Abstract
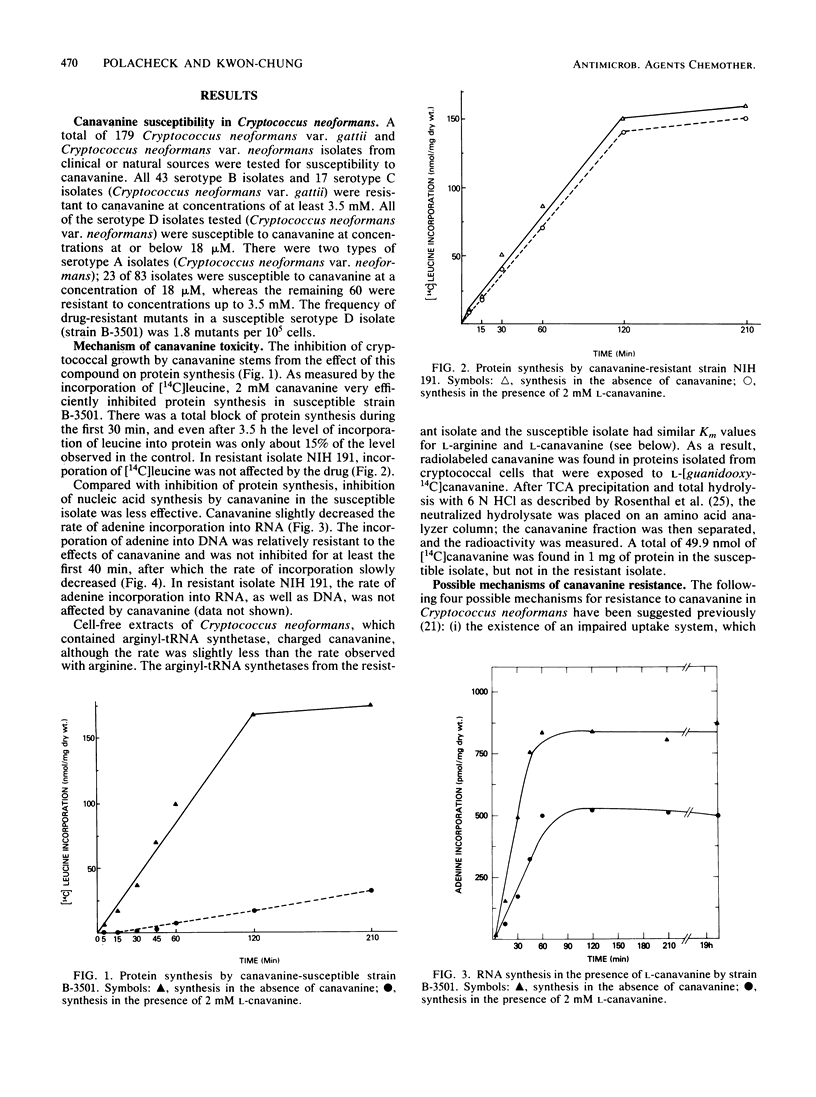
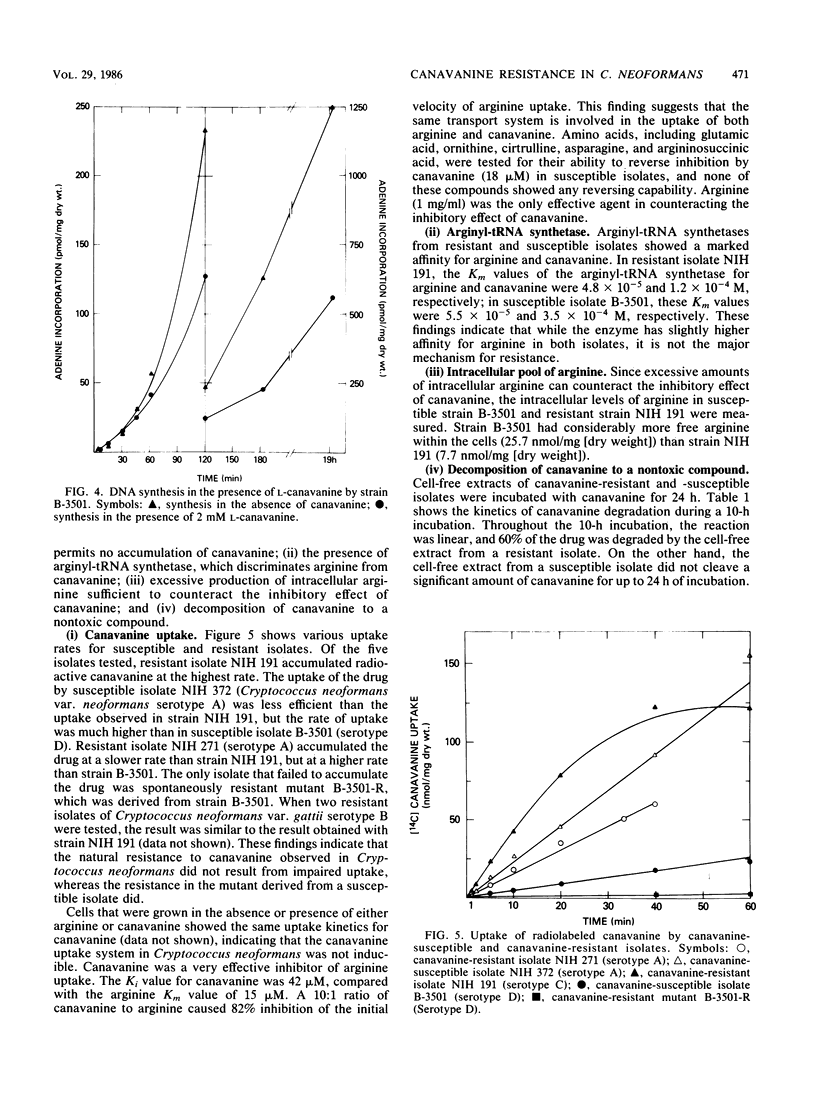
All of the isolates of Cryptococcus neoformans var. gattii which we tested were resistant to greater than or equal to 3.5 mM canavanine. All of the serotype D isolates and 28% of the serotype A isolates of C. neoformans var. neoformans tested were susceptible to less than or equal to 18 microM canavanine, whereas the remaining 72% of the serotype A isolates were as resistant as the C. neoformans gattii isolates. In the naturally resistant isolates, the mechanism of resistance appeared to be decomposition of canavanine to a nontoxic product. However, in a resistant mutant derived from a naturally susceptible isolate, the mechanism of resistance was an impaired uptake system for canavanine. The toxic effect of canavanine in Cryptococcus results from the incorporation of canavanine into the protein component that is essential for the synthesis of proteins and RNAs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLENDE C. C., ALLENDE J. E. PURIFICATION AND SUBSTRATE SPECIFICITY OF ARGINYL-RIBONUCLEIC ACID SYNTHETASE FROM RAT LIVER. J Biol Chem. 1964 Apr;239:1102–1106. [PubMed] [Google Scholar]
- Ackermann W. W., Cox D. C., Dinka S. Control of histone and DNA synthesis with canavanine, puromycin, and poliovirus. Biochem Biophys Res Commun. 1965 Jun 9;19(6):745–750. doi: 10.1016/0006-291x(65)90321-9. [DOI] [PubMed] [Google Scholar]
- Attias J., Schlesinger M. J., Schlesinger S. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. IV. Substitution of canavanine for arginine. J Biol Chem. 1969 Jul 25;244(14):3810–3817. [PubMed] [Google Scholar]
- Chrispeels M. J., Boyd R. F., Williams L. S., Neidhardt F. C. Modification of valyl tRNA synthetase by bacteriophage in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):463–475. doi: 10.1016/0022-2836(68)90421-x. [DOI] [PubMed] [Google Scholar]
- Dahlman D. L., Rosenthal G. A. Further studies of the effect of L-canavanine on the tobacco hornworm, Manduca sexta. J Insect Physiol. 1976;22(2):265–271. doi: 10.1016/0022-1910(76)90035-4. [DOI] [PubMed] [Google Scholar]
- Hare J. D. Reversible inhibition of DNA synthesis by the arginine analogue canavanine in hamster and mouse cells in vitro. Exp Cell Res. 1969 Nov;58(1):170–174. doi: 10.1016/0014-4827(69)90129-3. [DOI] [PubMed] [Google Scholar]
- KIHARA H., PRESCOTT J. M., SNELL E. E. The bacterial cleavage of canavanine to homoserine and guanidine. J Biol Chem. 1955 Nov;217(1):497–503. [PubMed] [Google Scholar]
- KIHARA H., SNELL E. E. The enzymatic cleavage of canavanine to O-ureidohomoserine and ammonia. J Biol Chem. 1957 May;226(1):485–495. [PubMed] [Google Scholar]
- KRUSE P. F., Jr, WHITE P. B., CARTER H. A., McCOY T. A. Incorporation of canavanine into protein of Walker carcinosarcoma 256 cells cultured in vitro. Cancer Res. 1959 Jan;19(1):122–125. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Bennett J. E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol. 1982 Mar;15(3):535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McMahon D., Blaschko W. Chloral hydrate inhibits protein synthesis in vivo. Biochim Biophys Acta. 1971 May 13;238(2):338–342. doi: 10.1016/0005-2787(71)90101-8. [DOI] [PubMed] [Google Scholar]
- McMahon D., Langstroth P. The effects of canavanine and of arginine starvation on macromolecular synthesis in Chlamydomonas reinhardi. J Gen Microbiol. 1972 Nov;73(2):239–250. doi: 10.1099/00221287-73-2-239. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Mehler A. H. The arginyl transfer ribonucleic acid synthetase of Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5490–5494. [PubMed] [Google Scholar]
- Polacheck I., Hearing V. J., Kwon-Chung K. J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Kwon-Chung K. J. Creatinine metabolism in Cryptococcus neoformans and Cryptococcus bacillisporus. J Bacteriol. 1980 Apr;142(1):15–20. doi: 10.1128/jb.142.1.15-20.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G. A., Dahlman D. L., Janzen D. H. A novel means for dealing with L-canavanine, a toxic metabolite. Science. 1976 Apr 16;192(4236):256–258. doi: 10.1126/science.1257764. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A., Dahlman D. L., Janzen D. H. L-Canaline Detoxification: A Seed Predator's Biochemical Mechanism. Science. 1978 Nov 3;202(4367):528–529. doi: 10.1126/science.202.4367.528. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A., Dahlman D. L., Robinson G. W. L-Arginine kinase from tobacco hornworm, Manduca sexta (L.). Purification, properties, and interaction with L-canavanine. J Biol Chem. 1977 Jun 10;252(11):3679–3683. [PubMed] [Google Scholar]
- Rosenthal G. A. Investigations of Canavanine Biochemistry in the Jack Bean Plant, Canavalia ensiformis (L.) DC: II. Canavanine Biosynthesis in the Developing Plant. Plant Physiol. 1972 Sep;50(3):328–331. doi: 10.1104/pp.50.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G. A. Is canavanine a natural component of mammalian metabolism? J Theor Biol. 1978 Mar 20;71(2):265–273. doi: 10.1016/0022-5193(78)90271-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A., Janzen D. H., Dahlman D. L. Degradation and detoxification of canavanine by a specialized seed predator. Science. 1977 May 6;196(4290):658–660. doi: 10.1126/science.854740. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A. The biological effects and mode of action of L-canavanine, a structural analogue of L-arginine. Q Rev Biol. 1977 Jun;52(2):155–178. doi: 10.1086/409853. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Russell A. S. A rapid and sensitive assay for arginase. Anal Biochem. 1980 Feb;102(1):206–212. doi: 10.1016/0003-2697(80)90340-1. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Canavanine death in Escherichia coli. J Mol Biol. 1965 Dec;14(2):474–489. doi: 10.1016/s0022-2836(65)80197-8. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Mechanism of canavanine death in Escherichia coli. I. Effect of canvainine on macromolecular synthesis. J Mol Biol. 1968 May 14;33(3):843–860. doi: 10.1016/0022-2836(68)90323-9. [DOI] [PubMed] [Google Scholar]


