Abstract
Aim
A population pharmacokinetic analysis was performed using plasma concentration data (n = 7025) from 380 patients to examine the relationship between ribavirin dose and its pharmacokinetics.
Methods
Ribavirin pharmacokinetics were described by a three-compartment model with sequential zero-order and a first-order absorption processes. Interoccasion variability and food effects were included.
Results
Lean body weight (range 41–91 kg) was the only covariate with a clinically significant influence on ribavirin pharmacokinetics, affecting clearance (15.3–23.9 l h−1) and the volume of the larger peripheral compartment.
Conclusion
The model provided a good description of the available data, confirmed by accurate estimates of parameter values and low residual variability (17%).
Keywords: hepatitis C, peginterferon alfa-2a (40KD), pharmacokinetics, population, ribavirin
Introduction
Current treatment for chronic hepatitis C infection is the combination of a pegylated interferon and ribavirin [1–3]. The recommended daily dose of ribavirin ranges from 800 to 1200 mg, dependent on hepatitis C virus genotype and bodyweight [1–3]. The optimal dose of ribavirin remains uncertain, but studies suggest that higher serum ribavirin concentrations result in higher response rates [4–6]. However, achieving the latter with higher doses of ribavirin must be balanced against potential increases in drug-related haemolysis. Thus, the relationships between ribavirin dose, its pharmacokinetics and treatment outcomes may be important clinically.
The objective of this work was to develop a population pharmacokinetic model for ribavirin and to explore the influence of covariates on its pharmacokinetics in patients with chronic hepatitis C infection receiving peginterferon alfa-2a (40KD) plus ribavirin.
Methods
Studies analysed
Data used in this analysis were obtained from subgroups of patients from two Phase III studies [7, 8] and patients recruited to three cross-over, bioequivalence Phase I studies. Subjects had either been previously infected or were currently infected with hepatitis C virus. All patients provided written, informed consent to participate in each of the studies. In each instance, institutional review boards of all the participating centres of all five of the studies approved the protocols and all the amendments. All studies were conducted according to the guidelines in the Declaration of Helsinki.
The objective of the three Phase I studies was to assess the pharmacokinetics of a single 600-mg oral dose of ribavirin. For the studies that contained two phases, the information about the duration of the washout period between the two single doses was taken into account. Patients in the Phase III studies received peginterferon alfa-2a (40KD) 180 µg week−1 plus either low-dose (800 mg day−1) or standard-dose ribavirin (1000–1200 mg day−1 by body weight). In the multiple-dose studies, samples were collected at steady state (weeks 8–48). Ribavirin plasma concentrations were quantified using a validated analytical procedure [9].
Data analysis
Ribavirin concentration measurements [n = 7025; collected over a range of times (0, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 16, 24, 32, 48, 72, 96, 120, 144 and 192 h) postdose] from 380 subjects were included in the analysis. The covariates that were assessed included race, sex, age, weight, height, lean body weight, body mass index, creatinine clearance (CLcr), serum creatinine, haematocrit and albumin.
All analyses were performed using NONMEM version V Level 1, FO method [10] and S-PLUS 2000 [11]. The Fortran compiler used was the Compaq Visual Fortran Standard Edition, Version 6.1. Xpose was used as an aid in model assessment [12]. Exploratory analysis indicated that ribavirin exhibited multicompartmental pharmacokinetics, in line with a previous study [13]. Two- and three-compartment models were fitted to the data. The latter was found to be superior and was used in all subsequent analyses.
A combined sequential zero-order then first-order process was found to best describe the absorption phase. The influence of meals on the absorption parameters was also investigated and included in the analysis.
Covariate analysis showed many possible influences on oral clearance (CL), but no obvious determinant on any of the volume terms in the model. Using predefined selection criteria plus a clinical significance level of a 20% change in parameter value for backward deletion, covariates were screened for their influence on CL. Only race and CLcr were found to have a significant effect at the predefined P < 0.0001 level, but did not meet the clinical significance criteria of a change in oral CL of 20%. For both CL and the volume of the second larger peripheral compartment (V2), only lean body weight remained in the final model, linearly influencing both CL and V2.
Evaluation using a jack-knife technique showed that the model was not particularly sensitive to any of the excluded data within each of the jack-knife runs.
Results
Of the 380 patients, the majority were male (64%), comprising 87% White and 9% Black subjects. The median (range) age was 44 (18–71) years and their median (range) weight and height were 80 (44–155) kg and 173 (147–198) cm, respectively, resulting in a median (range) body mass index of 26 (18–65) kg m−2. The median (range) lean body weight was 68 (41–91) kg. Serum albumin and creatinine concentrations [median (range)] were 4.2 (2.9–5.4) g dl−1 and 0.9 (0.5–1.5) mg dl−1, respectively. The median (range) haematocrit was 44.0 (33.9–52.5)% and creatinine clearance was 99 (34–173) ml min−1. Patients received a range of daily doses of ribavirin, namely 600 mg (n = 138), 800 mg (n = 60), 1000 mg (n = 55) and 1200 mg (n = 127).
The final population pharmacokinetic model used to describe ribavirin pharmacokinetics consisted of three compartments with a sequential zero-order then first-order absorption process. Interoccasion variability and food effects were included in the absorption model. Lean body weight was the only covariate that met the predefined covariate selection criteria and had a linear influence on both CL and V2. Goodness of fit plots showed that the final model provided a good description of the data (data not shown). Final population parameter values are presented in Table 1.
Table 1.
Final population pharmacokinetic parameter estimates for ribavirin
| Parameter | Estimate | SE (%) | Interindividual variability (%) | SE (%) | Interoccasion variability (%) | SE (%) |
|---|---|---|---|---|---|---|
| CL* (l h−1) | 19.8 | 1.8 | 16 | 29 | – | – |
| LBW on CL | 0.00869 | 20 | – | – | – | – |
| V1 (l) | 472 | 8.2 | 42 | 25 | – | – |
| Q2 (l h−1) | 34.4 | 3.5 | 19 | 35 | – | – |
| V2* (l) | 4910 | 5.6 | 37 | 31 | – | – |
| LBW on V2 | 0.011 | 32 | – | – | – | – |
| Q3 (l h−1) | 97.7 | 7.3 | 35 | 25 | – | – |
| V3 (l) | 871 | 4.9 | 18 | 43 | – | – |
| F1 (fasting and standard meal) | 1† | – | 22 | 14 | 20 | 11 |
| F1 (high-fat meal) | 1.46 | 4.8 | ||||
| D1 (fasting and standard meal) (h) | 0.498 | 8.5 | 8.5 | 710 | 55 | 35 |
| D1 (high-fat meal) (h) | 0.740 | 11 | ||||
| Ka (fasting) (h−1) | 1.45 | 10 | ||||
| Ka (standard meal) (h−1) | 0.767 | 35 | 30 | 98 | 69 | 16 |
| Ka (high-fat meal) (h−1) | 0.99 | 5.2 | ||||
| Residual error (%) | 17.0 | 5.5 | – | – | – | – |
For a subject with a lean body weight (LBW) of 67 kg.
Fixed value. CL = 19.8 × [1 + 0.00869 × (LBW − 67)] l h−1; V2 = 4910 × [1 + 0.011 × (LBW − 67)] l.
LBW range is 41–91 kg. CL will vary from 15.3 to 23.9 l h−1over that range. LBW range is 41–91 kg. V2 will vary from 3506 to 6206 l over that range. CL, Clearance; D1, duration of the zero-order input part of the absorption model; F1, bioavailability; Ka, absorption rate constant; V1/ V2/V3, volume of distribution of the first, second or third compartment; Q2/Q3, intercompartmental clearance from the second or third compartment.
The component of the final population pharmacokinetic model that describes the absorption phase is complex. Although a standard meal did not affect ribavirin bioavailability (F1), administration of ribavirin with a high-fat meal increased bioavailability by 46% relative to the fasting state. A high-fat meal prolonged the duration of the zero-order input part of the absorption model, with D1 increasing from 0.498 h (fasting and standard meal) to 0.740 h. The type of meal also influenced the first-order input part of the absorption model (Table 1).
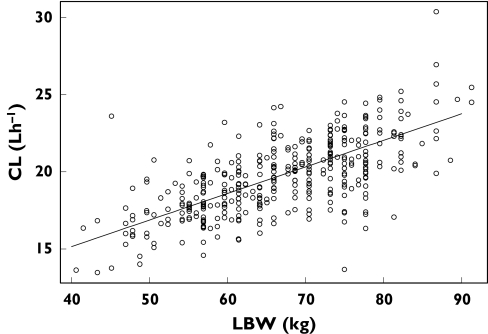
The CL was estimated to be 19.8 l h−1 for a person with a lean body weight of 67 kg. The relationship between CL and lean body weight in the final model is shown in Figure 1.The half-lives of the drug in the three compartments were 1.7, 19.4 and 303 h. Based upon the t1/2,z of 303 h steady state was predicted to be achieved by 7–11 weeks (4–6 half-lives), but may be attained earlier. The value for apparent volume of distribution at steady-state (Vdss/F) was estimated to be 6253 l.
Figure 1.
Ribavirin clearance (CL) vs. lean body weight (LBW). The black line is the estimated relationship, as described by CL = 19.8 × [1 + 0.00869 × (LBW − 67)] l h−1
Interindividual variability in CL and F1 were 16% and 22%, respectively. The low residual variability (17%) showed that the final model provided a good description of the data. It is likely that the major component of the residual variability was due to variability in the analytical assay used to quantify ribavirin [9].
Discussion
The final model used to describe ribavirin pharmacokinetics consisted of three disposition compartments with a sequential zero-order then first-order absorption process. The model provided a good description of the data based on the goodness of fit plots and the accuracy of the parameter estimates. There was some overprediction of ribavirin concentrations between 800 and 1200 ng ml−1, which were similar to the values of the peak concentrations in two of the Phase I studies. This implies that there was a minor model misspecification with respect to absorption, but this was judged not to have affected the estimation or the influence of the covariates on CL.
The estimate of CL (19.8 l h−1) for a typical individual with a lean body weight of 67 kg is consistent with the values obtained using noncompartmental analysis. The value of Vdss/F (6253 l) is also consistent with previous results, reported to be > 4000 l (Roche, data on file) and probably reflects the extensive distribution of ribavirin into nonplasma compartments. The estimate of V1 (472 l) is large, consistent with distribution into erythrocytes [14]. We also consider that the t1/2,z of 303 h calculated from the parameters of the population model is a robust estimate given the long time period over which the data were collected.
A complex absorption function was developed to prevent any potential misspecification of the absorption process affecting estimation of the distribution and elimination parameters [15]. A high-fat meal increased relative bioavailability by a factor of 1.46, consistent with the value obtained from a previous noncompartmental analysis (Roche, data on file). Other than a different Ka value for the standard meal, none of the remaining parameters associated with absorption was found to differ from those in the fasting state, suggesting that a standard meal has little or no effect on ribavirin absorption. The variability in relative bioavailability was similar between and within individuals. However, for the zero- and first-order input parameters the variability was much larger within than between individuals. The absorption of ribavirin was highly variable under the conditions studied and was probably responsible for most of the observed variability in exposure.
Little published information is available on the population pharmacokinetics of ribavirin. However, the results of a two-stage pharmacokinetic analysis of serum ribavirin data [6] are consistent with our study in that an influence of weight on CL was observed. Lindahl et al. [6] also reported a gender effect, which may be due to differences in weight between males and females.
In a previous analysis on a small number of samples, CL was found to be linearly dependent upon both weight and CLcr over the range 5–144 ml min−1 [16]. The latter finding may be a result of more patients with renal impairment being present in the dataset. Our study, which included more patients, found no effect of CLcr on CL, suggesting that any influence is apparent only when CLcr falls below 34 ml min−1. Only two patients with CLcr < 40 ml min−1 were included in our study.
Lean body weight was the only covariate that met the predefined criteria for an influence on CL or volume (V2). Although lean body weight was statistically the best ‘size’ determinant of clearance in our study, there was a high degree of correlation between the effect of total weight and lean body weight on clearance. The model predicts that clearance will vary from 15.3 to 23.9 l h−1 over the lean body weight range in the dataset (41–91 kg), decreasing the exposure relative to dose as weight increases. This decrease is relatively modest and the recommended dose of 1000 mg for patients < 75 kg and 1200 mg for patients ≥ 75 kg compensates for this effect, ensuring adequate exposure in patients with hepatitis C virus genotype 1. Our findings are also consistent with data showing that ribavirin dose kg−1 is predictive of both ribavirin efficacy and safety [17].
Competing interests
F.D., M.L. and K.J. are employees of F. Hoffmann-La Roche. J.R.W. and E.S. from Exprimo have been contracted to perform the analysis on behalf of F. Hoffmann-La Roche.
References
- 1.National Institutes of Health Consensus Development Conference Statement. Management of hepatitis C: 2002 June 10–12, 2002. Hepatology. 2002;36(5 Suppl. 1):S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 2.Cacoub P. [Treatment of hepatitic C virus infection after the French 2002 Consensus Conference] Rev Med Interne. 2002;23:889–92. doi: 10.1016/s0248-8663(02)00714-2. [DOI] [PubMed] [Google Scholar]
- 3.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 4.Jen JF, Glue P, Gupta S, Zambas D, Hajian G. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit. 2000;22:555–65. doi: 10.1097/00007691-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Larrat S, Stanke-Labesque F, Plages A, Zarski JP, Bessard G, Souvignet C. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob Agents Chemother. 2003;47:124–9. doi: 10.1128/AAC.47.1.124-129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindahl K, Stahle L, Bruchfeld A, Schvarcz R. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology. 2005;41:275–9. doi: 10.1002/hep.20563. [DOI] [PubMed] [Google Scholar]
- 7.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM PEGASYS International Study Group. Peginterferon alfa-2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Torres M, Torriani FJ, Soriano V, Borucki MJ, Lissen E, Sulkowski M, Dieterich D, Wang K, Gries J-M, Hoggard PG, Back DJ. APRICOT Study Group. Effect of ribavirin on intracellular and plasma pharmacokinetics of nucleoside reverse transcriptase inhibitors in patients with HIV-HCV co-infection: results of a randomized clinical study. Antimicrob Agents Chemother. 2005;49:3997–4008. doi: 10.1128/AAC.49.10.3997-4008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beal SL, Sheiner LB. NONMEM Users Guides. San Francisco: NONMEM Project Group, University of California at San Francisco; 1998. [Google Scholar]
- 11.MathSoft Inc. S-Plus 2000Modern Statistics and Advanced Graphics. Seattle, WA: Mathsoft; 2000. [Google Scholar]
- 12.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comp Meth Prog Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 13.Preston SL, Drusano GL, Glue P, Nash J, Gupta SK, McNamara P. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother. 1999;43:2451–6. doi: 10.1128/aac.43.10.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homma M, Matsuzaki Y, Inoue Y, Shibata M, Mitamura K, Tanaka N, Kohda Y. Marked elevation of erythrocyte ribavirin levels in interferon and ribavirin-induced anemia. Clin Gastroenterol Hepatol. 2004;2:337–9. doi: 10.1016/s1542-3565(04)00064-3. [DOI] [PubMed] [Google Scholar]
- 15.Wade JR, Kelman AW, Howie CA, Whiting B. Effect of misspecification of the absorption process on subsequent parameter estimation in population analysis. J Pharmacokinet Biopharm. 1993;21:209–22. doi: 10.1007/BF01059771. [DOI] [PubMed] [Google Scholar]
- 16.Bruchfeld A, Lindahl K, Schvarcz R, Stahle L. Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit. 2002;24:701–8. doi: 10.1097/00007691-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Snoeck E, Wade J, Duff F, Lamb M, Jorga K. Predicting SVR and anemia in HCV-patients treated with PEG-IFN alfa-2a (40KD:) plus ribavirin. Br J Clin Pharmacol; submitted for publication. [DOI] [PMC free article] [PubMed]