Abstract
Observational data suggest that periodic breathing is more common in subjects with low FETCO2, high apnoeic thresholds or high chemoreflex sensitivity. It is, however, difficult to determine the individual effect of each variable because they are intrinsically related. To distinguish the effect of isolated changes in chemoreflex sensitivity, mean FETCO2 and apnoeic threshold, we employed a modelling approach to break their obligatory in vivo interrelationship. We found that a change in mean CO2 fraction from 0.035 to 0.045 increased loop gain by 70 ± 0.083% (P < 0.0001), irrespective of chemoreflex gain or apnoea threshold. A 100% increase in the chemoreflex gain (from 800 l min−1 (fraction CO2)−1) resulted in an increase in loop gain of 275 ± 6% (P < 0.0001) across a wide range of values of steady state CO2 and apnoea thresholds. Increasing the apnoea threshold FETCO2 from 0.02 to 0.03 had no effect on system stability. Therefore, of the three variables the only two destabilizing factors were high gain and high mean CO2; the apnoea threshold did not independently influence system stability. Although our results support the idea that high chemoreflex gain destabilizes ventilatory control, there are two additional potentially controversial findings. First, it is high (rather than low) mean CO2 that favours instability. Second, high apnoea threshold itself does not create instability. Clinically the apnoea threshold appears important only because of its associations with the true determinants of stability: chemoreflex gain and mean CO2.
Periodic breathing is self-sustaining oscillations of cardiac and respiratory parameters, with cyclical periods of apnoea and hyperpnoea approximately once per minute. It occurs in some patients with chronic heart failure (Hanly et al. 1989b; Sin et al. 1999; Silva et al. 2001), and is an adverse prognostic indicator (Hanly & Zuberi-Khokhar, 1996; Lanfranchi et al. 1999; Sin et al. 2000; Bradley & Floras, 2003; Corra et al. 2006). This unstable pattern of control arises from overshoot of the time-delayed negative feedback mechanisms controlling ventilation (Douglas & Haldane, 1909; Cherniack et al. 1979). This results from excessive and/or delayed ventilatory responses to alterations in CO2 levels, which leads to a vicious circle of inappropriate feedback responses. Physiological variables that are known to interact to determine system stability include chemoreflex delay and gain, alveolar volume and cardiac output (Francis et al. 1999a; Francis et al. 2000b).
Many clinical studies both from our group and others have found that accompanying the respiratory oscillations in periodic breathing there are significant haemodynamic oscillations, and that system stability determines both respiratory and cardiac oscillations (Faber et al. 1990; Davies et al. 2000; Francis et al. 2000a).
Previously it was thought that these haemodynamic oscillations may have a causative role in periodic breathing (Ben-Dov et al. 1992). This was based on the observation that the amplitude of  oscillations is larger than the amplitude of
oscillations is larger than the amplitude of 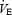 oscillations in periodic breathing, and the
oscillations in periodic breathing, and the  oscillations occur earlier in the periodic breathing cycle. This led the authors to conclude that ventilation alone cannot be driving the oscillations in
oscillations occur earlier in the periodic breathing cycle. This led the authors to conclude that ventilation alone cannot be driving the oscillations in  , and therefore the cardiac system must have a role (Ben-Dov et al. 1992). However, subsequent theoretical work by our group (Francis et al. 1999b) used a more rigorous mathematical approach to analyse the effect of cyclic fluctuations in ventilation on
, and therefore the cardiac system must have a role (Ben-Dov et al. 1992). However, subsequent theoretical work by our group (Francis et al. 1999b) used a more rigorous mathematical approach to analyse the effect of cyclic fluctuations in ventilation on  and
and 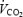 . We reanalysed the data of Ben-Dov et al. (1992) using this new mathematical approach, and found that the
. We reanalysed the data of Ben-Dov et al. (1992) using this new mathematical approach, and found that the  and
and 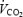 oscillations could be entirely explained by the fluctuations in alveolar ventilation. Clinical work by our group (Francis et al. 1999a) which specifically controlled for the confounding factors in the work of Ben-Dov et al. (1992) demonstrated that simple oscillations in ventilation in volunteers produce exactly the oscillations in
oscillations could be entirely explained by the fluctuations in alveolar ventilation. Clinical work by our group (Francis et al. 1999a) which specifically controlled for the confounding factors in the work of Ben-Dov et al. (1992) demonstrated that simple oscillations in ventilation in volunteers produce exactly the oscillations in  and
and 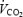 that would be expected. Therefore although haemodynamic oscillations may be present in periodic breathing, they are not necessary in the genesis of the instability and therefore it is reasonable in modelling studies to consider cardiac output as broadly stable in the vicinity of the steady state.
that would be expected. Therefore although haemodynamic oscillations may be present in periodic breathing, they are not necessary in the genesis of the instability and therefore it is reasonable in modelling studies to consider cardiac output as broadly stable in the vicinity of the steady state.
There are two opposing hypotheses about the influence of a subject's carbon dioxide levels on ventilatory stability. Observational clinical studies have reported an association between low mean arterial CO2 or high apnoeic thresholds and unstable ventilatory control (Skatrud & Dempsey, 1983; Modarreszadeh et al. 1995; Javaheri & Corbett, 1998). Moreover, since apnoea is preceded by hyperventilation driving arterial CO2 below an ‘apnoea threshold’, it has been argued a high apnoea threshold can encourage instability (Cherniack et al. 1966; Naughton et al. 1993).
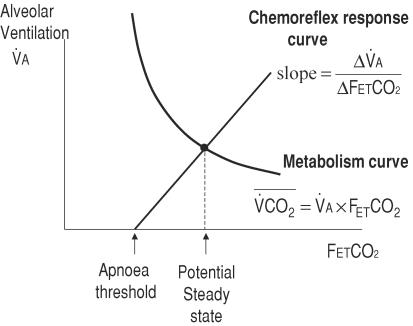
An alternative hypothesis is that the apnoeic threshold is not itself an independent determinant of system stability, but that it only appears to be so because it is intimately related to the chemoreflex gain and mean CO2. In a system with a linear chemoreflex response, the apnoeic threshold is geometrically constrained to be linked to mean FETCO2 and the chemoreflex gain (Fig. 1). The chemoreflex slope equals the potential steady state ventilation divided by the difference between potential steady state CO2 and the apnoeic threshold CO2. This makes it extremely difficult to extricate the influence of each independent variable on ventilatory control by clinical observation.
Figure 1. The relationship between the potential steady state, chemoreflex gain and the apnoea threshold.
The potential steady state is the point of intercept of the chemoreflex response and the curve of metabolic production of CO2 (isometabolic curve). If the chemoreflex response curve is constrained to be linear, the intercept (Capn) is determined by the steady state CO2  and
and  .
.
In contrast to clinical studies, mathematical models allow the effect of individual parameters to be assessed independently. However, although there have been several such models of periodic breathing, these typically include an enforced linear chemoreflex relationship meaning that it is still impossible to examine the independent effect of the apnoeic threshold on ventilatory stability.
We present a mathematical model deliberately designed to allow any combination of potential steady state CO2 and ventilation, chemoreflex gain and apnoeic threshold. The aim of this study was to apply this model to assess the stability of the resultant control system, separating the relative contributions to ventilatory stability of potential steady state CO2, chemoreflex slope and apnoeic threshold.
Methods
Chemoreflex control of ventilation
Ventilatory stability depends on the interplay of two physiological mechanisms. The first is chemoreflex gain: the effect that a change in end-tidal CO2 has on ventilation. This corresponds to controller gain in standard control theory. The second is exhalation gain, corresponding to plant gain – the effect that a change in ventilation has on end-tidal carbon dioxide (FETCO2).
The central role of the potential steady state
In ventilatory control the two key variables are ventilation and CO2. Mean FETCO2 is linked to ventilation according to a hyperbolic curve (Fig. 1) if CO2 exhalation is to match metabolic production of CO2  in the body.
in the body.
If a particular alveolar ventilation rate (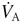 ) is maintained in the medium term, FETCO2 is destined to become
) is maintained in the medium term, FETCO2 is destined to become  (the isometabolic curve). Ventilation in turn depends on FETCO2 through the chemoreflex response curve. Given these constraints, there is only one combination of FETCO2 and ventilation which can be sustained: this is called the potential steady state. Regardless of whether the system is stable or unstable, the potential steady state values of FETCO2 and alveolar ventilation can be defined to be the crossing point of these two lines. Whether this potential steady state will be achieved is determined by the configuration of the control system.
(the isometabolic curve). Ventilation in turn depends on FETCO2 through the chemoreflex response curve. Given these constraints, there is only one combination of FETCO2 and ventilation which can be sustained: this is called the potential steady state. Regardless of whether the system is stable or unstable, the potential steady state values of FETCO2 and alveolar ventilation can be defined to be the crossing point of these two lines. Whether this potential steady state will be achieved is determined by the configuration of the control system.
In general, system stability is dependent on its behaviour around the potential steady state. If the system configuration responds to a small deviation away from the potential steady state by moving progressively closer back to the potential steady state, then the system is stable (Fig. 2A).
Figure 2. Ventilatory stability.
An initial perturbation in ventilation or FETCO2 away from the steady state leads to oscillations in both of these variables. Whether these oscillations magnify into periodic breathing or decay away back to the steady state depends on system stability. In a stable system (A), despite an initial perturbation in FETCO2 away from the steady state, oscillations decay away and values return to close to the potential steady state. In an unstable system (B), with even a small initial perturbation in respiratory parameters, oscillations in FETCO2 and ventilation increase in amplitude, with resulting periodic breathing.
If, on the other hand, the system configuration is such that its responses to a small deviation cause progressively larger deviations from the potential steady state, then the system will be unstable. In this case the steady state exists only in potential form – i.e. a central point around which system oscillations occur (Fig. 2B).
Measurement of system stability
System instability of the model was measured with each potential combination of input parameters by calculating the loop gain (the scale factor by which the amplitude of oscillations increased or decreased on each cycle) in response to a small initial perturbation in ventilation or FETCO2. Values greater than 1 indicate spontaneously expanding oscillations characteristic of unstable control, whereas values less than 1 indicate spontaneously decaying oscillations, i.e. stable control.
Loop gain was calculated by dividing the amplitude of one oscillation in ventilation by the amplitude of the previous oscillation. The value that we take for loop gain is an average of the values for loop gain for the second to fourth oscillations prior to the system reaching its final outcome pattern.
In an unstable system, the presence of apnoeas prevents the size of oscillations increasing beyond a particular amplitude, and therefore eventually the ratio of amplitudes of consecutive oscillations becomes 1.
The model
A simple iterative model can be used to map system behaviour in response to a perturbation (in the form of a small transient change in ventilation or FETCO2 from potential steady state), and thereby determine whether ventilatory control will be stable. The model creates a chemoreflex response curve using our chosen values of chemoreflex gain, potential steady state FETCO2 and apnoeic threshold. Since the chemoreflex response is created synthetically in the model, any desired shape of response can be created, which allows us to separately adjust chemoreflex gain and apnoea threshold (Fig. 3).
Figure 3. A special curved shape of the chemoreflex response allowing chemoreflex slope and apnoea threshold to be changed independently.
The inset shows a magnified region near the potential steady state, indicating 3 chemoreflex slopes. The main figure shows 3 possible apnoea thresholds. Allowing the chemoreflex response to be curved permits any of the chemoreflex slopes to be combined with any of the apnoea thresholds, giving a total of 9 different chemoreflex response curves.
Clinical data suggest that although the chemoreflex response curve is linear near potential steady state values of FETCO2, it is non-linear (concave) near the apnoea threshold (Mohan & Duffin, 1997).
The model plots the response in FETCO2 and ventilation to the perturbation, given the particular chemoreflex response curve characteristics. The ventilation and FETCO2 can therefore oscillate with ever-decreasing amplitude back to the potential steady state (loop gain < 1; stable control), or the oscillations can increase in amplitude (loop gain > 1; unstable control).
To prevent unnecessary duplication of entities representing CO2 levels, in this model we use just two variables. Arterial and end-tidal CO2 move largely in parallel, so as long as ventilation and cardiac output are close to their potential steady state, one can be used as a proxy for the other, providing we remain aware that there may be an offset between the two. The FETCO2 has additional meaning as (in combination with alveolar ventilation) it completely determines the amount of CO2 that is exhaled by the body, and simultaneously (in combination with alveolar volume) it describes the volume of CO2 stored in the lungs. We therefore choose to use end-tidal CO2 fraction (FETCO2) as the single variable to represent CO2 status. To describe chemoreflex responses in these terms, we must recognize that chemoreflexes sense not current FETCO2, but rather the level of CO2 in the blood that was in contact with lung CO2 several seconds previously (equivalent to the chemoreflex delay, δ). We can represent this time-delayed value of CO2 using a subscript (ct−δ).
We incorporated this information into the equation based on a standard single-compartment model (Francis et al. 2000b) to describe how the rate of change in FETCO2 depends on the rate of CO2 production by metabolism following removal by ventilation and circulatory buffering (Francis et al. 2000b). The potential steady state FETCO2 is represented by  , potential steady state ventilation as
, potential steady state ventilation as  , and the current value of CO2 and ventilation at time t, as v and c, respectively. The buffering of CO2 by the circulation and extrapulmonary stores depends on β, the solubility of CO2 in blood, and
, and the current value of CO2 and ventilation at time t, as v and c, respectively. The buffering of CO2 by the circulation and extrapulmonary stores depends on β, the solubility of CO2 in blood, and  , the mean cardiac output.
, the mean cardiac output.
The dependency of rate of change of FETCO2 on the other variables can be derived to be as follows (Francis et al. 1999a;2000b):
| (1) |
We calculated and plotted the fixed curve for the set of potential steady state pairs of CO2 and alveolar ventilation based on the requirement that their mutual product must equal metabolic production of CO2  . We created a modifiable function (‘chemoreflex function’) which described the chemoreflex response: this could be configured to give a large gain (such as in a patient with heart failure), or small gain (such as in a normal subject). Our values for chemoreflex gain were taken from clinical data measured by several different groups, using a range of methodologies (Khoo et al. 1995; Mohan et al. 1999; Van den Aardweg & Karemaker, 2002; Beecroft et al. 2006).
. We created a modifiable function (‘chemoreflex function’) which described the chemoreflex response: this could be configured to give a large gain (such as in a patient with heart failure), or small gain (such as in a normal subject). Our values for chemoreflex gain were taken from clinical data measured by several different groups, using a range of methodologies (Khoo et al. 1995; Mohan et al. 1999; Van den Aardweg & Karemaker, 2002; Beecroft et al. 2006).
We initialized the model from its potential steady state, and then introduced a small perturbation in CO2. The model then iteratively calculated successive pairs of values of v (using the chemoreflex function and the appropriate previous value of c) and dc/dt (using eqn (1)). Thus the course of v and c could be plotted for several minutes of simulated time. The details of the model are given as accompanying online Supplemental material.
We used standard values for the cardiorespiratory parameters that were held constant throughout our investigations: cardiac output ( )= 3.51 min−1,
)= 3.51 min−1,  = 0.21 min−1, chemoreflex delay (δ) = 0.33 min (Gabrielsen et al. 2002; Turner et al. 2004).
= 0.21 min−1, chemoreflex delay (δ) = 0.33 min (Gabrielsen et al. 2002; Turner et al. 2004).
Results
Study 1. Linear chemoreflex response
The simplest shape of the chemoreflex response is a linear function (Fig. 1). Changing any one of chemoreflex gain (S),  or Capn necessitates a change in one of the others.
or Capn necessitates a change in one of the others.
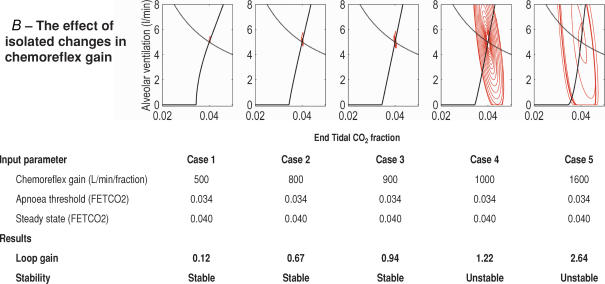
Study 1A. Changing chemoreflex gain and apnoea threshold
First we assessed the effect of altering the chemoreflex gain programmed into the model, whilst maintaining the potential steady state CO2 constant. Because in this study the shape of the chemoreflex response is linear, when chemoreflex gain is altered, the apnoea threshold must change (as shown in Fig. 1).
The simulation was run 5 times with chemoreflex gain taking values of 500, 900, 950, 1000 and 1500 (1000 l min−1 fraction−1FETCO2 is equivalent to 1.3 l min−1 mmHg−1 or 9.9 l min−1 kPa−1). These values range from those observed in normal subjects to ones that would be typical for a patient with heart failure and periodic breathing (Javaheri, 1999; Francis et al. 2000b).
We found that increased chemoreflex gain and apnoeic threshold readily destabilized ventilatory control (Fig. 4A). In the unstable systems, chemoreflex slope also determined the amplitude of oscillations in ventilation and CO2 in the final outcome pattern.
Figure 4. The effect on ventilatory stability of changing apnoea threshold, chemoreflex gain and steady state carbon dioxide in a linear control system.
It is impossible to separate the independent effects of these 3 variables on system stability, when the chemoresponse is linear.
However, from this study in isolation, it is impossible to identify which of these two parameters is responsible for the alterations in ventilatory stability.
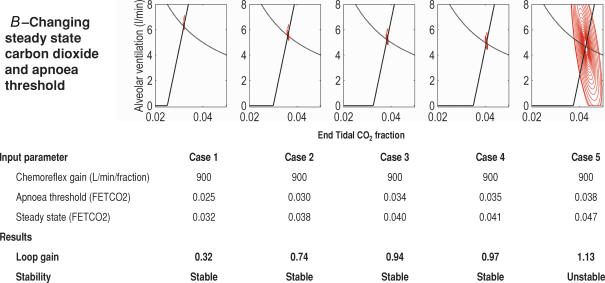
Study 1B. Changing the apnoea threshold and potential steady state values of CO2 and ventilation
We then used the model to investigate the effect of changing both the levels of potential steady state CO2 and the apnoea threshold, whilst maintaining an unchanging chemoreflex gain. Again, due to the linearity of the model, a higher potential steady state CO2 at a fixed chemoreflex gain required a higher apnoea threshold.
As the values of apnoea threshold and potential steady state increased, ventilatory control destabilized (Fig. 4B).
Study 1C. Changing chemoreflex gain and potential steady state values of CO2 and ventilation
In the third study, we altered the third possible pair of these three physiological variables: potential steady state CO2 and chemoreflex gain. As the chemoreflex gain was increased and potential steady state CO2 reduced, the system became more unstable (Fig. 4C).
Potential explanations for Studies 1A, 1B and 1C
From studying the results of Study 1, an observer might propose three possible explanations:
an increase in chemoreflex gain is destabilizing, an increase in apnoea threshold is destabilizing, and the level of CO2 at potential steady state has no effect;
an increase in both the levels of CO2 at potential steady state and at the apnoea threshold are destabilizing, and the chemoreflex gain has no effect;
an increase in the chemoreflex gain and a decrease in the potential steady state value of CO2 are destabilizing and the apnoea threshold has no effect.
The only way to determine which of these explanations is correct is to study the effect of changing each of the three variables separately. To vary  , Capn and S independently requires a specially devised model, which we created for Study 2.
, Capn and S independently requires a specially devised model, which we created for Study 2.
Study 2. Separately investigating the independent effects of chemoreflex gain, apnoea threshold and potential steady state
In order to change one of the variables ( , Capn or S) without altering the others, we introduced curvature into the shape of the chemoreflex response near its lower end. This allows it to pass through any chosen apnoea threshold, while maintaining the chosen value of chemoreflex slope around the potential steady state (Fig. 3).
, Capn or S) without altering the others, we introduced curvature into the shape of the chemoreflex response near its lower end. This allows it to pass through any chosen apnoea threshold, while maintaining the chosen value of chemoreflex slope around the potential steady state (Fig. 3).
Study 2A. Effect of the apnoeic threshold in isolation on system stability
We tried a range of values for apnoea threshold fraction (0.0200–0.0375), whilst maintaining the chemoreflex gain at 950 l min−1 fraction−1 and the potential steady state fractional CO2 at 0.04. These values were selected because in the models of Study 1 they were found to be at the borderline of stability, which meant they were most likely to be informative in Study 2: small changes in system stability would be readily detectable.
We found apnoea threshold had no effect on system stability (Fig. 5A): β = 0.032, P ≥ 0.99.
Figure 5. The effect on ventilatory stability of changing apnoea threshold, chemoreflex gain and steady state carbon dioxide levels independently.
With a curved chemoreflex response, it is possible to demonstate that whilst steady state CO2 and chemoreflex gain both affect stability, apnoea threshold has no influence on it.
Study 2B. Effect of chemoreflex gain in isolation on system stability
We then changed chemoreflex sensitivity, maintaining potential steady state CO2 and apnoea threshold unchanged.
We found that even subtle changes in chemoreflex sensitivities result in large shifts of system stability (Fig. 5B): the 25% increase in chemoreflex gain between cases 2 and 4 causes loop gain to increase by 82%. The unstandardized β coefficient on linear regression was 0.02 (standardized β = 0.998), P ≤ 0.0001.
Study 2C. Effect of potential steady state CO2 in isolation on system stability
We set the chemoreflex gain at 900 l min−1 fraction−1, and the apnoea threshold at 0.035 whilst changing the potential steady state level of CO2 (and hence ventilation) over a narrow range. As the potential steady state value of CO2 increased, respiratory control was found to become more unstable (Fig. 5C). The unstandardized β regression coefficient on linear regression was 62.3 (standardized β = 1.0), P ≤ 0.0001.
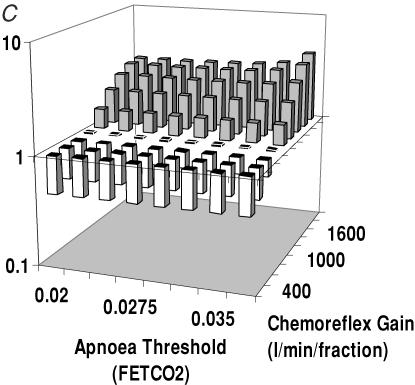
Study 3. Independently varying two parameters
To visualize the independent effects of chemoreflex gain and apnoea threshold, we tested a range of combinations of values of these variables, displaying the resulting loop gains on a 3D plot.
When potential steady state CO2 fraction increased from 0.035 to 0.045, with constant chemoreflex gain, mean loop gain increased by 70 ± 0.083%, P < 0.0001 (Fig. 6A). This was true irrespective of the apnoea threshold, giving additional evidence that the apnoea threshold does not influence system stability.
Figure 6. The effect of independently varying two parameters.
A, the effect of  and Capn on system stability. We maintained the chemoreflex at a fixed value but used different combinations of potential steady state CO2 (
and Capn on system stability. We maintained the chemoreflex at a fixed value but used different combinations of potential steady state CO2 ( ) and apnoeic threshold (Capn). This showed that as
) and apnoeic threshold (Capn). This showed that as  increased, system stability decreased. Capn however, had no effect on stability. B, the effect of
increased, system stability decreased. Capn however, had no effect on stability. B, the effect of  and chemoreflex gain on system stability. We maintained the apnoeic threshold at a constant value and found that as either the chemoreflex gain and
and chemoreflex gain on system stability. We maintained the apnoeic threshold at a constant value and found that as either the chemoreflex gain and  increase, there is a reduction in system stability. C, the effect of Capn and chemoreflex gain on system stability. Again increases in chemoreflex gain were found to increase system instability, and again apnoeic threshold did not affect stability.
increase, there is a reduction in system stability. C, the effect of Capn and chemoreflex gain on system stability. Again increases in chemoreflex gain were found to increase system instability, and again apnoeic threshold did not affect stability.
When chemoreflex gain was increased from 800 to 1600 l min−1 fraction−1 with potential steady state CO2 held constant (Fig. 6C), the loop gain increased by 275 ± 6% (P < 0.0001). Again, there was no change in loop gain over a wide range of apnoea thresholds.
In Fig. 6B the chemoreflex gain and potential steady state CO2 were changed in combination, without changing apnoea threshold. Increasing chemoreflex gain from 800 to 1600 l min−1 (fraction CO2)−1, increased mean loop gain by 248 ± 24% (P < 0.0001). When potential steady state CO2 fraction increased from 0.035 to 0.045 there was a mean loop gain increase of 44 ± 26% (P = 0.03).
Therefore, supporting our findings in Study 2, we found using multiple combinations of parameters of  , Capn and chemoreflex gain, only
, Capn and chemoreflex gain, only  and chemoreflex gain influenced system stability. Apnoea threshold had no independent effect on stability.
and chemoreflex gain influenced system stability. Apnoea threshold had no independent effect on stability.
Study 4. The effect of initial perturbation size on ventilatory stability
Although the results of studies 1, 2 and 3 have shown us which of the three variables tested contribute to system stability, the model has also demonstrated that the relevant part of the chemoreflex response is the ‘average slope’ over the range of FETCO2 which the patient experiences.
Any steep portions of the chemoreflex response curve, near the central potential steady state point, contribute to instability. What constitutes ‘near’ the potential steady state is simply the range of FETCO2 which the patient experiences, and includes values both lower and higher than the steady state FETCO2.
Hypothetically therefore if a chemoreflex response curve has a ‘convex’ shape, it would have an especially steep segment just above the apnoea threshold, and this additional contribution to ‘average’ slope could make a significant impact on stability. In this hypothetical state, it is conceivable that a system could be just stable if initiated in the shallow-sloped region close to the steady state point, but be unstable if initiated at apnoea (giving the patient exposure to the steep part of the curve near apnoea).
However, real physiological measurements of the chemoreflex response curve performed by several previous workers, using a variety of techniques including modified Read's rebreathing and dynamic end-tidal forcing, have shown no evidence of a steepening of the chemoreflex response near the apnoea threshold. In fact, if there is any curvature of the chemoreflex response, the available data indicate that the slope at lower FETCO2 is typically flatter (Mohan et al. 1999), as shown in Fig. 1 of Jensen et al. (2005).
We therefore tested the effect of different-sized initial perturbations on ventilatory stability, first in a system that was ‘unstable’ with a small initial perturbation, and subsequently in a ‘stable’ system.
We found that in an ‘unstable’ system a small perturbation (Fig. 7A, case 1) will result in an initial loop gain > 1, whereas a large perturbation (Fig. 7A, case 5) may result in an initial loop gain of < 1. In both situations however, the final outcome pattern of the system is the same.
Figure 7. Effect on stability of different initial perturbations sizes in FETCO2, with system configuration otherwise kept identical.
A, an ‘unstable’ chemoresponse system: it develops a consistent pattern of oscillations irrespective of the size of the initial perturbation. From a small initial perturbation (top right) the loop gain is clearly initially > 1, while from a large initial oscillation (top left) the loop gain is clearly initially < 1. A system that has loop gain > 1 from a small perturbation definitely cannot be stable. In contrast a loop gain < 1 from large perturbation does not guarantee stability: it might indicate that the system will settle into oscillations of smaller size than the initial perturbation. B, a ‘stable’ chemoresponse system: irrespective of the initial perturbation, ventilation and FETCO2 revert back towards steady state values. Loop gain is < 1 for all perturbation sizes including the small perturbations which are the type of stimuli that truly distinguish stable from unstable systems. Thus loop gain is only a straightforward indicator of stability when calculated for small disturbances.
In the ‘stable’ system, however, the initial loop gain is always < 1, regardless of perturbation size.
Clearly therefore loop gain is a useful summary of system stability only when measured for small initial perturbations. Perturbation size does not affect the final outcome pattern, but large initial perturbations may settle to smaller long-term oscillations in the final outcome pattern (Table 1).
Table 1.
Value of loop gain for different initial perturbation sizes
| Stable control system | Unstable control system | |
|---|---|---|
| Loop gain | Loop gain always <1 | If initial perturbation larger than oscillations in final outcome pattern, oscillations must initially shrink, i.e. Loop Gain <1. |
| If initial perturbation smaller than oscillations in final outcome pattern, oscillations must initially shrink, i.e. Loop Gain >1. |
Discussion
This modelling approach gives a unique opportunity to distinguish individual contributions of different variables which in clinical practice are inextricably linked.
In the first phase of this study, using a model with linear properties, we observed that ventilatory control becomes more unstable if chemoreflex gain increases (with potential steady state CO2 falling), if chemoreflex gain increases (with the apnoeic threshold also increasing) or if both potential steady state and apnoeic levels of CO2 rise together. Potentially there might be three contradictory explanations for these data:
high chemoreflex gain and high Capn each destabilize control (with
 having no effect);
having no effect);high steady state CO2
 and high Capn each destabilize control (but chemoreflex gain has no effect);
and high Capn each destabilize control (but chemoreflex gain has no effect);high chemoreflex gain and
 each destabilize control (but Capn has no effect).
each destabilize control (but Capn has no effect).
The only way to separate these possibilities was to modify the chemoreflex function to remove its constraint of a linear chemoreflex slope. Doing so yielded two potentially controversial findings:
high, rather than low, levels of CO2 favour system instability;
apnoea threshold itself has no influence on ventilatory instability.
Resolving the apparent conflict with clinical studies: chemoreflex measurement may be the key
These findings superficially appear to conflict with widely held clinical opinion, which is that low average CO2 levels predispose to unstable ventilatory control (Skatrud & Dempsey, 1983; Naughton et al. 1993; Xie et al. 1994; Javaheri, 1999). For example, it was reported that low arterial CO2 in heart failure patients is a powerful predictor of central sleep apnoea (Javaheri & Corbett, 1998).
Yet the results of our study indicate that low potential steady state CO2 is stabilizing rather than destabilizing. We believe this apparent inconsistency between model findings and clinical observations is because the clinical observations rarely include the quantitatively most important determinant of respiratory control stability: chemoreflex gain. Clinical studies more often measure  and/or Capn (Modarreszadeh et al. 1995; Javaheri & Corbett, 1998). Dempsey et al. (2004) and Dempsey (2005) concluded that the key determinants of system stability are plant gain, chemoreflex gain and ‘CO2’ reserve (the difference between steady state CO2 and the apnoea threshold), but clinical data cannot separate these parameters.
and/or Capn (Modarreszadeh et al. 1995; Javaheri & Corbett, 1998). Dempsey et al. (2004) and Dempsey (2005) concluded that the key determinants of system stability are plant gain, chemoreflex gain and ‘CO2’ reserve (the difference between steady state CO2 and the apnoea threshold), but clinical data cannot separate these parameters.
If chemoreflex gain is not measured, then the only observable differences between the stable and unstable patients may be small differences in Capn and  . For example, in Fig. 8, there is a twofold difference in the chemoreflex gain between the subject modelled in the left panel and that in the right, but that this is manifest as only a 14% difference in Capn. This may give the false impression that high Capn is mechanistically important in causing instability, whereas in reality it is the large difference in chemoreflex gain that is important. The apnoeic threshold (Capn) has no independent effect on stability, and indeed, in isolation, low
. For example, in Fig. 8, there is a twofold difference in the chemoreflex gain between the subject modelled in the left panel and that in the right, but that this is manifest as only a 14% difference in Capn. This may give the false impression that high Capn is mechanistically important in causing instability, whereas in reality it is the large difference in chemoreflex gain that is important. The apnoeic threshold (Capn) has no independent effect on stability, and indeed, in isolation, low  would actually favour stability rather than instability.
would actually favour stability rather than instability.
Figure 8. A small change in the apnoeic threshold hides a large change in the chemoreflex gain.
If the potential steady state is maintained at a constant value, and only the apnoeic threshold is measured, it would appear that there has only been a small change in the input parameters. Closer inspection, however, shows that although the apnoea threshold has only increased by 14%, the chemoreflex gain doubled.
This distinction is not simply academic, because incorrect belief in a stabilizing effect of increased potential steady state CO2 may result in incorrect design of treatment strategies.
Our work and that of others since the beginnings of mathematical modelling of periodic breathing (Mackey & Glass, 1977) have consistently found that  levels and chemoreflex gain have a multiplicative effect on stability: a 1% rise in chemoreflex gain has an identical effect on system stability as a 1% increase in
levels and chemoreflex gain have a multiplicative effect on stability: a 1% rise in chemoreflex gain has an identical effect on system stability as a 1% increase in  (Francis et al. 2000b). The potential confusion arises because clinically, very large differences in chemoreflex gain may accompany small differences in
(Francis et al. 2000b). The potential confusion arises because clinically, very large differences in chemoreflex gain may accompany small differences in  , and therefore the strong destabilizing effect of the high chemoreflex will certainly overcome the small change in
, and therefore the strong destabilizing effect of the high chemoreflex will certainly overcome the small change in  .
.
The reason why small changes in  can conceal large changes in chemoreflex gain is due to the mandatory relationships between the chemoreflex gain,
can conceal large changes in chemoreflex gain is due to the mandatory relationships between the chemoreflex gain,  and the apnoeic threshold under the constraint of a linear chemoreflex response. From the triangular shape of Fig. 1, we can readily derive this relationship:
and the apnoeic threshold under the constraint of a linear chemoreflex response. From the triangular shape of Fig. 1, we can readily derive this relationship:
Small changes in  , because it is effectively a ‘squared’ term on the denominator, can be associated with large changes in chemoreflex gain.
, because it is effectively a ‘squared’ term on the denominator, can be associated with large changes in chemoreflex gain.
How does supplemental inhaled CO2 assist stability?
A second conundrum is the well recognized observation that raising ambient CO2 concentration can help to stabilize ventilatory control in some patients (Badr et al. 1994; Steens et al. 1994; Lorenzi-Filho et al. 1999). This would appear to contradict our model results, which indicate that raised levels of potential steady state CO2 are destabilizing.
This apparent paradox can be readily explained if one considers that an elevation in inspired CO2 concentration will firstly result in a net reduction in the proportion of CO2 produced by metabolism that is exhaled with each breath. As inspired CO2 concentration increases, each litre of alveolar ventilation per minute at the same FETCO2 excretes less net CO2 from the body. This is equivalent to a rightward shift of the isometabolic curve by FICO2. Quantitatively the curve of metabolically sustainable states changes from
to
The location of the new potential steady state (the new crossing point of the chemoreflex response curve with the new isometabolic curve) therefore moves rightwards and upwards as shown in Fig. 9. At this new potential steady state, the higher average ventilation means that a 1 l min−1 disturbance in ventilation has a smaller absolute effect on FETCO2, i.e. the plant gain has fallen. The result is a smaller total loop gain, i.e. a more stable system (Francis et al. 2000b).
Figure 9. A higher inspired level of CO2 narrows the gap between end-tidal CO2 levels and inspired CO2.
The result is that ventilation rises, and so for a constant metabolic production of CO2 there is a new potential steady state and a fall in plant gain.
This effect of an increased concentration of inhaled CO2 has a similar stabilizing effect on ventilatory control to pharmacologically induced hyperventilation (Nakayama et al. 2002).
How might supplemental oxygen assist stability?
The administration of oxygen during sleep has been shown to reduce sleep apnoea (Hanly et al. 1989a; Ponikowski et al. 1999). This could be due to a reduction in the net chemoreflex gain. First, the degree of CO2 sensitivity increases with hypoxia, and therefore one would expect elimination of hypoxia to reduce CO2 chemoreflex gain. Second, oscillations in arterial oxygen may contribute to some degree to the oscillations in ventilation. Elimination of these oscillations by hyperoxia may therefore further favour stability.
Do our findings conflict with previous modelling studies?
There have been previous modelling studies which also used approaches that allow investigation of the effect of chemoreflex gain and the apnoea threshold on ventilatory stability. For example, our group has previously created an analytical model to identify the causative factors that lead to periodic breathing, and then we performed clinical validation in human subjects (Francis et al. 2000b). The clinical data identified chemoreflex gain as a powerful destabilizing factor on ventilatory stability, and the theoretical analysis also indicated that high potential steady state levels of CO2 are a destabilizing factor.
Khoo et al. (1982, 1991) have created other mathematical models and also assessed the effects of chemoreflex gain and potential steady state CO2 levels on ventilatory stability. They found that system instability is increased by hypercapnia and large chemoreflex gain amongst other factors.
Carley & Shannon (1988b) developed a mathematical model using a frequency domain approach. This model too indicated that high ventilation enhanced system stability, whereas high CO2 was destabilizing.
Miyamoto et al. (2004) clinically validated the concept of the CO2 regulatory system being divided into controller gain (the ventilatory response to inspired CO2), and plant gain (the arterial CO2 response to changes in ventilation). They were able to measure controller gain and plant gain (the reciprocal of the slope of the hyperbola at the potential steady state), and therefore estimate total loop gain. They too found that at higher ventilation plant gain is lower and the system more stable.
The effect of raised inspired CO2 on ventilatory stability has also been assessed by Topor et al. (2004) using a comprehensive computational model. They deduced that raised FICO2 stabilizes control by increasing mean ventilation.
The independent role of the apnoeic threshold has less often been studied in mathematical models. Vielle (2000) used a model that could alter both the chemoreflex gain and CO2 levels, and again found that elevated values of either parameter led to decreased stability. He used Capn threshold as an index for CO2 levels, but as  was not described separately, Capn acted as a proxy for
was not described separately, Capn acted as a proxy for  . Any change in the apnoeic threshold would therefore be automatically associated with a similar increment in potential steady state CO2. Therefore his finding that a high apnoeic threshold leads to system instability needs to be qualified as it is related to a joint increase in potential steady state CO2 and chemoreflex gain. His study does not identify which of the two is the true culprit.
. Any change in the apnoeic threshold would therefore be automatically associated with a similar increment in potential steady state CO2. Therefore his finding that a high apnoeic threshold leads to system instability needs to be qualified as it is related to a joint increase in potential steady state CO2 and chemoreflex gain. His study does not identify which of the two is the true culprit.
Recently, in a review of the mechanisms of periodic breathing, Cherniack & Longobardo (2006) have warned of the importance of studying the behaviour of the respiratory control system near the potential steady state, rather than just focusing on the apnoea threshold.
Taken together, the results of these previous studies are all consistent with the conclusions that we have found in this modelling study. Increased chemoreflex gain and increased mean FETCO2 are each able to decrease system stability. Ours is the first model to deliberately assess the independent influence of chemoreflex gain, potential steady state CO2 and the apnoeic threshold on stability. We have aimed to provide a model that is simple to understand, so that the clinical implications can readily be appreciated.
Limitations of the study
For simplicity, we used only a single variable to represent blood gas variation, in keeping with several previous models (Vielle, 2000). During periodic breathing at rest, CO2 and O2 oscillations are in almost perfect antiphase (Faber et al. 1990) and therefore these oscillations can be satisfactorily treated as a single variable, with tacit recognition that the chemoreflex responds both to a rise in CO2 and correspondingly to a fall in O2. Moreover, near the potential steady state, chemoreflex responses to hypoxia are significantly smaller than hypercapnic responses for the same change in partial pressure (Carley & Shannon, 1988a; Maayan et al. 1992), and therefore the hypoxic contribution should be small.
For simplicity again, we use FETCO2 levels as a proxy for all CO2 levels in the body, making the assumption that changes in one are tracked in parallel by changes in the others (in particular, arterial). This simplification is equivalent to assuming no significant physiological dead space. However, even if there is significant physiological dead space, the principal consequence is that the chemoreflex gains expressed in terms of changes in FETCO2 would be numerically smaller than their conventional expression in terms of arterial CO2 changes. It would have no impact on the relative contributions of the three parameters to system stability, and therefore no effect on the conclusions of this study. The venous CO2 is taken to not fluctuate significantly during periodic breathing. This substantially simplifies the mathematics and is supported by physiological measurements (Vielle & Chauvet, 1998).
Our third simplification is to not separate peripheral and central components of the chemoreflex. Although there are multiple clusters of individual chemosensitive cells located in the aorta, the carotids and the brainstem, we describe the ‘net’ response, with a single effective delay and gain that represents a summation of the responses of all these individual receptors. Treating each component separately substantially increases the complexity and obscurity of the model, but cannot change the conclusions.
We have intentionally used the simplest possible units for each physical quantity in this study. This eliminates the need for arbitrary constants to convert between units. The simplest measure of end-tidal CO2 level is its fraction, which is dimensionless. As a result, our unit of chemoreflex gain is ‘l min−1 (fraction of end-tidal CO2)−1’, synonymous with ‘l min−1 atm−1’, which is unconventional but fulfils the aim of being the simplest possible unit.
Finally, the purpose of this model is not to examine all cardiac and respiratory influences on system stability, but to extricate the independent effects of chemoreflex sensitivity, apnoea threshold and steady state CO2, which cannot be separated clinically. This paper therefore adds to previously published clinical studies and mathematical models which aim to comprehensibly identify all influences on system stability – cardiac (including cardiac output and circulation time), respiratory and central (Khoo et al. 1982, 1991; Francis et al. 2000b; Vielle, 2000; Topor et al. 2004; Cherniack & Longobardo, 2006).
Conclusions
We have found that in contrast to beliefs arising from clinical observation, cardiorespiratory stability in heart failure is not independently affected by the apnoeic threshold. Moreover, in a second apparent conflict with clinical observation, we have found that it is a high (rather than low) potential steady state level of CO2 that favours instability. In this paper we have explained both of these apparent paradoxes. They arise from the powerful effect of chemoreflex gain, which is infrequently addressed – perhaps because it is not automatically available from routine clinical data.
Acknowledgments
C.H.M. was supported by a Research Training Fellowship from the Wellcome Trust (077049/Z/05/Z) and the Coronary Flow trust. D.P.F. (FS/04/079), Z.I.W. (FS/05/068) and J.E.D. (FS/05/006) were supported by the British Heart Foundation and K.W. received support from the Foundation for Circulatory Health.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.116764
http://jp.physoc.org/cgi/content/full/jphysiol.2006.116764/DC1 and contains supplemental material.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Badr MS, Grossman JE, Weber SA. Treatment of refractory sleep apnea with supplemental carbon dioxide. Am J Respir Crit Care Med. 1994;150:561–564. doi: 10.1164/ajrccm.150.2.8049848. [DOI] [PubMed] [Google Scholar]
- Beecroft J, Duffin J, Pierratos A, Chan CT, Mcfarlane P, Hanly PJ. Enhanced chemo-responsiveness in patients with sleep apnoea and end-stage renal disease. Eur Respir J. 2006;28:151–158. doi: 10.1183/09031936.06.00075405. [DOI] [PubMed] [Google Scholar]
- Ben-Dov I, Sietsema KE, Casaburi R, Wasserman K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am Rev Respir Dis. 1992;145:776–781. doi: 10.1164/ajrccm/145.4_Pt_1.776. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: Central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- Carley DW, Shannon DC. Relative stability of human respiration during progressive hypoxia. J Appl Physiol. 1988a;65:1389–1399. doi: 10.1152/jappl.1988.65.3.1389. [DOI] [PubMed] [Google Scholar]
- Carley DW, Shannon DC. A minimal mathematical model of human periodic breathing. J Appl Physiol. 1988b;65:1400–1409. doi: 10.1152/jappl.1988.65.3.1400. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Longobardo GS, Levine OR, Mellins R, Fishman AP. Periodic breathing in dogs. J Appl Physiol. 1966;21:1847–1854. doi: 10.1152/jappl.1966.21.6.1847. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, von Euler EC, Homma I, Kao FF. Experimentally induced Cheyne-Stokes breathing. Respir Physiol. 1979;37:185–200. doi: 10.1016/0034-5687(79)90070-7. [DOI] [PubMed] [Google Scholar]
- Corra U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: Prognostic importance and interdependence. Circulation. 2006;113:44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- Davies LC, Francis DP, Crisafulli A, Concu A, Coats AJ, Piepoli M. Oscillations in stroke volume and cardiac output arising from oscillatory ventilation in humans. Exp Physiol. 2000;85:857–862. [PubMed] [Google Scholar]
- Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Smith CA, Przybylowski T, Chenuel B, Xie A, Nakayama H, Skatrud JB. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C, Haldane J. The causes of periodic or Cheyne-Stokes breathing. J Physiol. 1909;38:401–419. doi: 10.1113/jphysiol.1909.sp001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber J, Lorimier P, Sergysels R. Cyclic haemodynamic and arterial blood gas changes during Cheyne-Stokes breathing. Intensive Care Med. 1990;16:208–209. doi: 10.1007/BF01724804. [DOI] [PubMed] [Google Scholar]
- Francis DP, Davies LC, Piepoli M, Rauchhaus M, Ponikowski P, Coats AJS. Origin of oscillatory kinetics of respiratory gas exchange in chronic heart failure. Circulation. 1999b;100:1065–1070. doi: 10.1161/01.cir.100.10.1065. [DOI] [PubMed] [Google Scholar]
-
Francis DP, Davies LC, Willson K, Piepoli M, Seydnejad SR, Ponikowski P, Coats AJ. Impact of periodic breathing on
 and
and 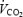 : a quantitative approach by Fourier analysis. Respir Physiol. 1999a;118:247–255. doi: 10.1016/s0034-5687(99)00074-2. [DOI] [PubMed] [Google Scholar]
: a quantitative approach by Fourier analysis. Respir Physiol. 1999a;118:247–255. doi: 10.1016/s0034-5687(99)00074-2. [DOI] [PubMed] [Google Scholar] - Francis DP, Davies LC, Willson K, Ponikowski P, Coats AJ, Piepoli M. Very-low-frequency oscillations in heart rate and blood pressure in periodic breathing: role of the cardiovascular limb of the hypoxic chemoreflex. Clin Sci (Lond) 2000a;99:125–132. [PubMed] [Google Scholar]
- Francis DP, Willson K, Davies LC, Coats AJS, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000b;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A, Videbaek R, Schou M, Damgaard M, Kastrup J, Norsk P. Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci (Lond) 2002;102:247–252. [PubMed] [Google Scholar]
- Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. The effect of oxygen on respiration and sleep in patients with congestive heart failure. Ann Intern Med. 1989a;111:777–782. doi: 10.7326/0003-4819-111-10-777. [DOI] [PubMed] [Google Scholar]
- Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. Respiration and abnormal sleep in patients with congestive heart failure. Chest. 1989b;96:480–488. doi: 10.1378/chest.96.3.480. [DOI] [PubMed] [Google Scholar]
- Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- Javaheri S, Corbett WS. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med. 1998;128:204–207. doi: 10.7326/0003-4819-128-3-199802010-00006. [DOI] [PubMed] [Google Scholar]
- Jensen D, Wolfe LA, O'Donnell DE, Davies GA. Chemoreflex control of breathing during wakefulness in healthy men and women. J Appl Physiol. 2005;98:822–828. doi: 10.1152/japplphysiol.01208.2003. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Gottschalk A, Pack AI. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol. 1991;70:2014–2024. doi: 10.1152/jappl.1991.70.5.2014. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Yang F, Shin JJ, Westbrook PR. Estimation of dynamic chemoresponsiveness in wakefulness and non-rapid-eye-movement sleep. J Appl Physiol. 1995;78:1052–1064. doi: 10.1152/jappl.1995.78.3.1052. [DOI] [PubMed] [Google Scholar]
- Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal cheyne-stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- Lorenzi-Filho G, Rankin F, Bies I, Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 1999;159:1490–1498. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- Maayan C, Carley DW, Axelrod FB, Grimes J, Shannon DC. Respiratory system stability and abnormal carbon dioxide homeostasis. J Appl Physiol. 1992;72:1186–1193. doi: 10.1152/jappl.1992.72.3.1186. [DOI] [PubMed] [Google Scholar]
- Mackey MC, Glass L. Oscillation and chaos in physiological control systems. Science. 1977;197:287–289. doi: 10.1126/science.267326. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Inagaki M, Takaki H, Kawada T, Yanagiya Y, Sugimachi M, Sunagawa K. Integrated characterization of the human chemoreflex system controlling ventilation, using an equilibrium diagram. Eur J Appl Physiol. 2004;93:340–346. doi: 10.1007/s00421-004-1219-x. [DOI] [PubMed] [Google Scholar]
- Modarreszadeh M, Bruce EN, Hamilton H, Hudgel DW. Ventilatory stability to CO2 disturbances in wakefulness and quiet sleep. J Appl Physiol. 1995;79:1071–1081. doi: 10.1152/jappl.1995.79.4.1071. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Amara CE, Cunningham DA, Duffin J. Measuring central-chemoreflex sensitivity in man: rebreathing and steady-state methods compared. Respir Physiol. 1999;115:23–33. doi: 10.1016/s0034-5687(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Mohan R, Duffin J. The effect of hypoxia on the ventilatory response to carbon dioxide in man. Respiration Physiol. 1997;108:101–115. doi: 10.1016/s0034-5687(97)00024-8. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165:1251–1260. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, Coats AJS, Piepoli M. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- Silva R, Alves R, Mady C, Lorenzi-Filho G. Breathing disorders in patients with congestive heart failure. Sleep. 2001;24:A291. [Google Scholar]
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61–66. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol. 1983;55:813–822. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- Steens RD, Millar TW, Su X, Biberdorf D, Buckle P, Ahmed M, Kryger MH. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep. 1994;17:61–68. doi: 10.1093/sleep/17.1.61. [DOI] [PubMed] [Google Scholar]
- Topor ZL, Pawlicki M, Remmers JE. A computational model of the human respiratory control system: responses to hypoxia and hypercapnia. Ann Biomed Eng. 2004;32:1530–1545. doi: 10.1114/b:abme.0000049037.65204.4c. [DOI] [PubMed] [Google Scholar]
- Turner MS, Bleasdale RA, Mumford CE, Frenneaux MP, Morris-Thurgood JA. Left ventricular pacing improves haemodynamic variables in patients with heart failure with a normal QRS duration. Heart. 2004;90:502–505. doi: 10.1136/hrt.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med. 2002;165:1041–1047. doi: 10.1164/ajrccm.165.8.2104100. [DOI] [PubMed] [Google Scholar]
- Vielle B. A new explicit stability criterion for human periodic breathing. J Math Biol. 2000;41:546–558. doi: 10.1007/s002850000061. [DOI] [PubMed] [Google Scholar]
- Vielle B, Chauvet G. Delay equation analysis of human respiratory stability. Math Biosci. 1998;152:105–122. doi: 10.1016/s0025-5564(98)10028-7. [DOI] [PubMed] [Google Scholar]
- Xie A, Wong B, Phillipson EA, Slutsky AS, Bradley TD. Interaction of hyperventilation and arousal in the pathogenesis of idiopathic central sleep apnea. Am J Respir Crit Care Med. 1994;150:489–495. doi: 10.1164/ajrccm.150.2.8049835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.