Abstract
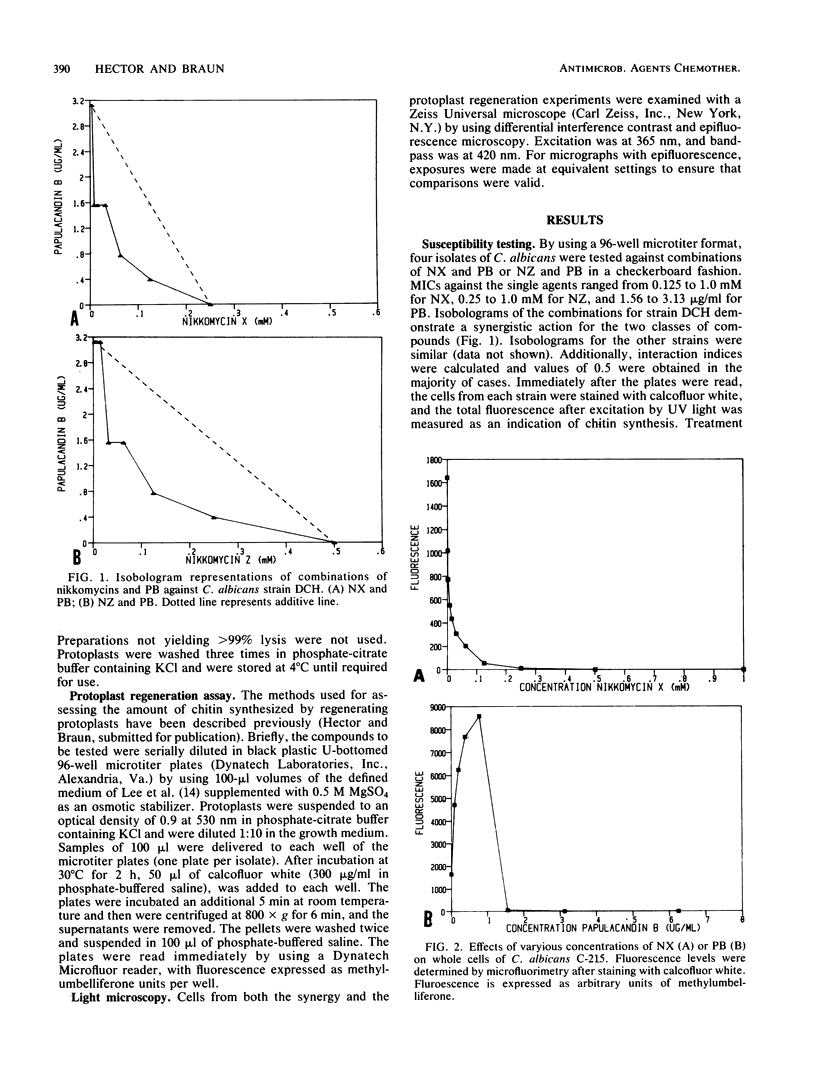
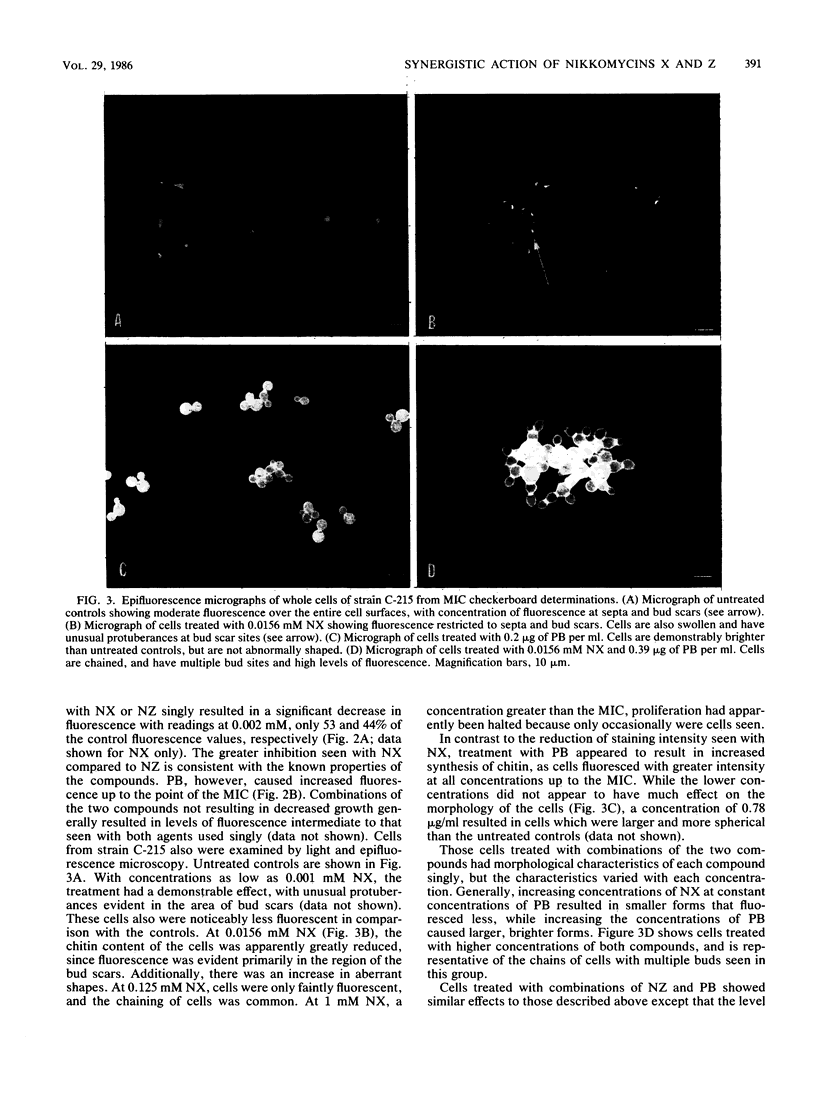
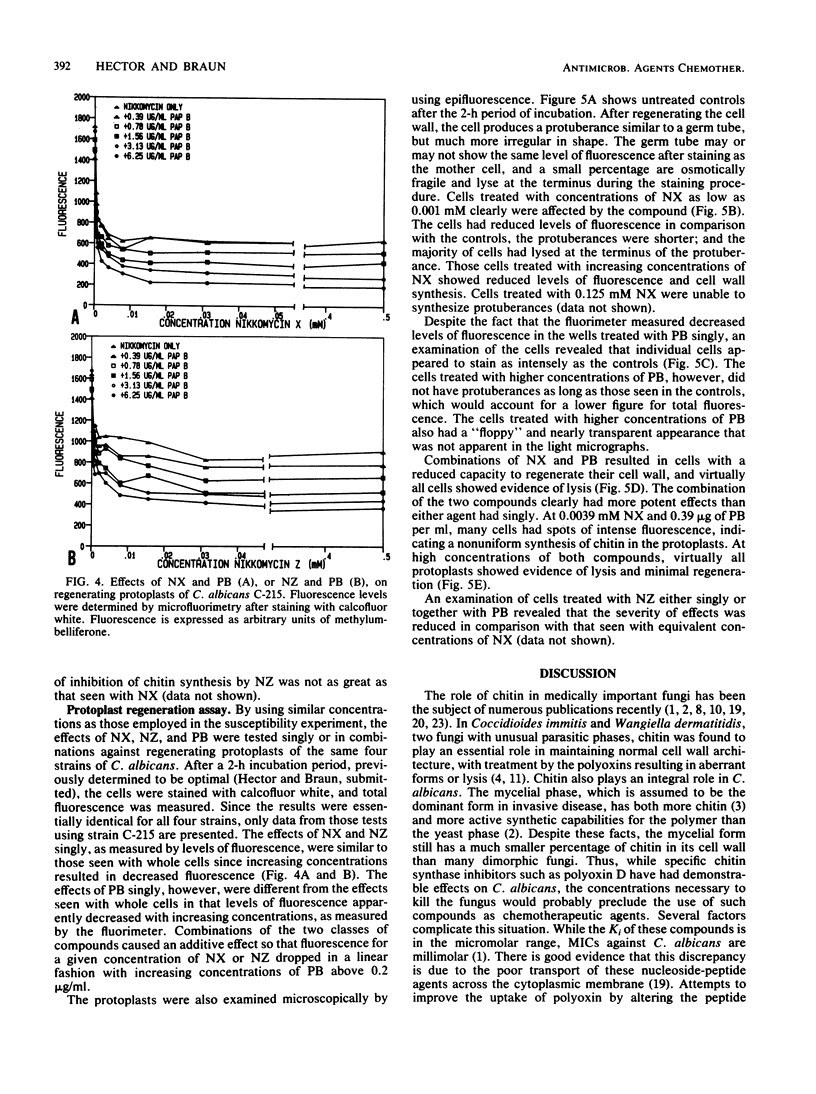
Combinations of nikkomycin X (NX) or nikkomycin Z (NZ), known inhibitors of chitin synthesis in fungi, together with papulacandin B (PB), an inhibitor of beta-glucan synthesis, were tested for synergistic activity against four isolates of Candida albicans by using the broth microdilution checkerboard technique and a method to assess the regeneration of cell wall material in protoplasts. The construction of isobolograms from the data generated by the checkerboard determinations revealed a synergistic effect for the two classes of compounds against all strains. The combination of NX and PB was more effective than the combination of NZ and PB, perhaps reflecting the lower Ki value of NX. While the presence of NX and NZ reduced chitin synthesis, as determined by staining with calcofluor white and assaying with a microfluorimeter, cells treated with PB demonstrated an increased synthesis of chitin. Protoplast regeneration experiments using similar concentrations of the two classes of compounds resulted in comparable findings. The combination of NX and PB resulted in a greater inhibition of chitin synthesis than did equivalent combinations of NZ and PB. These data suggest that combinations of agents active against cell wall synthesis in fungi may prove more useful as chemotherapeutic agents than such compounds used singly.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. M., Covert N. L., Shenbagamurthi P., Steinfeld A. S., Naider F. Polyoxin D inhibits growth of zoopathogenic fungi. Antimicrob Agents Chemother. 1983 Jun;23(6):926–929. doi: 10.1128/aac.23.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P. C., Calderone R. A. Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J Bacteriol. 1978 Mar;133(3):1472–1477. doi: 10.1128/jb.133.3.1472-1477.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Davis T. E., Jr, Domer J. E., Li Y. T. Cell wall studies of Histoplasma capsulatum and Blastomyces dermatitidis using autologous and heterologous enzymes. Infect Immun. 1977 Mar;15(3):978–987. doi: 10.1128/iai.15.3.978-987.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorza M. V., Rico H., Gozalbo D., Sentandreu R. Cell wall composition and protoplast regeneration in Candida albicans. Antonie Van Leeuwenhoek. 1983 Nov;49(4-5):457–469. doi: 10.1007/BF00399324. [DOI] [PubMed] [Google Scholar]
- Endo A., Kakiki K., Misato T. Mechanism of action of the antifugal agent polyoxin D. J Bacteriol. 1970 Oct;104(1):189–196. doi: 10.1128/jb.104.1.189-196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Payne C. M., Jones J. F. Human polymorphonuclear-leukocyte inhibition of incorporation of chitin precursors into mycelia of Coccidioides immitis. J Infect Dis. 1984 Mar;149(3):404–412. doi: 10.1093/infdis/149.3.404. [DOI] [PubMed] [Google Scholar]
- Garcia Mendoza C., Novaes Ledieu M. Chitin in the new wall of regenerating protoplasts of Candida utilis. Nature. 1968 Dec 7;220(5171):1035–1035. doi: 10.1038/2201035a0. [DOI] [PubMed] [Google Scholar]
- Hector R. F., Pappagianis D. Inhibition of chitin synthesis in the cell wall of Coccidioides immitis by polyoxin D. J Bacteriol. 1983 Apr;154(1):488–498. doi: 10.1128/jb.154.1.488-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecká M. Lysis of growing cells of Saccharomyces cerevisiae induced by papulacandin B. Folia Microbiol (Praha) 1984;29(2):115–119. doi: 10.1007/BF02872926. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Mizoguchi J., Saito T., Mizuno K., Hayano K. On the mode of action of a new antifungal antibiotic, aculeacin A: inhibition of cell wall synthesis in Saccharomyces cerevisiae. J Antibiot (Tokyo) 1977 Apr;30(4):308–313. doi: 10.7164/antibiotics.30.308. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Yagi A., Satoi S., Takada M., Hayashi M. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J Antibiot (Tokyo) 1977 Apr;30(4):297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- Naider F., Shenbagamurthi P., Steinfeld A. S., Smith H. A., Boney C., Becker J. M. Synthesis and biological activity of tripeptidyl polyoxins as antifungal agents. Antimicrob Agents Chemother. 1983 Nov;24(5):787–796. doi: 10.1128/aac.24.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. H., Lange C. F., Hashimoto T. "Nonfibrillar" chitin associated with walls and septa of Trichophyton mentagrophytes arthrospores. J Bacteriol. 1983 May;154(2):965–975. doi: 10.1128/jb.154.2.965-975.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Shenbagamurthi P., Smith H. A., Becker J. M., Steinfeld A., Naider F. Design of anticandidal agents: synthesis and biological properties of analogues of polyoxin L. J Med Chem. 1983 Oct;26(10):1518–1522. doi: 10.1021/jm00364a030. [DOI] [PubMed] [Google Scholar]
- Sietsma J. H., Wessels J. G. Solubility of (1 leads to 3)-beta-D/(1 leads to 6)-beta-D-glucan in fungal walls: importance of presumed linkage between glucan and chitin. J Gen Microbiol. 1981 Jul;125(1):209–212. doi: 10.1099/00221287-125-1-209. [DOI] [PubMed] [Google Scholar]
- Yadan J. C., Gonneau M., Sarthou P., Le Goffic F. Sensitivity to nikkomycin Z in Candida albicans: role of peptide permeases. J Bacteriol. 1984 Dec;160(3):884–888. doi: 10.1128/jb.160.3.884-888.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]