Abstract
Metastases are the most common malignant liver lesions and the most common indication for hepatic imaging. Specific characterization of liver metastases in patients with primary non-hepatic tumors is crucial to avoid unnecessary diagnostic work-up for incidental benign liver lesions. Magnetic resonance (MR) is rapidly emerging as the imaging modality of choice for detection and characterization of liver lesions due to the high specificity resulting from optimal lesion-to-liver contrast and no radiation exposure. Improvements in breath-hold T1-weighted fast spoiled gradient echo and rapid T2-weighted single shot echo-train acquisition enable imaging of the liver in a single breath-hold with high spatial resolution. Most metastases are hypo- to isointense on T1 and iso- to hyperintense on T2-weighted images. MR contrast agents provide critical tumor characterization and can be safely used in patients with iodine contrast allergy and renal failure. Other agents, including newly developing gadolinium-chelates or iron oxide agents may provide additional benefits in selected applications. The degree and nature of tumor vascularity form the basis for liver lesion characterization based on enhancement properties. Liver metastases may be hypovascular or hypervascular. Colon, lung, breast and gastric carcinomas are the most common tumors causing hypovascular liver metastases, and typically show perilesional enhancement. Neuroendocrine tumors including carcinoid and islet cell tumors, renal cell carcinoma, breast, melanoma, and thyroid carcinoma are tumors most commonly causing hypervascular hepatic metastases, which may develop early enhancement with variable degrees of washout and peripheral rim enhancement.
Keywords: Liver metastasis, magnetic resonance imaging
Introduction
Evaluation of liver metastases is one of the most common indications for magnetic resonance (MR) imaging of the liver. Metastases are the most common malignant liver lesions and are about 18–40 times more common that primary liver tumors[1]. It has been established that complete surgical resection of liver metastases prolongs survival in eligible surgical candidates. Hence, detection, absolute quantification and localization of liver metastases are crucial as the findings alter the clinical outcome of the disease and patient management[2]. Furthermore, the rising cost of health care also makes a compelling case for a single comprehensive imaging test for evaluation of liver metastases to aid the clinician in making patient treatment decisions.
Although, intraoperative ultrasound can accurately detect liver lesions, it increases the total duration of surgery and it has limited ability to distinguish benign lesions from malignant lesions[3]. Computed tomographic arterial portography (CTAP) is thought to be a highly sensitive technique for detection of liver lesions. However, CTAP is an invasive test with limited ability to characterize lesions and has high false positive rates[3]. Prior reports indicate that the sensitivity of unenhanced and gadolinium-chelate-enhanced MR imaging is superior to contrast-enhanced computed tomography (CT) scanning, although it is comparable or slightly inferior to CTAP for detection of liver metastases[4–6]. With the recent advances in MR contrast agents, MR may replace CTAP. MR imaging has several advantages over CT such as no risks from radiation exposure and no adverse reactions to iodinated contrast agents. Indeed, MR is rapidly evolving as the primary imaging modality for the detection and characterization of liver lesions including metastases in many centers[2]. In this review, the MR imaging features of the common metastatic liver lesions are discussed after a brief review of current MR imaging techniques.
MR imaging techniques
MR imaging of the liver initially relied upon standard spin-echo (SE) T1-weighted (T1W) and T2-weighted (T2W) methods, representing sequences that acquire data over a long time window relative to respiratory movement[7]. State-of-the-art MR techniques allow shorter acquisition time sequences that can be completed within a single breath-hold, including T1W fast spoiled gradient echo (SGE) and breath-hold half-Fourier transform single shot spin echo (HASTE or SSFSE) methods. The single shot spin echo sequences are slice selective, performing all of the preparation and acquisition for an individual slice in approximately one second, with the central k-space data acquired over a fraction of that time. As the image contrast is derived from central k-space, single shot techniques are remarkably motion insensitive, and have breathing-independent characteristics that are useful in non-compliant patients. T1W gradient echo, either two-dimensional (2D) or three-dimensional (3D) sequences, tend to be motion sensitive as these techniques use interleaved phase lines. The phase lines are collected from each image slice one phase line at a time moving from slice to slice. This leads to the observation that even transient motion occurring during only a fraction of the acquisition will affect all the slices.
T1W techniques with motion insensitive properties are also available. These use the same basic concept of acquiring 2D data with rapid filling of central k-space by preparing and analyzing one slice at a time, but based upon SGE sequences using an inversion or saturation pre-pulse to generate the T1 contrast. These sequences have been referred to as turbo fast low angle shot (tFLASH) and fast inversion recovery motion insensitive (FIRM). Another development has been the application of 3D gradient echo sequences such as volumetric interpolated breath-hold examination (VIBE). This approach facilitates generation of high-resolution images of the liver, particularly out-of-plane resolution, with the ability to generate near isotropic voxel sizes in the order of 2.0–2.5 mm. Such an approach allows better evaluation of hepatic vascular anatomy, and generates volumetric data sets that can be used for multiplanar reconstruction. Currently, one of the limitations of high spatial resolution 3D techniques may be related to reduced soft-tissue contrast relative to 2D gradient echo sequences acquired with thicker sections.
Intravenous contrast material
Gadolinium chelate is an extracellular agent causing T1-shortening resulting in marked elevation of the signal on T1W images. The liver is unique in having a dual blood supply, receiving 70–80% of afferent blood flow from the portal vein, and the remainder from the hepatic artery. Hepatic metastases develop varying degrees of hepatic arterial blood supply depending on the characteristics of the primary tumor. Thus, liver metastases may be classified as hypervascular and hypovascular. Liver metastases that have predominantly hepatic arterial blood supply (hypervascular) are conspicuous in the hepatic arterial dominant phase of liver enhancement. Hypovascular liver metastases are predominantly supplied by portal venous branches, and demonstrate slower and less intense enhancement making them more conspicuous in portal venous phase images. Hence, MR imaging for liver metastases should include dynamic multiphase post-gadolinium SGE imaging.
The timing delay between initiation of contrast administration and initiation of the SGE scan for optimal hepatic arterial dominant phase SGE images is critical, with a narrow time window of between 20 and 30 seconds (s) noted for most patients. This is variable and would depend on the cardiac output of the patient. Visually, optimal arterial phase enhancement may be verified on the resulting images with ideal results showing contrast enhancement of the central portal veins, while the hepatic veins remain completely unenhanced[8,9]. The optimal time for the portal venous dominant phase is less critical, and generally the scanning delay is 55–70 s, with ideal images showing recent filling of the hepatic veins. Scanning for delayed phase may be performed anytime between 2 and 4 minutes (min).
Tissue specific MR contrast agents are also available such as mangafodipir (Mn-DPDP), a hepatocyte-specific positive agent, and iron oxide particles such as ferumoxides, reticuloendothelial system-negative agents[10,11]. Mn-DPDP, a T1-shortening agent, is taken up by normal hepatocytes, through the same transport mechanism used by circulating bile salts, and secreted into the bile duct canaliculi without being metabolized. After intravenous administration, an enhancement plateau in the liver is reached within 10 min which may persist for several hours and result in an elevated signal on T1W SGE images in normal liver and bile ducts, rendering liver metastases, which lack functional hepatocytes, as relatively lower signal foci. The presumed mechanism of enhancement of normal liver is due to uptake of manganese ions within the mitochondria of the hepatocytes[1]. However, Mn-DPDP lacks critical dynamic enhancement characteristics. Mn-DPDP is taken up by the functioning hepatocytes in focal nodular hyperplasia and regenerative nodules, so that they appear iso-intense to normal liver parenchyma. However, metastases lack the ability to take up Mn-DPDP and will appear hypo-intense compared with enhancing liver parenchyma on T1W images. Currently, the Food and Drug Administration has not approved Mn-DPDP for diagnostic clinical imaging in the United States.
Several other contrast agents are being formulated in an attempt to combine the benefits of the dynamic enhancing properties of extracellular agents with tissue characterization capability based on hepatocyte uptake of hepatocyte-specific agents. Gadoxetate disodium (Gd-EOB) and gadobenate dimeglumine (Gd- BOPTA) offer optimal enhancement of the liver in dynamic early phase images as well as in delayed hepatobiliary phase images followed by excretion into the bile ducts[1]. Thus, these agents enable focal liver lesion detection and characterization based on hepatocyte specific uptake.
Iron oxide particles such as ferumoxides are taken up by functioning Kupffer's cells, concentrating within the intracellular space over time. When ferumoxides are placed in a magnetic field, they create strong local field inhomogeneities with shortening of both T2 and T2-star relaxation[1,12]. A plateau of decreased signal intensity can be observed from 30 min lasting for up to 6 h[12]. As metastases do not contain Kupffer's cells, they lack the capacity to phagocytose the iron oxide particles. Hence, the MR signal of liver metastases remains unchanged on T2W and T2-star images after intravenous injection of ferumoxides, whereas the normal liver shows marked signal loss. Thus, ferumoxides could substantially increase the lesion-to-liver contrast for liver metastases and improve detection[13].
MR imaging appearance
Unenhanced MR imaging
Liver metastases are variable in their T1 and T2 signal intensities but are usually prolonged resulting in hypo- to iso-intensity on T1W images and iso- to hyper-intensity on T2W images[7]. Liver metastases tend to lose signal in heavily T2W (TE > 160) images unlike hemangioma and cysts. However, liver metastases with liquefactive necrosis resulting in cystic appearance, neuroendocrine tumors, sarcoma and melanoma may be hyper-intense on long TE T2W images[1]. About 25% of liver metastases and in particular colorectal cancer metastases may show a hyper-intense halo of viable tumor surrounding central hypo-intensity due to necrosis[14]. Metastases are also described to show the doughnut sign on T1W images and the target sign on T2W images[15]. The doughnut sign shows a hypo-intense rim surrounding an irregular or ovoid center of even lower signal intensity. The target sign consists of a hyper-intense center due to necrosis surrounded by a relatively less intense rim of viable tumor.
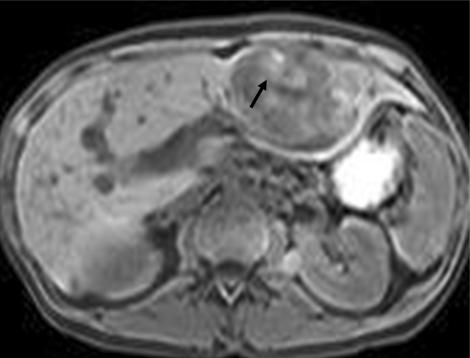
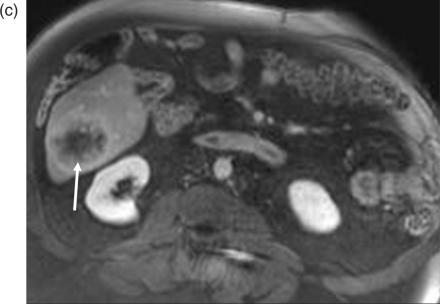
A hyper-intense appearance on T1W images has been described for various liver metastases due to internal content of paramagnetic substance[14]. A high T1 signal has been reported for liver metastases from melanoma (Fig. 1) due to melanin and extracellular methemoglobin, colonic adenocarcinoma due to hemorrhage, ovarian adenocarcinoma due to protein content, myeloma and pancreatic mucinous cystadenocarcinoma[16].
Figure 1.
Melanoma metastasis in liver. Lesion demonstrates heterogenous areas of elevated signal (arrow) on the T1W gradient echo image, characteristic of melanin-containing tumors such as melanoma.
Contrast-enhanced MR imaging
The pattern of tumor vascularity is exploited for detection of liver metastases in gadolinium chelate-enhanced MR imaging. Hyper-vascular liver metastases are usually from primary tumors such as neuroendocrine tumors including carcinoid, islet cell tumors, renal cell carcinoma, melanoma and thyroid carcinoma (Fig. 2). These hypervascular metastases show peak enhancement in hepatic arterial phase images. Hypo-vascular liver metastases are generally from lung, breast, stomach and colorectal carcinoma. Although, breast carcinoma metastases are generally hypo-vascular, occasionally they can be hyper-vascular (Fig. 3). Hypo-vascular liver metastases are conspicuous in the portal venous phase as they appear hypo-intense when compared to the enhancing normal liver parenchyma (Figs. 4 and 5). Cystic liver metastases with no enhancement may be seen with papillary cystic ovarian tumors (Fig. 6).
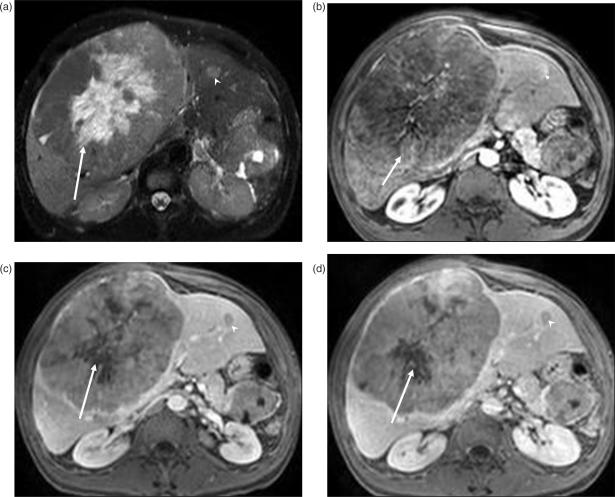
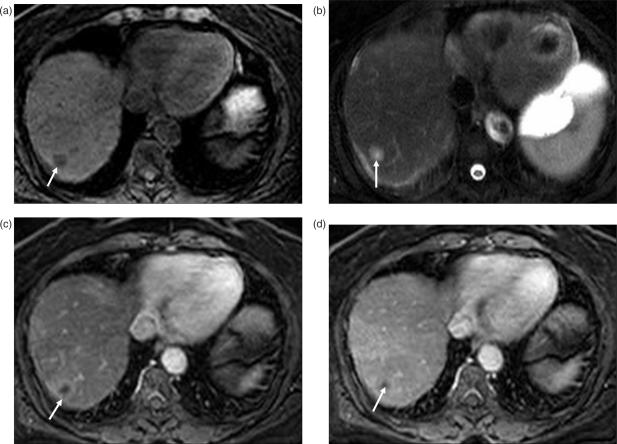
Figure 2.
Islet cell tumor metastases. (a) T2W image demonstrates a well-circumscribed large mass replacing the right lobe of the liver. The central (arrow) hyperintense area represents necrosis as the metastases has rapidly outgrown its blood supply. Post-contrast T1W images reveal heterogenous enhancement of the peripheral viable portion of the metastases in arterial phase (arrow in (b)). In portal venous (c) and delayed phase (d), the lesion is iso- to hypo-intense to the liver, with the central necrotic portion (arrow) remaining unenhanced. Another small metastatic lesion (arrowhead) is seen in the left lobe of the liver.
Figure 3.
Hypervascular liver metastasis from breast carcinoma: (a) The lesion (arrow) is hypointense on the T1W image and (b) iso- to hyperintense (arrow) on the T2W image. (c) Arterial phase gadolinium-enhanced T1W gradient echo image shows enhancement of the lesion (arrow). The lesion becomes iso-intense to the liver in the portal venous phase (d).
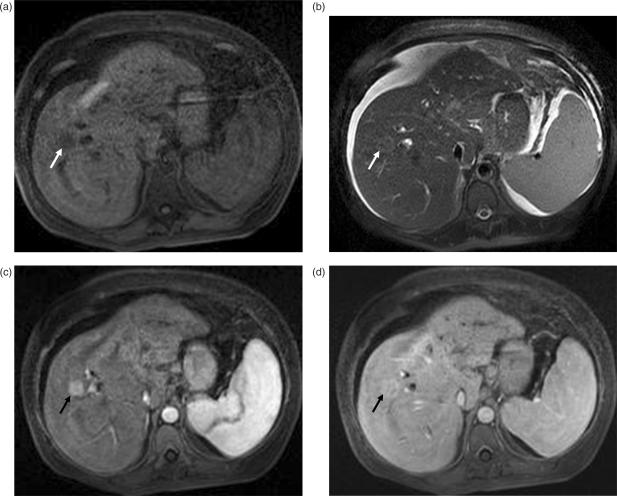
Figure 4.
Colon carcinoma metastasis. (a) T1W image shows a well-circumscribed hypo-intense mass (arrow) in the right lobe of the liver. (b) In post-contrast arterial phase T1W image, the mass remains hypo-intense with a subtle continuous rim of perilesional enhancement (arrow). (c) Portal venous phase images show heterogenous peripheral enhancement (arrow) with no enhancement of the center due to necrosis.
Figure 5.
Hypovascular liver metastases from pancreatic adenocarcinoma. (a) T1W image shows hypo-intense lesion (arrow) in segment VII of the liver, which is hyper-intense (arrow) on the T2W image (b). (c) Lesion demonstrates subtle concentric perilesional enhancement (arrow) on post-contrast arterial phase image. (d) On portal venous phase image perilesional enhancement becomes iso-intense to background liver and the center of the lesion (arrow) does not enhance due to necrosis.
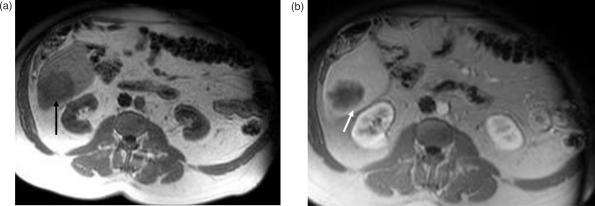
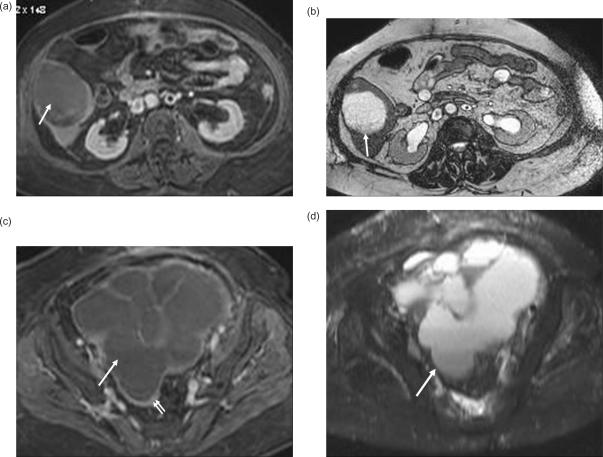
Figure 6.
Cystic liver metastasis from papillary cystic ovarian tumor. (a) Post-contrast T1W image in portal venous phase shows a hypo-intense non-enhancing lesion in segment VI of the liver, which is homogenously hyper-intense on T2W image (b). (c) Post-contrast T1W image of the pelvis shows a lobulated multi-septate cystic mass (arrow) with thin peripheral rim enhancement (double arrows). This cystic mass is hyper-intense (arrow) on the T2W image (d).
Semelka et al. reported nearly equivalent detection of 62 liver metastases in 20 surgically staged patients using gadolinium chelate-enhanced MR imaging, when compared to spiral CTAP[4]. Liver metastases can show transient peripheral continuous rim enhancement in arterial phase. This is due to abundant viable cells in the periphery and fibrosis or necrosis in the center of the metastases[17]. However, recent sophisticated histologic investigations reveal that the peripheral rim enhancement is mainly at the extralesional area due to desmoplastic reaction, inflammation and vascular proliferation[18]. Peripheral continuous rim enhancement is thought to be a reliable feature to differentiate liver metastases from hemangioma, which shows discontinuous or nodular peripheral rim enhancement, similar in intensity to aorta. Soyer et al. demonstrated that hemangiomas can be differentiated from neuroendocrine tumor metastases with a specificity of 98% using both unenhanced T2W and gadolinium chelate-enhanced MR images[19].
Hepatobiliary MR contrast agents have a long temporal imaging window, with satisfactory images obtained for several hours after injection of contrast material. Generally, post-contrast GRE T1W images are acquired 20–60 min after injection of hepatobiliary agents. The sensitivity of MR imaging enhanced with hepatobiliary agents (Mn-DPDP, Gd-BOPTA and Gd-DOB-DTPA) is greater than that of unenhanced MR imaging for non-hepatocellular tumors[1]. Torres et al. reported that the sensitivity of Mn-DPDP-enhanced MR imaging was superior to unenhanced MR imaging for liver metastases in 189 patients[20]. Hamm et al. reported that additional liver lesions were detected with Mn-DPDP-enhanced MR imaging when compared to unenhanced MR imaging[21].
Baron et al. reported that Gd-BOPTA-enhanced MR images and CTAP had equal sensitivity for malignant lesions in 15 patients[22]. Caudana et al. reported that the contrast-to-noise ratio was significantly increased and a significantly greater number of small metastases were detected with post-contrast Gd-BOPTA images that unenhanced MR images[23]. Reimer et al. evaluated the dynamic enhancement patterns of various liver lesions with Gd-EOB-DTPA-enhanced MR imaging[24]. They reported that metastases demonstrated heterogenous uptake during the early phase, washout on delayed phase (>3 min) and the highest lesion-to-liver contrast at 20–45 min. Vogl et al. reported that in patients with liver metastases 56% more lesions can be seen with Gd-EOB-DTPA-enhanced MR imaging than with unenhanced MR imaging[25]. In addition, they found that the detection of additional metastatic lesions in Gd-DOB-DTPA-enhanced MR imaging was independent of the dose of contrast agent used.
Superparamagnetic iron oxides (SPIO) enable detection of liver lesions as small as 3 mm on MR imaging[1]. Liver metastases generally do not take up iron oxides due to lack of normal Kupffer's cells and hence, appear hyper-intense in the background of dark normal liver on T2 and T2-star images. Several studies have compared ferumoxide-enhanced MR imaging with CTAP for detection of liver metastases[1]. Seneterre et al. reported that ferumoxide MR imaging is comparable in sensitivity to CTAP for detection of liver metastases[26]. In this study only a few small metastases were included. Only five patients had metastatic lesions less than 10 mm in size. Limited literature is available for evaluation of small metastatic lesions using ferumoxide-enhanced MR imaging. Oudkerk et al. reported that the sensitivity of ferumoxide-enhanced MR imaging was poor for detection of small liver metastases less than 10 mm in size[27]. Hagspiel et al. reported that the sensitivity of ferumoxide-enhanced MR imaging was inferior to intra-operative ultrasound (56% vs. 80%) in their study of 14 patients with surgical correlation[28]. Recently, Kim et al. reported that the diagnostic performance of Gd-BOPTA-enhanced MR imaging was comparable to SPIO-enhanced MR imaging for detection of liver metastases in their study of 23 patients[29].
Recent advances
Investigators are now evaluating the role of diffusion weighted MR imaging (DWI) for detection of liver metastases. Koh et al. evaluated 40 patients with colorectal hepatic metastases with DWI. They found that liver metastases had greater apparent diffusion coefficients (ADC) when compared to that of background liver[30]. Nasu et al. compared the diagnostic performance of DWI with SPIO-enhanced MR images for detection of liver metastases[31]. They found that DWI when evaluated in conjunction with T1W and T2W MR images were superior to SPIO-enhanced MR imaging for detection of liver metastases in their study of 24 patients.
Conclusions
Optimal detection of liver metastases can alter patient management and result in significant cost savings by reducing the number of unnecessary laparotomies for unresectable disease. MR imaging with gadolinium chelates offers an accurate non-radiation based imaging test for detection of liver metastases. Liver specific MR contrast agents (hepatobiliary and reticuloendothelial agents) offer greater lesion-to-liver contrast than the conventional extracellular agents (gadolinium chelates). Liver specific MR contrast agents may be used in selected clinical situations when the goal is to achieve the highest detection rate for liver focal lesions, for example, when a patient is being evaluated for curative liver resection. Although, ferumoxide-enhanced MR imaging is comparable to CTAP for detection of liver metastases, it has limited specificity. Hence, the metastatic lesions should be confirmed on intra-operative ultrasound.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- [1].Imam K, Bluemke DA. MR imaging in the evaluation of hepatic metastases. Magn Reson Imaging Clin N Am. 2000;8:741–56. [PubMed] [Google Scholar]
- [2].Ward J. New MR techniques for the detection of liver metastases. Cancer Imaging. 2006;6:33–42. doi: 10.1102/1470-7330.2006.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rummeny EJ, Marchal G. Liver imaging. Clinical applications and future perspectives. Acta Radiol. 1997;38:626–30. doi: 10.1080/02841859709172392. [DOI] [PubMed] [Google Scholar]
- [4].Semelka RC, Cance WG, Marcos HB, Mauro MA. Liver metastases: comparison of current MR techniques and spiral CT during arterial portography for detection in 20 surgically staged cases. Radiology. 1999;213:86–91. doi: 10.1148/radiology.213.1.r99oc3386. [DOI] [PubMed] [Google Scholar]
- [5].Soyer P, Levesque M, Caudron C, Elias D, Zeitoun G, Roche A. MRI of liver metastases from colorectal cancer vs. CT during arterial portography. J Comput Assist Tomogr. 1993;17:67–74. doi: 10.1097/00004728-199301000-00012. [DOI] [PubMed] [Google Scholar]
- [6].Schima W, Kulinna C, Langenberger H, Ba-Ssalamah A. Liver metastases of colorectal cancer: US, CT or MR? Cancer Imaging. 2005;5A:S149–56. doi: 10.1102/1470-7330.2005.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saini S, Nelson RC. Technique for MR imaging of the liver. Radiology. 1995;197:575–7. doi: 10.1148/radiology.197.3.7480718. [DOI] [PubMed] [Google Scholar]
- [8].Martin DR, Danrad R, Hussain SM. MR imaging of the liver. Radiol Clin North Am. 2005;43:861–86. viii. doi: 10.1016/j.rcl.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [9].Martin DR, Friel HT, Danrad R, De Becker J, Hussain SM. Approach to abdominal imaging at 1.5 Tesla and optimization at 3 Tesla. Magn Reson Imaging Clin N Am. 2005;13:241–54. v–vi. doi: 10.1016/j.mric.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [10].Hahn PF, Saini S. Liver-specific MR imaging contrast agents. Radiol Clin North Am. 1998;36:287–97. doi: 10.1016/s0033-8389(05)70022-1. [DOI] [PubMed] [Google Scholar]
- [11].Ji H, Ros PR. Magnetic resonance imaging. Liver-specific contrast agents. Clin Liver Dis. 2002;6:73–90. doi: 10.1016/s1089-3261(03)00067-9. [DOI] [PubMed] [Google Scholar]
- [12].Bellin MF, Zaim S, Auberton E, et al. Liver metastases: safety and efficacy of detection with superparamagnetic iron oxide in MR imaging. Radiology. 1994;193:657–63. doi: 10.1148/radiology.193.3.7972804. [DOI] [PubMed] [Google Scholar]
- [13].Vogl TJ, Hammerstingl R, Schwarz W, et al. Superparamagnetic iron oxide-enhanced versus gadolinium-enhanced MR imaging for differential diagnosis of focal liver lesions. Radiology. 1996;198:881–7. doi: 10.1148/radiology.198.3.8628887. [DOI] [PubMed] [Google Scholar]
- [14].Sica GT, Ji H, Ros PR. Computed tomography and magnetic resonance imaging of hepatic metastases. Clin Liver Dis. 2002;6:165–79. vii. doi: 10.1016/s1089-3261(03)00071-0. [DOI] [PubMed] [Google Scholar]
- [15].Wittenberg J, Stark DD, Forman BH, et al. Differentiation of hepatic metastases from hepatic hemangiomas and cysts by using MR imaging. AJR Am J Roentgenol. 1988;151:79–84. doi: 10.2214/ajr.151.1.79. [DOI] [PubMed] [Google Scholar]
- [16].Kelekis NL, Semelka RC, Woosley JT. Malignant lesions of the liver with high signal intensity on T1-weighted MR images. J Magn Reson Imaging. 1996;6:291–4. doi: 10.1002/jmri.1880060206. [DOI] [PubMed] [Google Scholar]
- [17].Yu JS, Rofsky NM. Hepatic metastases: perilesional enhancement on dynamic MRI. AJR Am J Roentgenol. 2006;186:1051–8. doi: 10.2214/AJR.04.1698. [DOI] [PubMed] [Google Scholar]
- [18].Semelka RC, Hussain SM, Marcos HB, Woosley JT. Perilesional enhancement of hepatic metastases: correlation between MR imaging and histopathologic findings-initial observations. Radiology. 2000;215:89–94. doi: 10.1148/radiology.215.1.r00mr2989. [DOI] [PubMed] [Google Scholar]
- [19].Soyer P, Gueye C, Somveille E, Laissy JP, Scherrer A. MR diagnosis of hepatic metastases from neuroendocrine tumors versus hemangiomas: relative merits of dynamic gadolinium chelate-enhanced gradient-recalled echo and unenhanced spin-echo images. AJR Am J Roentgenol. 1995;165:1407–13. doi: 10.2214/ajr.165.6.7484575. [DOI] [PubMed] [Google Scholar]
- [20].Torres CG, Lundby B, Sterud AT, McGill S, Gordon PB, Bjerknes HS. MnDPDP for MR imaging of the liver. Results from the European phase III studies. Acta Radiol. 1997;38:631–7. doi: 10.1080/02841859709172393. [DOI] [PubMed] [Google Scholar]
- [21].Hamm B, Vogl TJ, Branding G, et al. Focal liver lesions: MR imaging with Mn-DPDP – initial clinical results in 40 patients. Radiology. 1992;182:167–74. doi: 10.1148/radiology.182.1.1309218. [DOI] [PubMed] [Google Scholar]
- [22].Baron RL, Peterson MS. Liver tumor detection: comparison of Gd-BOPTA-enhanced MR, contrast-enhanced CT, and CT arterial portography (abstract) Radiology. 1995;197:415. [Google Scholar]
- [23].Caudana R, Morana G, Pirovano GP, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium benzyloxypropionictetra-acetate (BOPTA) – preliminary results of phase II clinical application. Radiology. 1996;199:513–20. doi: 10.1148/radiology.199.2.8668804. [DOI] [PubMed] [Google Scholar]
- [24].Reimer P, Rummeny EJ, Daldrup HE, et al. Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Radiol. 1997;7:275–80. doi: 10.1007/s003300050150. [DOI] [PubMed] [Google Scholar]
- [25].Vogl TJ, Kummel S, Hammerstingl R, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59–67. doi: 10.1148/radiology.200.1.8657946. [DOI] [PubMed] [Google Scholar]
- [26].Seneterre E, Taourel P, Bouvier Y, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200:785–92. doi: 10.1148/radiology.200.3.8756932. [DOI] [PubMed] [Google Scholar]
- [27].Oudkerk M, van den Heuvel AG, Wielopolski PA, Schmitz PI, Borel Rinkes IH, Wiggers T. Hepatic lesions: detection with ferumoxide-enhanced T1-weighted MR imaging. Radiology. 1997;203:449–56. doi: 10.1148/radiology.203.2.9114103. [DOI] [PubMed] [Google Scholar]
- [28].Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471–8. doi: 10.1148/radiology.196.2.7617863. [DOI] [PubMed] [Google Scholar]
- [29].Kim YK, Ko SW, Hwang SB, Kim CS, Yu HC. Detection and characterization of liver metastases: 16-slice multidetector computed tomography versus superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur Radiol. 2006;16:1337–45. doi: 10.1007/s00330-005-0140-y. [DOI] [PubMed] [Google Scholar]
- [30].Koh DM, Scurr E, Collins DJ, et al. Colorectal hepatic metastases: quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006;16:1898–905. doi: 10.1007/s00330-006-0201-x. [DOI] [PubMed] [Google Scholar]
- [31].Nasu K, Kuroki Y, Nawano S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–30. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]