Abstract
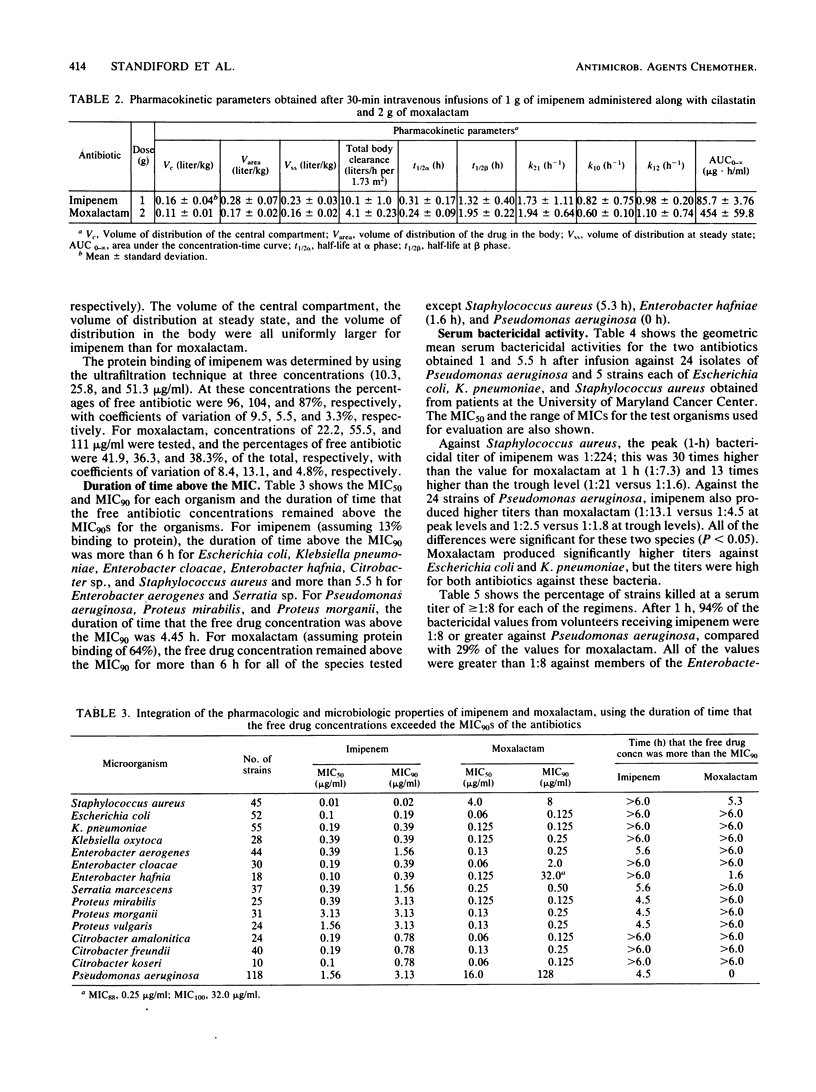
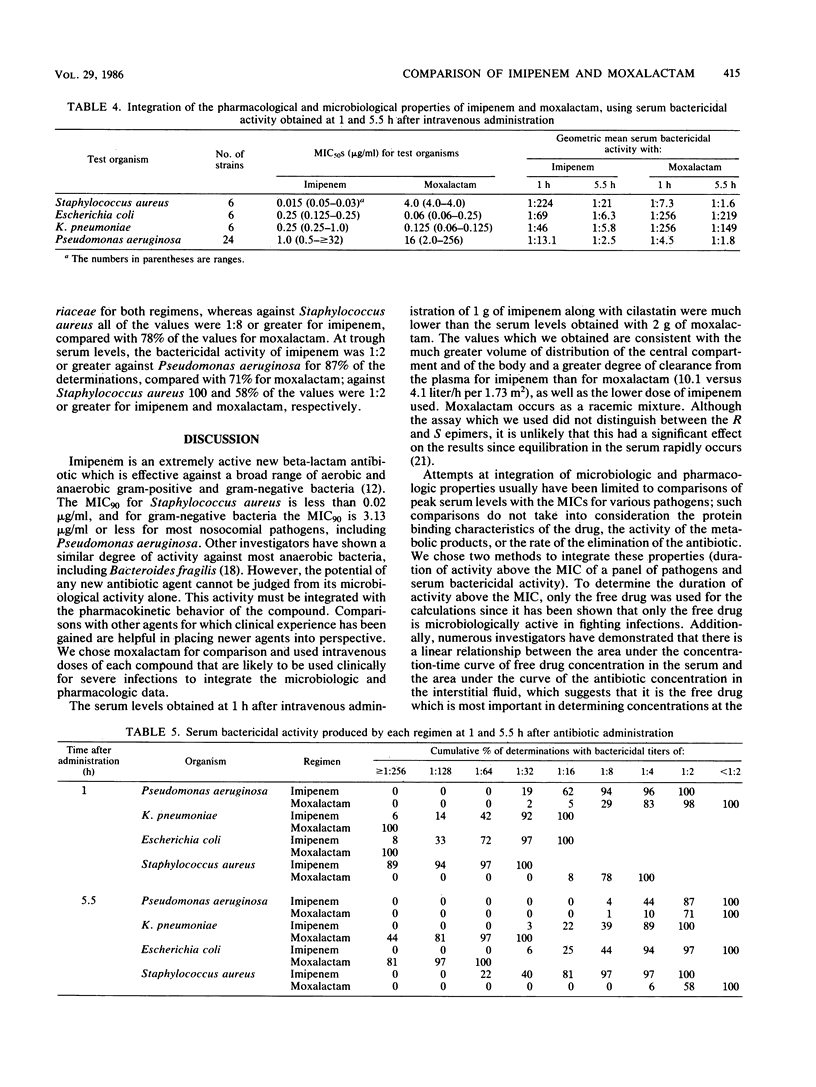
We administered 1 g of imipenem along with equal amounts of cilastatin (a dehydropeptidase I inhibitor) or 2 g of moxalactam intravenously over a period of 30 min to six volunteers in a crossover manner 1 week apart. The antibiotic concentrations and pharmacokinetics for each drug were determined and integrated with the microbiologic activity by measuring the duration of time that the free drug concentrations remained above the MICs for 90% of 581 clinical isolates and by measuring serum bactericidal activities against organisms which commonly infect granulocytopenic cancer patients. Moxalactam produced serum levels at 1 h after infusion of 99.9 micrograms/ml; these levels were four times greater than the plasma levels of imipenem (22.8 micrograms/ml). The trough (5.5-h) moxalactam serum levels were 10 times greater than those of imipenem (18.5 and 1.7 micrograms/ml, respectively). Essentially all of the imipenem was unbound to protein, whereas 36 to 42% of the moxalactam was unbound. Moxalactam produced free antibiotic concentrations that were above the MIC for 90% of the strains tested for more than 6 h against all of the species tested except Staphylococcus aureus (5.3 h), Enterobacter hafnia (1.6 h), and Pseudomonas aeruginosa (0 h). The imipenem concentrations were above the MIC for 90% of the strains tested for 5.6 h or more against all of the bacteria tested except Proteus spp. and Pseudomonas aeruginosa (4.5 h). The geometric mean peak bactericidal titers from volunteers receiving imipenem were more than 1:8 against all bacteria and were significantly higher than the titers from volunteers receiving moxalactam against S. aureus (1:7.3) and Pseudomonas aeruginosa (1:4.5). These data, in addition to information obtained from animal models, indicate that imipenem is a promising new candidate for carefully controlled clinical trials as a single agent for therapy of serious infections, including empiric therapy for fever in granulocytopenic cancer patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Bustamante C. I., Drusano G. L., Tatem B. A., Standiford H. C. Postantibiotic effect of imipenem on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Nov;26(5):678–682. doi: 10.1128/aac.26.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio D. Z., Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979 Mar;9(2):115–134. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Ryan P. A., Standiford H. C., Moody M. R., Schimpff S. C. Integration of selected pharmacologic and microbiologic properties of three new beta-lactam antibiotics: a hypothesis for rational comparison. Rev Infect Dis. 1984 May-Jun;6(3):357–363. doi: 10.1093/clinids/6.3.357. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick B. J., Standiford H. C. A comparative evaluation of moxalactam: antimicrobial activity, pharmacokinetics, adverse reactions, and clinical efficacy. Pharmacotherapy. 1982 Jul-Aug;2(4):197–212. doi: 10.1002/j.1875-9114.1982.tb03187.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Calia F. M., Snyder M. J., Warren J. W., Schimpff S. C. Imipenem therapy of Pseudomonas aeruginosa bacteraemia in neutropenic rats. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):89–96. doi: 10.1093/jac/12.suppl_d.89. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Daneau D., Swings G., Weerts D. Antibacterial activity in serum and urine as a therapeutic guide in bacterial infections. J Infect Dis. 1974 Feb;129(2):187–193. doi: 10.1093/infdis/129.2.187. [DOI] [PubMed] [Google Scholar]
- Miner D. J., Coleman D. L., Shepherd A. M., Hardin T. C. Determination of moxalactam in human body fluids by liquid chromatographic and microbiological methods. Antimicrob Agents Chemother. 1981 Aug;20(2):252–257. doi: 10.1128/aac.20.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Klastersky J. Significance of serum bactericidal activity in gram-negative bacillary bacteremia in patients with and without granulocytopenia. Am J Med. 1984 Mar;76(3):429–435. doi: 10.1016/0002-9343(84)90662-4. [DOI] [PubMed] [Google Scholar]
- Standiford H. C., Drusano G. L., Fitzpatrick B., Tatem B., Schimpff S. C. Bactericidal activity of ceftazidime in serum compared with that of ticarcillin combined with amikacin. Antimicrob Agents Chemother. 1984 Sep;26(3):339–342. doi: 10.1128/aac.26.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford H. C., Johnson D. E., Schimpff S. C. Role of antimicrobial synergism in infected granulocytopenic patients. Eur J Cancer Clin Oncol. 1984 Aug;20(8):1007–1010. doi: 10.1016/0277-5379(84)90101-9. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Gorbach S. L. In vitro activity of N-formimidoyl thienamycin (MK0787). Antimicrob Agents Chemother. 1980 Oct;18(4):642–644. doi: 10.1128/aac.18.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompsett R., Shultz S., McDermott W. The Relation of Protein Binding to the Pharmacology and Antibacterial Activity of Penicillins X, G, Dihydro F, and K. J Bacteriol. 1947 May;53(5):581–595. doi: 10.1128/jb.53.5.581-595.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Cadge B., Durham S. R., Baker S. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J Infect Dis. 1980 Jul;142(1):77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]
- Wise R., Wills P. J., Bedford K. A. Epimers of moxalactam: in vitro comparison of activity and stability. Antimicrob Agents Chemother. 1981 Jul;20(1):30–32. doi: 10.1128/aac.20.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]



