Abstract
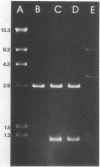
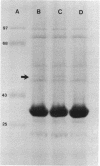
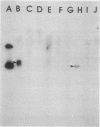
High-level chloramphenicol resistance in Pseudomonas aeruginosa may be due to enzymatic inactivation, ribosomal mutation, or a permeability barrier. We investigated the nonenzymatic resistance mechanism encoded by Tn1696, a transposon found in P. aeruginosa. A 1-megadalton DNA fragment from Tn1696 was cloned which mediated expression of chloramphenicol resistance in Escherichia coli. Comparison of the effects of chloramphenicol on in vitro translation revealed no difference between the susceptible recipient strain and the resistant transformant containing the cloned gene. The rate of chloramphenicol uptake was slower in the resistant strain, suggesting a permeability barrier to the antibiotic. In addition, sodium dodecyl sulfate-polyacrylamide gel electrophoresis of outer membranes demonstrated the absence of a 50,000-dalton protein in the resistant strain. DNA homology was evident between Tn1696 and chloramphenicol-resistant isolates of Haemophilus influenzae possessing altered outer membrane permeability. We conclude that chloramphenicol resistance encoded by Tn1696 is due to a permeability barrier and hypothesize that the gene from P. aeruginosa may share a common ancestral origin with these genes from other gram-negative organisms.
Full text
PDF
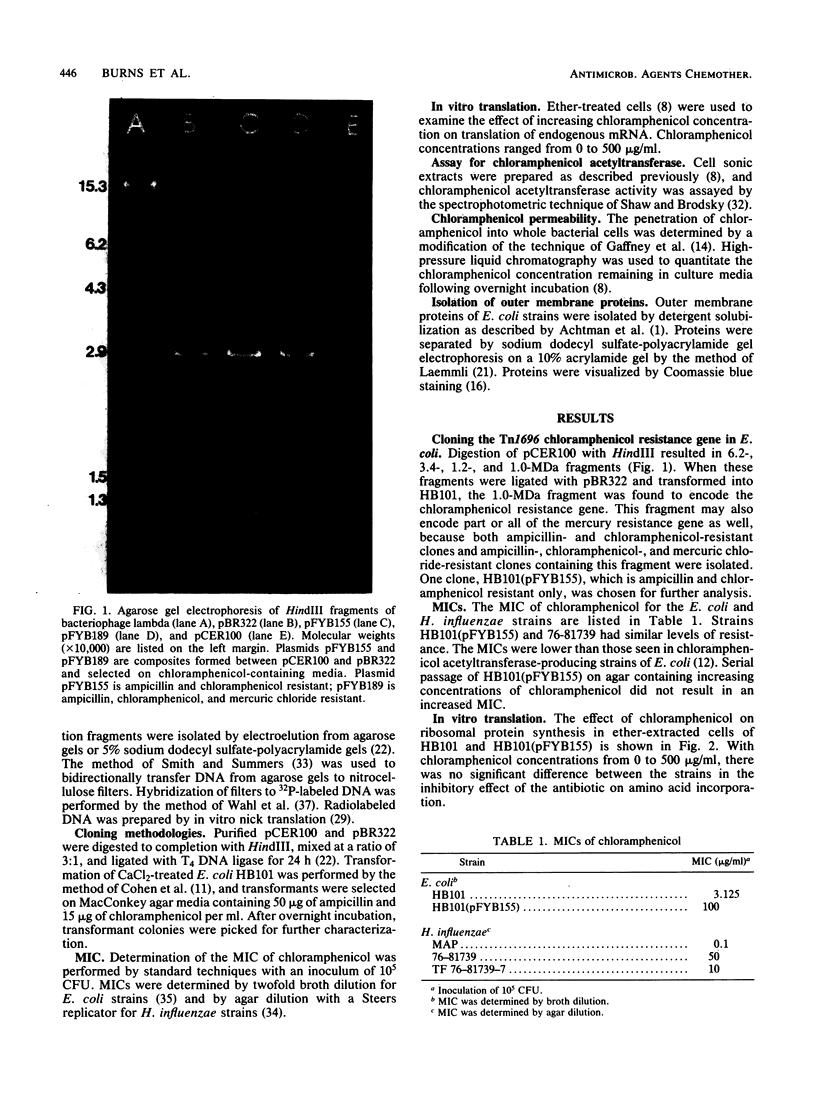



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. M., Henkin T. M., Chambliss G. H., Bott K. F. New chloramphenicol resistance locus in Bacillus subtilis. J Bacteriol. 1984 Apr;158(1):386–388. doi: 10.1128/jb.158.1.386-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G. A., Fahnestock S. R. Chloramphenicol resistance mutation in Escherichia coli which maps in the major ribosomal protein gene cluster. J Bacteriol. 1979 Mar;137(3):1315–1323. doi: 10.1128/jb.137.3.1315-1323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., O'Hara K., Wong S. Lipopolysaccharide changes in impermeability-type aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):250–255. doi: 10.1128/aac.26.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Chopra I., Eccles S. J. Diffusion of tetracycline across the outer membrane of Escherichia coli K-12: involvement of protein Ia. Biochem Biophys Res Commun. 1978 Jul 28;83(2):550–557. doi: 10.1016/0006-291x(78)91025-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. R factor tetracycline and chloramphenicol resistance in Escherichia coli K12 cmlB mutants. J Gen Microbiol. 1975 Oct;90(2):303–310. doi: 10.1099/00221287-90-2-303. [DOI] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin J. E., Ingram J. M. Chloramphenicol-resistant variants of Pseudomonas aeruginosa defective in amino acid transport. Can J Biochem. 1980 Oct;58(10):1165–1171. doi: 10.1139/o80-156. [DOI] [PubMed] [Google Scholar]
- Iyobe S., Sagai H., Mitsuhashi S. Tn2001, a transposon encoding chloramphenicol resistance in Pseudomonas aeruginosa. J Bacteriol. 1981 Apr;146(1):141–148. doi: 10.1128/jb.146.1.141-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., O'Hara K. Mechanism of chloramphenicol-resistance mediated by kR102 factor in Pseudomonas aeruginosa. J Antibiot (Tokyo) 1976 Feb;29(2):176–180. doi: 10.7164/antibiotics.29.176. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus D., Nierhaus K. H. Identification of the chloramphenicol-binding protein in Escherichia coli ribosomes by partial reconstitution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2224–2228. doi: 10.1073/pnas.70.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Takata R., Tanaka K., Tamaki M. Chloramphenicol resistant mutants of Bacillus subtilis. Mol Gen Genet. 1973 Dec 20;127(2):163–173. doi: 10.1007/BF00333664. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Swenson C. D., Owens L. M., Smith A. L. Characterization of chloramphenicol-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Oct;18(4):610–615. doi: 10.1128/aac.18.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., McNeill W. F., Farrar W. E., Jr Transposable plasmid deoxyribonucleic acid sequence in Pseudomonas aeruginosa which mediates resistance to gentamicin and four other antimicrobial agents. J Bacteriol. 1979 Sep;139(3):877–882. doi: 10.1128/jb.139.3.877-882.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]