSA, and 5 μg/ml human HDL, to remove any apolipoprotein adherent to the cell surface. The cells then were chased for 90 min at 37°C in 0.5 ml of DGGB containing 50 μg/ml HDL, with or without 0.3 mM 8-Br-cAMP. After this chase, the media were saved and the cells were washed once with PBS/BSA and twice with PBS and dissolved by incubation in 0.5 ml of 0.2 M NaOH for determination of the residual cell associated radioactivity. Total and trichloroacetic acid (TCA)-soluble radioactivity in the medium was determined as described above. The total cell uptake was calculated as the medium plus cell-associated radioactivity at the end of the chase period. The amount of degradation was determined as the TCA-soluble fraction of the medium, and the amount of resecreted apolipoprotein was the difference between the total and degraded radioactivity in the medium. An aliquot of the medium was spun in an ultracentrifuge to obtain an HDL fraction (1.050 < d < 1.21).
Electron Microscopy of apoAI Bound to Cells.
RAW264 cells were incubated for 24 hr with 100 μg/ml of acetylated LDL, with or without the addition of 0.3 mM 8-Br-cAMP. The cells were washed and incubated at 4°C for 90 min with 20 μg/ml of unlabeled human apoAI in DGGB. After washing, the cells were incubated for an additional 60 min at 4°C with a 1:250 dilution in DGGB of anti-human apoAI IgG (Incstar, Stillwater, MN). The cells were washed and incubated for 50 min at 4°C with a 1:20 dilution in DGGB of protein A colloidal gold (Pierce). The cells were fixed in 2.5% glutaraldehyde for transmission electron microscopic analysis at The Rockefeller University core laboratory. All photos were originally at ×65,000 magnification.
Statistics.
Statistical analyses were performed by using prism software (GraphPad, San Diego).
RESULTS
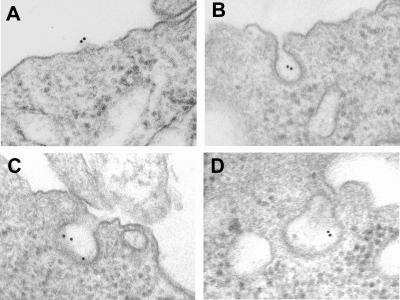
8-Br-cAMP induced cholesterol efflux from RAW264 cells to apoAI in a dose-dependent manner that was saturable at about 10 μg/ml (data not shown), similar to our published data for cAMP-mediated efflux to apoE (10). In the absence of 8-Br-cAMP, apoAI did not lead any cholesterol efflux over the background level. We previously had demonstrated that 8-Br-cAMP treatment induces apoE and apoAI binding to RAW264 cells (10). Ultrastructural analysis via transmission electron microscopy was performed to localize the site of the cAMP-induced binding of apoAI. RAW264 cells were pretreated with or without 8-Br-cAMP and were incubated with unlabeled apoAI. The bound apoAI was visualized with antibody to apoAI and protein A-colloidal gold. The results were striking; virtually no gold particles were detected in samples from the untreated cells (not shown), whereas gold particles were easily seen in the cAMP-pretreated samples (Fig. 1). The gold particles were seen associated only with the plasma membrane (Fig. 1A), or more commonly in what appear to be coated pits or coated vesicles (Fig. 1 B–D).
Figure 1.
Ultrastructural analysis of apoAI binding to cAMP-treated RAW264 cells. The cAMP-induced apoAI binding site on RAW264 cells was localized by indirect immunogold labeling, as described in Methods. Binding occurred on the cell surface (A) or in coated pits (B–D). No binding was observed on cells not treated with 8-Br-cAMP (not shown).
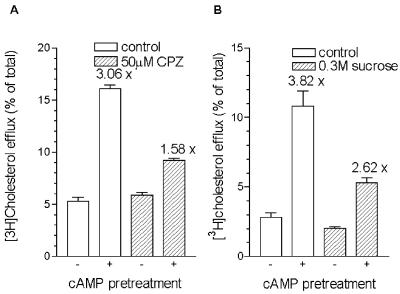
To confirm that coated pits play a role in RAW264 cells in the cAMP-mediated cholesterol efflux to apoAI, chlorpromazine and hypertonic shock were used as two different pharmacological treatments that inhibit clathrin-coated pit formation and receptor-mediated endocytosis (13, 14). Initial experiments determined times and doses of chlorpromazine and sucrose that were reported to inhibit endocytosis and did not increase cytotoxicity above 8%. 8-Br-cAMP was added as a pretreatment, so that the effects of these treatments could be ascertained in 2- to 4-hr chase periods. Chlorpromazine (50 μM) led to a marked reduction in the cAMP-mediated cholesterol efflux to apoAI during a 4-hr chase, without affecting the basal efflux level (Fig. 2A). Sucrose (0.3 M) also decreased the cAMP-mediated cholesterol efflux to apoAI in a 2-hr chase period, although the basal efflux level was also slightly decreased by the hypertonic medium (Fig. 2B). Chlorpromazine also reduced cAMP-induced cholesterol efflux to apoE (data not shown).
Figure 2.
Decrease of cAMP-dependent cholesterol efflux to apoAI by inhibitors of endocytosis. Cholesterol-loaded and -labeled RAW264 cells were pretreated with or without 0.3 mM 8-Br-cAMP for 16 hr and subsequently chased with 10 μg/ml apoAI in DGGB in the absence or presence of 50 μM chlorpromazine (CPZ) (A) or 0.3 M sucrose (B). The cells were washed and pretreated for 15 min with or without chlorpromazine or sucrose, and then chased with apoAI in DGGB (open columns) or in DGGB containing chlorpromazine or sucrose (hatched columns) for 4 hr (A) or 2 hr (B). n = 3 ± SD. The numbers over the columns are the fold increase in efflux caused by 0.3 mM 8-Br-cAMP.
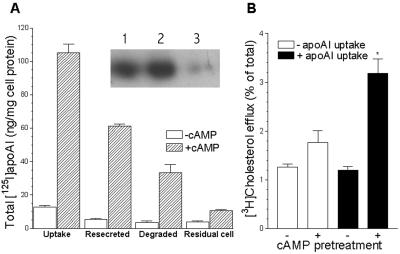
To determine whether there was resecretion of the apolipoprotein after cellular uptake, RAW cells were incubated with labeled apoAI or apoE for 1 hr, and after extensive washing in the presence of HDL, the cells were chased for 90 min with unlabeled HDL to compete for reuptake of any resecreted apolipoproteins. The cAMP pretreatment greatly increased total [125I]apoAI cell uptake (Fig. 3A). After the subsequent 90-min chase, 58% of the cell-associated label was resecreted into the medium as protein-associated radioactivity, and 32% was degraded, whereas only 10% remained associated with the cells (Fig. 3A). The chase medium and an aliquot of chase medium of HDL density from cAMP-pretreated cells were run on SDS/PAGE and radioactivity in intact apoAI was apparent by autoradiography (Fig. 3A, Inset). In a similar resecretion experiment with [125I]apoE2, 33% was resecreted, 35% degraded, and 32% remained with the cells after the 90-min chase (data not shown). In a related experiment, we allowed cholesterol-loaded cells, treated with or without 8-Br-cAMP, to take up apoAI for 1 hr, and after thorough washing we detected significant cAMP-dependent [3H]cholesterol efflux during a subsequent 30-min chase without exogenous apoAI (Fig. 3B). The low level of cholesterol efflux in this experiment is caused by the short incubation and chase periods. Thus, not only is cell-associated apoAI secreted back into the media, but cholesterol also is released at the same time in the absence of additional acceptors.
Figure 3.
Resecretion of apoAI and cholesterol efflux from cAMP-treated RAW264 cells. (A) Total cell uptake of [125I]apoAI and the amount resecreted, degraded, and remaining in the cells after a 90-min chase were determined, as described in Methods, with (hatched columns) or without (open columns) pretreatment with 0.3 mM 8-Br-cAMP. (Inset) SDS/PAGE autoradiography of [125I]apoAI from an aliquot of the media after the 90-min chase (lane 1), from an aliquot of the HDL fraction from the 90-min chase media (lane 2), and from apoAI not incubated with cells (lane 3), demonstrating that intact apoAI was recovered from the chase media after resecretion from cells pretreated with 0.3 mM 8-Br-cAMP. (B) Cholesterol efflux from cells first pretreated for 16 hr with or without 0.3 mM 8-Br-cAMP, then incubated for 1 hr with or without 10 μg/ml apoAI to allow uptake of apoAI into the cells, then washed four times in cold PBS/BSA and once in PBS/BSA at 37°C, and finally chased for 30 min at 37°C in DGGB without additional acceptors. ∗, n = 6 ± SD, P < 0.001 compared with all other columns by Newman–Keuls Multiple Comparison test after ANOVA.
We next examined the role of extracellular calcium ions in cholesterol efflux to apoAI and HDL. We previously had shown that HDL mediates a dose-dependent cholesterol efflux that has both cAMP-independent and cAMP-dependent components (10). The removal of calcium from the medium significantly inhibited the cAMP-induced cholesterol efflux to both apoAI and HDL, but did not inhibit the cAMP-independent cholesterol efflux to HDL (Fig. 4). In these experiments, cytotoxicity was never greater then 7%, as assayed by lactate dehydrogenase release (data not shown). Calcium removal also significantly inhibited the cAMP-induced cholesterol efflux to apoE (data not shown), confirming the similarity of the efflux pathway that we observed previously for these two apolipoprotein acceptors (10). In a calcium dose-response study, the half-maximal inhibitory effect on cAMP-mediated cholesterol efflux to apoAI was observed between 30 and 100 μM Ca2+ (data not shown).
Figure 4.
Calcium requirement for 8-Br-cAMP-stimulated cholesterol efflux. Cholesterol-loaded and -labeled RAW264 cells were incubated for 24 hr with 10 μg/ml apoAI (A) or with 10 μg/ml HDL (B) in normal calcium-containing DGGB (open columns) or in calcium-free DGGB (solid columns), with or without 0.3 mM 8-Br-cAMP, as indicated. n = 3 ± SD. The numbers over the bars are the fold increase in efflux by 8-Br-cAMP. Significance of 8-Br-cAMP effect on cholesterol efflux within the same calcium condition determined by t test; ∗, P ≤ 0.005; ∗∗, P < 0.001; #, not significant. Basal efflux without apoAI or HDL was not stimulated by cAMP and was <10% (not shown).
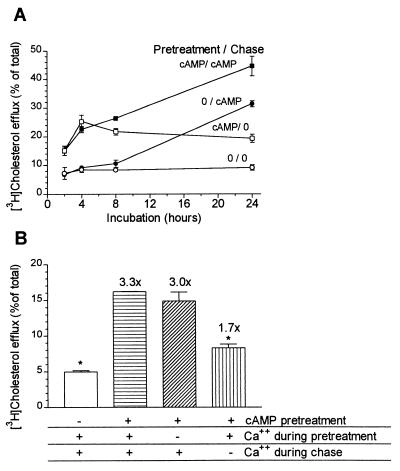
To determine at which stage calcium was needed in the cAMP-mediated cholesterol efflux to apoAI, a series of time-course experiments were performed with 8-Br-cAMP added in four different regimens (Fig. 5). If 8-Br-cAMP was added only during the chase phase, there was a time lag of about 8 hr before increased cholesterol efflux to apoAI was observed (Fig. 5A), similar to what we have observed previously for efflux to apoE (10). However, if the cAMP analogue was added during a 16-hr pretreatment period and again during the chase phase, induced cholesterol efflux was apparent by 2 hr, which was the first time point examined, and increased throughout the 24-hr chase (Fig. 5A). If cAMP was omitted during the chase, having been present during the pretreatment, cholesterol efflux increased through the first 4 hr, but did not increase further afterward (Fig. 5A). Thus, 8-Br-cAMP addition during the pretreatment phase was still effective to induce cholesterol efflux for 4 hr after being washed out. We took advantage of this temporal separation of the cAMP induction phase, during the pretreatment, from the chase phase to determine which phase depended on calcium. Fig. 5B shows the results of this experiment in which 8-Br-cAMP, if added, was present only during the pretreatment phase, and cholesterol efflux to apoAI was followed during the subsequent 4-hr chase. Calcium-free medium was used either during the pretreatment or the 4-hr chase phase. The absence of calcium from the medium during the apoAI chase inhibited cholesterol efflux significantly (P < 0.01), whereas its absence during the cAMP pretreatment had no significant effect on cholesterol efflux (Fig. 5B).
Figure 5.
Characterization of Ca2+ requirement for 8-Br-cAMP-mediated cholesterol efflux to apoAI. (A) Time course of 8-Br-cAMP induced cholesterol efflux with various 8-Br-cAMP treatment regimens. Cells were treated with or without 0.3 mM 8-Br-cAMP during the 16-hr cholesterol loading and labeling period (pretreatment) and/or during the subsequent chase with 10 μg/ml apoAI. ○, efflux without 8-Br-cAMP; ●, efflux with 8-Br-cAMP added only during apoAI chase; □, efflux with 8-Br-cAMP added only during pretreatment; ■, efflux with 8-Br-cAMP added both during the pretreatment and apoAI chase. n = 3 ± SD. (B) Calcium is required for cholesterol efflux only during the apoAI chase. RAW264 cells were cholesterol loaded and labeled for 16 hr and subsequently treated with (lined columns) or without (open column) 0.3 mM 8-Br-cAMP during the 24-hr pretreatment. The cells then were chased for 4 hr with 10 μg/ml apoAI. Calcium-free conditions were used either during the pretreatment (diagonal-line column), or during the apoAI chase (vertical-line column). The numbers above the columns show folds increase in cholesterol efflux compared with the cells treated without cAMP. n = 3 ± SD. Columns significantly differ by ANOVA, P < 0.001; ∗, P < 0.01 compared with cAMP-positive control (horizontal-line column) in Dunnett’s multiple comparison test.
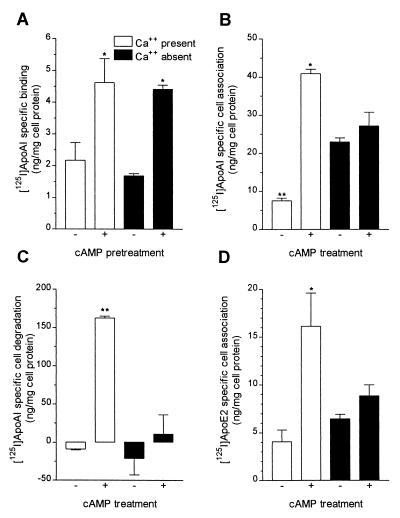
Because extracellular calcium apparently was not necessary for cAMP-mediated signal transduction during the pretreatment phase, we next determined which step in this efflux pathway required calcium. We previously had shown that cAMP treatment induced apoAI or apoE binding to RAW264 cells at 4°C (10); thus, specific binding of [125I]apoAI in the presence or absence of calcium was assayed in cells that had been pretreated with or without 8-Br-cAMP. Pretreatment with cAMP induced specific binding of apoAI to RAW264 cells, confirming our earlier findings, and, the absence of calcium during the binding experiment did not inhibit apoAI binding to the cells (Fig. 6A, representative of four independent experiments). Next, we checked whether apoAI cell association and degradation at 37°C were affected by calcium removal. 8-Br-cAMP pretreatment significantly induced both apoAI cellular association and degradation (P < 0.001), and these cAMP inductions both were inhibited by the removal of calcium (Fig. 6 B and C). However, calcium removal also increased basal apoAI cell association in the absence of 8-Br-cAMP pretreatment (Fig. 6B). We also examined the effect of calcium-free medium on the cell association of apoE2, which was induced by cAMP treatment. This induction of apoE2 cell association also was blocked in the calcium-free media; however, unlike apoAI association, there was no significant increase in the basal cell association in the absence of calcium (Fig. 6D). We also examined the effect of calcium on resecretion of apolipoproteins from cells. In an experiment analogous to that shown in Fig. 3A, if calcium was omitted solely during the 90-min chase of cAMP-treated cells preloaded with labeled apoAI or apoE, there was no effect on resecretion of either apoAI or apoE (data not shown). Thus, extracellular calcium appears indispensable for apolipoprotein endocytosis, but not for resecretion.
Figure 6.
Calcium requirement for cellular apoAI binding, association, and degradation. (A) Cholesterol-loaded cells were pretreated for 16 hr with or without 0.3 mM 8-Br-cAMP and then were incubated with 3,000 ng/ml [125I]apoAI, in the absence or presence of excess unlabeled apoAI, at 4°C for 1 hr to determine specific apoAI binding as described in Methods. Calcium-containing (open columns) or calcium-free (solid columns) medium was used during the 1-hr binding period. n = 3 ± SE, overall P < 0.01 by ANOVA; ∗, P < 0.05 compared with all other except each other in Newman-Keuls multiple comparison test. (B and C) Cells treated as in A were incubated with [125I]apoAI for 3 hr at 37°C to determine specific apoAI cell association (B) and degradation (C) as described in Methods. Calcium-containing (open columns) or calcium-free (solid columns) medium was used during the 3-hr incubation period. n = 3 ± SE, overall P < 0.001 by ANOVA for both B and C; ∗, P < 0.01 vs. all; ∗∗, P < 0.001 vs. all; remaining not significantly different from each other in Newman-Keuls multiple comparison test. (D) Cells treated as in A were incubated with [125I]apoE2 for 3 hr at 37°C to determine specific apoE2 cell association in calcium-containing (open columns) or calcium-free (solid columns) medium. n = 3 ± SE, overall P = 0.01 by ANOVA; ∗, P < 0.05 vs. all; remaining not significantly different from each other in Newman-Keuls multiple comparison test.
We investigated the calcium-dependent enzyme secretory phospholipase A2 (sPLA2) and its product lysophophatidylcholine (lyso PC) for their potential in altering cholesterol efflux. At doses up to and including 75 μg/ml, lyso PC had no effect on cholesterol efflux to apoAI (data not shown). At doses of 100 μg/ml and greater, lyso PC did lead to increased cholesterol efflux from RAW264 cells; however, this activity was completely concordant with its cytotoxic activity (data not shown). Furthermore, adding exogenous snake venom sPLA2 (90 units/ml) had no effect on cholesterol efflux from RAW264 cells (data not shown). Thus, sPLA2 or lyso PC are not likely to be involved in the cAMP-mediated induction of cholesterol efflux to apolipoproteins. We also examined whether cAMP could induce annexins I, V, VI, and VII, which are calcium and phospholipid binding proteins, as candidates for playing a role in this process. Western blot analysis of whole-cell extracts of RAW264 cells treated with or without 0.3 mM 8-Br-cAMP showed basal expression of annexins I, V, and VII, but no induction by the cAMP treatment, whereas annexin VI was not detected (data not shown). However, these data do not rule out the possibility that a member of the annexin gene family plays a calcium-dependent role in cholesterol efflux to apolipoproteins. Additionally, we examined whether 0.3 mM 8-Br-cAMP treatment induced the mRNA for the HDL receptor SR-B1; and rather than an increase, there was an ≈60% decrease in SR-B1 mRNA in Northern blots (data not shown).
DISCUSSION
We previously proposed that the cAMP-induced lipid efflux to apolipoproteins is mediated by a cAMP-induced receptor for apolipoproteins, which functions to transfer lipids from the cell to the apolipoprotein, followed by the release from the cell of the nascent lipoprotein particle (10). ApoAI and apoE were found to cross compete for binding to cAMP-treated cells (10). In the current study we primarily present data by using apoAI as the apolipoprotein acceptor, as apoAI exhibited less nonspecific binding than apoE in our cell culture experiments; although, our findings in this study apply equally to apoE, except as discussed below. Our ultrastructural data and the results of experiments with chlorpromazine and hypertonic media all point toward a role for coated pit-mediated endocytosis in this cholesterol efflux pathway; although, these inhibitors may have blocked cholesterol efflux by other mechanisms. We demonstrated resecretion of apoAI from the cells as well as cholesterol efflux resulting from prior cellular uptake of apoAI; however, the latter experiment cannot be used to determine the proportion of efflux caused by endocytosis and resecretion. Our current results also indicate that this efflux mechanism requires extracellular calcium, which also is needed for apoAI internalization, and further supports the role of endocytosis in this efflux pathway. Potential roles for calcium in the endocytosis pathway include binding to and activation of various members of the annexin gene family, which bind phospholipids in a calcium-dependent fashion, and some of which can play roles in membrane fusion and endocytosis (15, 16). The 50–200 μM calcium needed for activation of most members of the annexin gene family (17) is consistent with the dose-dependent inhibition of cholesterol efflux by calcium removal.
In 1985, Schmitz et al. (18) reported that HDL is bound to cholesterol-loaded mouse macrophages via a saturable receptor and then undergoes endocytosis in coated pits, but instead of degradation they observed resecretion, thus implying a receptor-mediated retroendocytosis pathway. Oram’s laboratory (19) did not find evidence for HDL internalization by using alternate methods. Garcia et al. (20) reported that HDL was taken up into clathrin-coated vesicles by HepG2 cells, in a process that was inhibited by sucrose hypertonic shock. HDL uptake by Caco-2 cells also occurs via clathrin-mediated endocytosis (21), and HDL retroendocytosis also is detected in these cells (22). There is also evidence of non-HDL apoE recycling and resecretion after the uptake of triglyceride-rich lipoproteins, or reconstituted proteoliposomes containing only apoE, into Hep3b cells (23). ApoE derived from very low density lipoprotein (VLDL) remnants is sorted into liver Golgi fractions in vivo, presumably for recycling (24). In addition, cholesterol efflux from human fibroblasts to HDL, and binding of HDL to the cells, also were stimulated by cAMP analogues or by reagents that increase endogenous cAMP (25). Additionally, the apolipoprotein endocytosis and resecretion pathway that we characterized in RAW264 cells may be similar to the pathways described for recycling of HDL- and VLDL-derived proteins in macrophages and other cell types.
Many membrane receptors, such as the LDL receptor and the transferrin receptor, are efficiently recycled via the receptor-mediated endocytosis pathway involving clathrin-coated pits and subsequently sorted, along with the bulk of the membrane lipids, to a cholesterol-enriched pericentriole tubule network (26). LDL is released from its receptor in the endosome and sorted to lysosomes for degradation. However, transferrin, after releasing its ferric ions in the acidified endosome, is recycled back to the plasma membrane, still bound to its receptor and then released from the cell to repeat its iron delivery function in additional cycles (26). Based on our current and previous data, we have formulated a revised model for the cAMP-induced lipid efflux pathway in RAW264 cells. Treatment with a cAMP analogue results in the induction of a receptor protein capable of binding apoE or apoAI. This induction requires new RNA synthesis and functional transport of the receptor through the trans-Golgi network (10, 27). Upon receptor binding to the apolipoprotein ligand, it is assembled into coated pits and endocytosed in a process that requires extracellular calcium and is inhibited by chlorpromazine. After endocytosis the receptor-ligand complex appears in endosomes. There sorting occurs and the receptor-ligand complex is directed for recycling back to the plasma membrane. In Hep3b cells, Beisiegel’s laboratory (23) found that the resecretion of labeled apoE is slower than the resecretion of transferrin, and that apoE and transferrin are sorted to separate compartments within the cell. We propose that the lipid transfer may occur in either the endosome or recycling compartments. Upon recycling to the plasma membrane, the nascent lipoprotein is released from the cell carrying with it phospholipids and free cholesterol.
The identity of the cAMP-induced apolipoprotein receptor in RAW264 cells is not known, although we tentatively have ruled out SR-B1, because its mRNA is down-regulated by cAMP treatment. Other potential apo receptor candidates are HBP (vigilin) (28) and HB2 (29), recently reviewed by Fidge (30). Both annexin I and annexin VII also can bind apoAI in vitro in a calcium-dependent fashion (31), and although these may play a role in this pathway, their expression in RAW264 cells did not depend on cAMP treatment. We also propose that this apolipoprotein-specific lipid efflux pathway is defective in Tangier disease (32) and in some subjects with familial HDL deficiency (33), although whether these specific defects reside within the receptor protein or in other protein mediators of lipid transfer or membrane recycling is not known. Because this apolipoprotein-mediated lipid efflux is likely to be a complex pathway, it is likely that two or more separate defects could decrease its activity.
The current results of the apoAI cell association and degradation experiments require further analysis. First, why did the specific apoAI cell association (Fig. 6B) increase in the absence of both calcium and cAMP compared with the control? We do not believe that the cAMP-induced receptor involved in cholesterol efflux appears on the cell surface under these conditions, as we previously have shown that cAMP induction of this receptor involves new transcription (10). Rather, we suspect that another apoAI binding activity is unmasked under calcium-free conditions. This analysis is bolstered by the results of apoE2-specific association, which was stimulated by cAMP treatment and also required extracellular calcium, but in this case basal association was not increased in the calcium-free medium (Fig. 6D). Second, in the current study we found increased apoAI degradation by the cAMP-treated cells (Fig. 6C). This finding is in contrast to our prior results with iodinated apoE2, where we observed increased specific cell association but no increased degradation (10). We repeated the apoE2 degradation experiment and confirmed our previous finding (not shown). We do not know the reason for the discrepancy between the degradation results with apoAI and apoE, but it might be related to varying affinity of these apolipoproteins for the same receptor, or it may be because of increased protein unfolding of apoAI vs. apoE2, and thus increased susceptibility to proteolysis, caused by either factors inherent to the protein sequence, or by the variable method of iodination of apoAI vs. apoE.
In conclusion, we have determined that cAMP-induced cholesterol efflux to apoAI is associated with the binding, uptake, and resecretion of apoAI, and that endocytosis inhibitors can largely block this pathway. This induced efflux and specific apoAI cell association depended on extracellular calcium. If apolipoprotein endocytosis is necessary for lipid efflux, as our data show, then the resecretion that we observed must be the final step in this efflux pathway. Finally, we speculate that the HDL retroendocytosis pathway proposed by Schmitz. et al. (18) is mediated by the binding of apolipoproteins and represents the same pathway characterized in the present study.
Acknowledgments
This research was supported by an Established Investigatorship from the American Heart Association (to J.D.S.) and by Grant PO1 HL54591 from the National Institutes of Health. Y.T. was supported, in part, by a grant from the Ito Foundation.
ABBREVIATIONS
- apoAI
apolipoprotein AI
- apoE
apolipoprotein E
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- DGGB
DMEM with glucose, glutamine, and BSA
Footnotes
A Commentary on this article begins on page 10950.
References
- 1.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.von Eckardstein A. Curr Opin Lipidol. 1996;7:308–319. doi: 10.1097/00041433-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkhem I, Andersson O, Diczfalusy U, Sevastik B, Xiu R J, Duan C, Lund E. Proc Natl Acad Sci USA. 1994;91:8592–8596. doi: 10.1073/pnas.91.18.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tall A R. Eur Heart J. 1998;19, Suppl. A:A31–A35. [PubMed] [Google Scholar]
- 5.Rothblat G H, Mahlberg F H, Johnson W J, Phillips M C. J Lipid Res. 1992;33:1091–1097. [PubMed] [Google Scholar]
- 6.Ji Y, Jian B, Wang N, Sun Y, Moya M L, Phillips M C, Rothblat G H, Swaney J B, Tall A R. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 7.Hara H, Yokoyama S. J Biol Chem. 1991;266:3080–3086. [PubMed] [Google Scholar]
- 8.Oram J F, Yokoyama S. J Lipid Res. 1996;37:2473–2491. [PubMed] [Google Scholar]
- 9.Phillips M C, Gillotte K L, Haynes M P, Johnson W J, Lund-Katz S, Rothblat G H. Atherosclerosis. 1998;137,Suppl.:S13–S17. doi: 10.1016/s0021-9150(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 10.Smith J D, Miyata M, Ginsberg M, Grigaux C, Shmookler E, Plump A S. J Biol Chem. 1996;271:30647–30655. doi: 10.1074/jbc.271.48.30647. [DOI] [PubMed] [Google Scholar]
- 11.Brinton E A, Eisenberg S, Breslow J L. J Clin Invest. 1989;84:262–269. doi: 10.1172/JCI114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markwell M A, Haas S M, Bieber L L, Tolbert N E. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang L H, Rothberg K G, Anderson R G. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daukas G, Zigmond S H. J Cell Biol. 1985;101:1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raynal P, Pollard H B. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 16.Lin H C, Sudhof T C, Anderson R G. Cell. 1992;70:283–291. doi: 10.1016/0092-8674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- 17.Niki I, Yokokura H, Sudo T, Kato M, Hidaka H. J Biochem (Tokyo) 1996;120:685–698. doi: 10.1093/oxfordjournals.jbchem.a021466. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz G, Robenek H, Lohmann U, Assmann G. EMBO J. 1985;4:613–622. doi: 10.1002/j.1460-2075.1985.tb03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oram J F, Johnson C J, Brown T A. J Biol Chem. 1987;262:2405–2410. [PubMed] [Google Scholar]
- 20.Garcia A, Barbaras R, Collet X, Bogyo A, Chap H, Perret B. Biochemistry. 1996;35:13064–13071. doi: 10.1021/bi952223l. [DOI] [PubMed] [Google Scholar]
- 21.Klinger A, Reimann F M, Klinger M H, Stange E F. Biochim Biophys Acta. 1997;1345:65–70. doi: 10.1016/s0005-2760(96)00164-6. [DOI] [PubMed] [Google Scholar]
- 22.Rogler G, Herold G, Stange E F. Biochim Biophys Acta. 1991;1095:30–38. doi: 10.1016/0167-4889(91)90041-u. [DOI] [PubMed] [Google Scholar]
- 23.Heeren J, Weber W, Beisiegel U. J Cell Sci. 1999;112:349–359. doi: 10.1242/jcs.112.3.349. [DOI] [PubMed] [Google Scholar]
- 24.Fazio S, Linton M F, Hasty A H, Swift L L. J Biol Chem. 1999;274:8247–8253. doi: 10.1074/jbc.274.12.8247. [DOI] [PubMed] [Google Scholar]
- 25.Middleton A, Middleton B. Biochim Biophys Acta. 1998;1391:117–132. doi: 10.1016/s0005-2760(97)00207-5. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh R N, Gelman D L, Maxfield F R. J Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- 27.Mendez A J, Uint L. J Lipid Res. 1996;37:2510–2524. [PubMed] [Google Scholar]
- 28.McKnight G L, Reasoner J, Gilbert T, Sundquist K O, Hokland B, McKernan P A, Champagne J, Johnson C J, Bailey M C, Holly R, et al. J Biol Chem. 1992;267:12131–12141. [PubMed] [Google Scholar]
- 29.Matsumoto A, Mitchell A, Kurata H, Pyle L, Kondo K, Itakura H, Fidge N. J Biol Chem. 1997;272:16778–16782. doi: 10.1074/jbc.272.27.16778. [DOI] [PubMed] [Google Scholar]
- 30.Fidge N H. J Lipid Res. 1999;40:187–201. [PubMed] [Google Scholar]
- 31.Brownawell A M, Creutz C E. Biochemistry. 1996;35:6839–6845. doi: 10.1021/bi952585t. [DOI] [PubMed] [Google Scholar]
- 32.Remaley A T, Schumacher U K, Stonik J A, Farsi B D, Nazih H, Brewer H J. Arterioscler Thromb Vasc Biol. 1997;17:1813–1821. doi: 10.1161/01.atv.17.9.1813. [DOI] [PubMed] [Google Scholar]
- 33.Marcil M, Yu L, Krimbou L, Boucher B, Oram J F, Cohn J S, Genest J J. Arterioscler Thromb Vasc Biol. 1999;19:159–169. doi: 10.1161/01.atv.19.1.159. [DOI] [PubMed] [Google Scholar]