Abstract
The lobula giant movement detector (LGMD) is an identified neuron in the locust visual system that responds preferentially to objects approaching on a collision course with the animal. For such looming stimuli, the LGMD firing rate gradually increases, peaks and decays towards the end of approach. The LGMD receives both excitatory and feed forward inhibitory inputs on distinct branches of its dendritic tree, but little is known about the contribution of feed forward inhibition to its response properties. We used picrotoxin, a GABA-ergic antagonist, to selectively block feed forward inhibition to the LGMD. We then computed differences in firing rate and membrane potential between control and picrotoxin conditions to study the activation of feed forward inhibition. For looming stimuli, a significant activation of inhibition was observed early, as objects exceeded on average ~23º in angular extent at the retina. Inhibition then increased in parallel with excitation over the remainder of approach trials. Experiments in which the final angular size of the approaching objects was systematically varied revealed that the relative activation of excitation and inhibition remains well balanced over most of the course of looming trials. Feed forward inhibition actively contributed to the termination of the response to approaching objects and was particularly effective for large or slowly moving objects. Suddenly appearing and receding objects activated excitation and feed forward inhibition nearly simultaneously, in contrast to looming stimuli. Under these conditions, the activation of excitation and feed forward inhibition was weaker than for approaching objects, suggesting that both are preferentially tuned to approaching objects. These results support a phenomenological model of multiplication within the LGMD and provide new constraints for biophysical models of its responses to looming and receding stimuli.
Keywords: looming, locust, LGMD, DCMD, collision avoidance, feed forward inhibition, synaptic integration
INTRODUCTION
Feed forward inhibition is ubiquitous in neuronal microcircuits (Sheperd, 2003). Its role in synaptic integration is likely to vary across different cell types. In the hippocampus and cerebellar cortex, in vitro experiments suggest that feed forward inhibition enhances coincidence detection in CA1 pyramidal cells and Purkinje cells (Pouille and Scanziani, 2001; Mittman et al., 2004). In cat visual cortex, whole-cell patch recordings in vivo have revealed an important role of inhibition in shaping the receptive field properties of simple and complex cells (Anderson et al., 2000; Borg-Graham et al., 1998; Hirsch et al., 1998). For such directionally selective cells, inhibition is activated by motion in the preferred direction in parallel with excitation and can sometimes result in a two- to threefold increase in membrane conductance (Monier et al., 2003). This in turn will lead to complex changes in the biophysical integration of synaptic inputs (Kuhn et al., 2004; Holt and Koch, 1997; Chance et al., 2002).
Little is known about the role of feed forward inhibition in shaping the receptive field properties of neurons sensitive to approaching objects (looming stimuli). Such neurons have been described in the visual system of several species, including monkeys, pigeons and insects (Fogassi et al., 1996; Cooke and Graziano, 2004; Sun and Frost, 1998; Wicklein and Strausfeld, 2000; Gabbiani et al., 2004). In the locust, one such identified, looming sensitive neuron is the lobula giant movement detector (LGMD; O’Shea and Williams, 1974). Its postsynaptic target, the descending contralateral movement detector (DCMD) neuron (Schlotterer, 1977; Rind and Simmons, 1992; Gabbiani et al., 2004) spikes in 1–1 correspondence with the LGMD and provides a convenient way of monitoring the LGMD output activity (Fig. 1A). The LGMD receives excitatory synaptic inputs sensitive to local motion onto a large dendritic fan (labelled A in Fig. 1A; O’Shea and Williams, 1974; Rowell, 1971; Krapp and Gabbiani, 2005). This excitatory input is affected by several inhibitory processes (Rowell and O’Shea, 1976; Rowell et al., 1977), and in particular by a phasic lateral inhibitory network effectively activated by translating and looming stimuli (Fig. 1A; O’Shea and Rowell, 1975; Gabbiani et al., 2002). In addition, the LGMD receives direct feed forward inhibition onto two separate dendritic subfields (labelled B and C in Fig. 1A; O’Shea and Williams, 1974; Rowell et al., 1977). This inhibition is most sensitive to large, transient changes in luminance (O'Shea and Rowell, 1975), with one subfield receiving inhibition in response to increases and the other subfield to decreases in luminance (separate ON and OFF channels onto subfields B and C, respectively; Rowell et al., 1977). Except for opposite contrast sensitivities, both types of feed forward inhibition exhibit similar response properties upon visual stimulation, including temporal activation kinetics and amplitudes at high contrasts (Rowell et al, 1977). The anatomical basis for the OFF-type inhibition is best understood: it is mediated by a bundle of approximately 500 axons originating from neurons whose cell bodies are located at the level of the second optic chiasm in the optic lobe (Elphick et al., 1996). Their receptive fields are thought to span approximately 8 × 12º (Rowell et al., 1977; Strausfeld and Naessel, 1981).
Figure 1.

Schematics of neuronal circuitry and stimulation configuration. A. The LGMD receives excitatory synaptic inputs (circles shaded in dark grey) on a large dendritic field (A). An important characteristic of this pathway is the presence of a lateral inhibitory network between excitatory afferent fibers presynaptic to the LGMD (inhibitory synapses are illustrated by circles shaded in light grey). ON and OFF feed forward inhibition impinges on two separate dendritic fields (B and C, respectively). B. Looming stimuli consisted of black squares on a bright background approaching at a constant speed v with half-size l. The angle subtended by the object at the retina is denoted by θ.
Feed forward inhibition is also activated by small field visual stimuli (Rowell et al., 1977), but its temporal pattern of activation in response to approaching objects is not well characterized. One biophysical model of the LGMD responses to looming stimuli assumed that activation occurs only immediately prior to collision, as the object covers most of one visual hemisphere, i.e., ~180º in angular extent (denoted by θ in Fig. 1B; Rind and Bramwell, 1996). A second, phenomenological, model suggests that activation of feed forward inhibition occurs considerably earlier and contributes to terminating the responses to looming stimuli (Hatsopoulos et al., 1995; Gabbiani et al., 1999, 2002). To characterize experimentally the time-course of activation of feed forward inhibition during object approach, we took advantage of the fact that the synapses made by afferent fibers onto the excitatory dendritic fan of the LGMD, as well as the lateral, presumed inhibitory, connections between these afferents in the lobula, are cholinergic (Rind and Simmons, 1998). Thus, local ejection onto the dorsal lobula of picrotoxin, a blocker of fast GABAA-meditated synaptic transmission (Rauh et al., 1990; Warzecha et al., 1993), will affect only feed forward inhibitory inputs to subfields B and C of the LGMD. Comparing the responses of the LGMD before and after picrotoxin ejection to approaching objects as well as to other types of stimuli allowed us to characterize the temporal activation dynamics of feed forward inhibition relative to that of excitation. Some of our results have been briefly reported earlier (Gabbiani et al., 2002).
MATERIALS AND METHODS
The preparation, electrophysiology, picrotoxin application and visual stimuli were similar to those described in earlier publications (Gabbiani et al., 1999, 2001; Gabbiani et al., 2002 and Supplementary material online).
Preparation
Experiments were done on adult locusts (mostly female) taken from the laboratory colony 3–4 weeks after their final moult. Animals were mounted dorsal side up on a custom-designed polycarbonate holder that allowed us to reproducibly align the stimulated eye with the stimulation apparatus. For extracellular DCMD recordings, an opening was made in the pronotum, the gut was removed and the connectives were exposed. A hook electrode consisting of two stainless steel wires 50 μm in diameter isolated except on the recording surface was positioned under the connective contralateral to the stimulated eye using a micromanipulator. The two wires were further isolated electrically with vacuum grease and the preparation was kept moist using locust saline. For intracellular LGMD recordings and picrotoxin application experiments, the entire head (except for the stimulated eye) was bathed in locust saline. The head capsule was opened dorso-frontally between the two eyes. Fatty tissue overlying the brain was removed and the muscles in the head capsule were sectioned. The exposed brain was de-sheathed with fine forceps. Protease (type XIV, Sigma-Aldrich, St. Louis, MO) was sometimes used to soften the brain’s protective sheath. A holder supported the brain during intracellular recordings to minimize movement.
Electrophysiology and Data Acquisition
Extracellular recordings from hook electrodes were amplified differentially 10,000 times using standard equipment (A-M Systems 1700, Carlsborg, WA; Brownlee 440, San Jose, CA). Intracellular recordings were obtained with sharp microlectrodes fabricated using a horizontal puller (P-97, Sutter Instruments, Novato, CA) from thick or thin wall borosilicate glass (1.2/0.68 or 1.2/0.9 mm outer/inner diameter, WPI, Sarasota, FL). They were filled with a 2 M potassium acetate aqueous solution (DC resistance: 30–70 MΩ). The tip of the electrodes was usually dipped in black ink to facilitate visualization. After penetration, the LGMD was identified through its spikes that are in 1-1 correspondence with those of the DCMD in the connective (Fig. 2A). Intracellular recordings typically lasted between 40–60 mins. Small hyperpolarizing currents (−0.5 to –1 nA) were used as needed to stabilize the recordings and did not alter the experimental results. The membrane potential with respect to the ground electrode placed in the bath solution was amplified ten times with an Axoclamp 2B (Axon Instruments, Union City, CA). Extracellular multielectrode recordings from the medulla were obtained using silicon probes fabricated by the Center for Neural Communication Technology of the University of Michigan (‘Michigan probes’). Each probe consisted of 16 recording pads arranged in tetrode configuration as illustrated in Fig. 2A of Pouzat et al. (2002). The recordings were amplified with a custom amplifier. All data were acquired at 12–14 bit resolution using A/D cards (Win30 or Powerdac series, United Electronics Industries, Canton, MA) controlled from a personal computer running the QNX operating system (QSSL, Ottawa, Canada). Intracellular recordings were sampled at 20kHz, extracellular hook electrode recordings at 10 kHz and extracellular Michigan probe recordings at 20 kHz.
Figure 2.

Effect of picrotoxin on the responses to visual stimuli of the LGMD and of neuronal populations recorded in the medulla. A. Five sample traces of intracellular recordings from the LGMD in response to a looming stimulus (l/|v| = 30 ms, illustrated in the top panels) are shown at the bottom. The recordings on the left and right were obtained before and after focal pressure ejection of picrotoxin on the dorsal lobula, respectively. The two extracellular traces above the top two intracellular recordings were acquired simultaneously from the contralateral connective. The large extracellular spikes, in one-to-one correspondence with those of the LGMD, are from the DCMD. The light grey shaded area marks the time of abrupt decrease of the LGMD membrane potential and is reproduced in B. B. Intracellular recording from the LGMD in response to a fast flow stimulus activated at the time marked by the arrowhead (duration 50 ms). Top: membrane potential responses when the screen remained at its background luminance level. Middle: In the control condition, the stimulus elicits brief EPSPs followed by long lasting IPSPs. Bottom: After focal ejection of picrotoxin on the dorsal lobula, strong excitation is unmasked (spikes are truncated). Each stimulus was presented 10 times; only 5 trials are depicted for clarity. C. Comparison of activity increase in the LGMD (abscissa) and in neuronal populations of the medulla (ordinate) induced by focal pressure ejection of picrotoxin on the dorsal lobula. Activity increase was assessed by averaging the response to 30 consecutive looming stimuli (l/|v|=25ms), spaced by 40–60s, in control condition and after picrotoxin ejection. Experiments were repeated on five locusts, each represented as a distinct symbol shape. Activity levels were estimated either as maximum firing frequency over the looming stimulus (open symbols) or as the total number of spikes (filled symbols), and normalized to control levels. In some experiments, activity was measured after successive ejections of picrotoxin. Activity in control conditions: LGMD, 18 (SD: 10) spikes/trial, 57 (SD: 25) spikes/s at peak, medulla population, 266 (SD: 127) spikes/trial, 152 (SD: 85) spikes/s at peak. Activity after picrotoxin ejection: LGMD, 32 (SD: 7) spikes/trial, 116 (SD: 37) spikes/s at peak, medulla population, 301 (SD: 188) spikes/trial, 193 (SD: 106) spikes/s at peak.
Picrotoxin application
Pipettes used for picrotoxin ejection were pulled and fire-polished to a final diameter of ~1 μm using a microforge (Narishige, Tokyo, Japan). They were backfilled with picrotoxin (5 mM; Sigma, St. Louis, MO) in aqueous solution containing 0.5% (w/v) of Fast green (Sigma) to allow visualization of the ejected liquid and to confirm visually the area affected by the ejection. The solution was heated to 35ºC and stirred prior to each experiment. The pipettes were connected to a pneumatic picopump (WPI) and the ejection pressure was calibrated prior to each experiment by measuring ejected volumes in mineral oil. The ejected quantities ranged from 2 to 20 nl. During an experiment the pipette was rapidly lowered with a micromanipulator towards the targeted site and retracted immediately after the ejection to minimize diffusion in the bath. During extracellular recordings the ejection pipette was placed in physical contact with the dorsal lobula whereas during intracellular recordings it was placed as close as possible to the dorsal lobula (~20 μm). This procedure allowed direct application of the drug to the neuropil since the optic lobe had been previously desheated (see Preparation above).
Visual stimulation
A video monitor controlled by a personal computer running the QNX operating system was used to generate the visual stimuli using a commercially available graphics library (MGL, Scitechsoft, Chico, CA). The monitor was refreshed at 200 frames/s and a synchronization pulse was issued for each frame refresh on the parallel port of the computer and acquired simultaneously with the electrophysiological data. During an experiment, the animal’s longitudinal axis was placed parallel to the screen at a distance of 15.8 cm. The centre of one eye was aligned with the centre of the screen. Under these conditions, the angle subtended by a screen pixel (0.22º) was well below the acceptance angle of a single ommatidium (~1.5º; Wilson, 1975). The approach of black squares (half-size l, constant speed v; ~0 cd/m2; Fig. 1B) on a white background (~90 cd/m2) was simulated by perspective projection as described in Gabbiani et al. (1999). The initial size of the objects on the screen was 1 pixel and the centre of expansion of these looming stimuli coincided with the centre of the screen. The time course of the angular size, θ (t), subtended by the object at the retina during such an approach is illustrated in Fig. 2A, for example (top panels). In this figure and subsequent ones, the discrete jumps in angular size visible towards the end of approach correspond to individual video refreshes of the monitor. By convention, the origin of the time axis corresponds to the projected collision time (t = 0, θ = 180º) and time before collision is negative as is the speed, v, for an approaching object. The time course of approach is uniquely characterized by the ratio l/|v| (in units of ms; Gabbiani et al., 1999). The values used (l/|v| = 10, 30 and 50 ms) correspond to approaches lasting 1.1, 3.4 and 5.7 s, respectively. The relation between the half-size of the object, l, its speed of approach, v, and the value of l/|v| is given in Table 1 of Gabbiani et al. (1999). The responses of the LGMD/DCMD to receding objects were studied by inverting these approach sequences. In these experiments, the black square appeared abruptly on the screen and stayed stationary for 2.5 s before the recession sequence started. The onset of recession was defined as time zero in such experiments (Fig. 6A and B, top panels). In other experiments, we used a fast optic flow stimulus, that has been described in Gabbiani et al. (2002 and Supplementary material), consisting of alternating white and gray bars (30% contrast) rapidly moving at a temporal frequency of 50 Hz. This stimulus is well suited to activate feed forward inhibition onto the LGMD. This rapid motion stimulus lasted 50 ms and was preceded and followed by 1s during which the screen was set to a white background.
Figure 6.
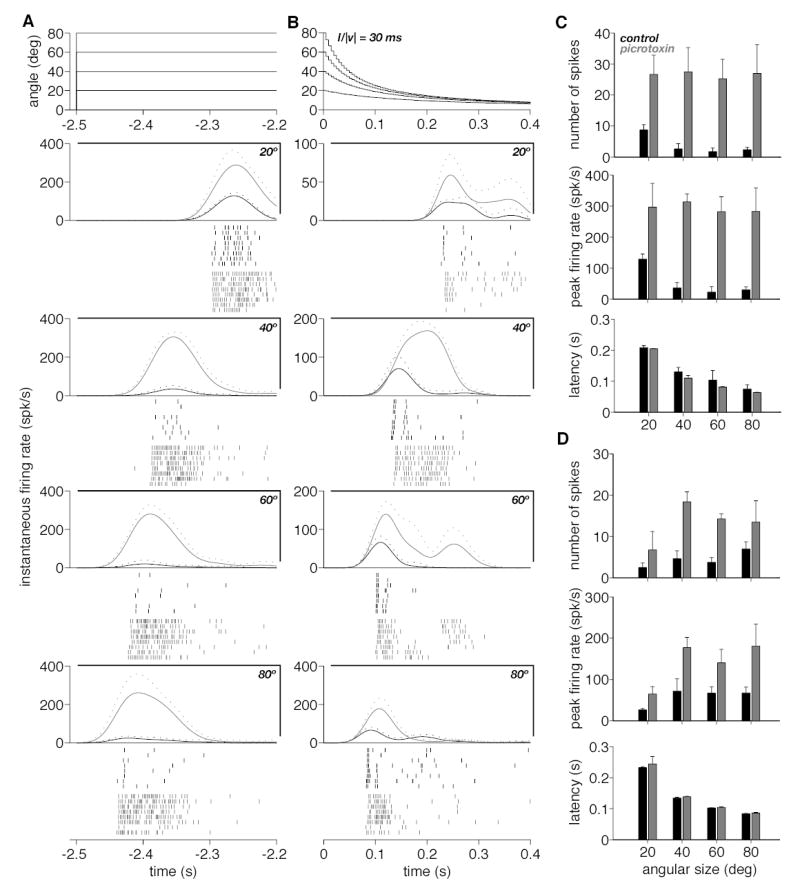
Responses of the DCMD to the appearance and recession of a black square before and after picrotoxin ejection. A. The angle subtended at the retina by the appearing stimuli is plotted as a function of time in the top panel. The instantaneous firing rate (mean and mean + SD, solid and dotted lines) before and after picrotoxin ejection (black and gray) is plotted separately for each angular size (20, 40, 60 and 80º) in the next four panels. The rasters of spikes below each panel correspond to the eight trials before and after picrotoxin ejection (black and gray). B. The angle subtended at the retina by the receding squares is plotted as a function of time on top (l/|v| = 30 ms). Instantaneous firing rate and spike rasters are plotted below using the same conventions as in A. C. The three panels from top to bottom give summary statistics for the number of spikes, the peak firing rate and the latency to the first spike following stimulus appearance, respectively (obtained from A). In each case, the mean over eight trials is given as a function of the angular size of the appearing stimulus. Black and gray bars correspond to trials before and after picrotoxin ejection, respectively. Error bars denote SD. D. Summary statistics for receding squares (obtained from B). Same plotting conventions as in C.
Experimental Protocols
To facilitate reference in the Results, each protocol is numbered in the following description.
P1. In a first series of experiments, we studied the time-course of the firing rate and the intracellular membrane potential of the LGMD during looming. Two looming stimuli were used with l/|v| values of 10 and 30 ms and a final approach angle of 80º. Each stimulus was presented pseudo-randomly five times before and after picrotoxin application with an inter-trial interval of 20 s. The inter-trial interval during intracellular recordings was shorter than during extracellular recordings (see P3-P9 below) to compensate for shorter recording times and acquire sufficient data for statistical analyses. This led to slightly increased habituation of the responses in some animals. During these experiments two additional stimuli combining the looming and fast-flow stimuli were also presented five times before and after picrotoxin application and the resulting data have been reported earlier (Gabbiani et al. 2002, Fig. 3a). The experiment was performed on six animals and typically lasted between 40 and 60 min. An additional group of four animals were tested with the same visual stimulation protocol. In these experiments, just the firing rate of the LGMD was monitored extracellularly via DCMD hook electrode recordings.
Figure 3.
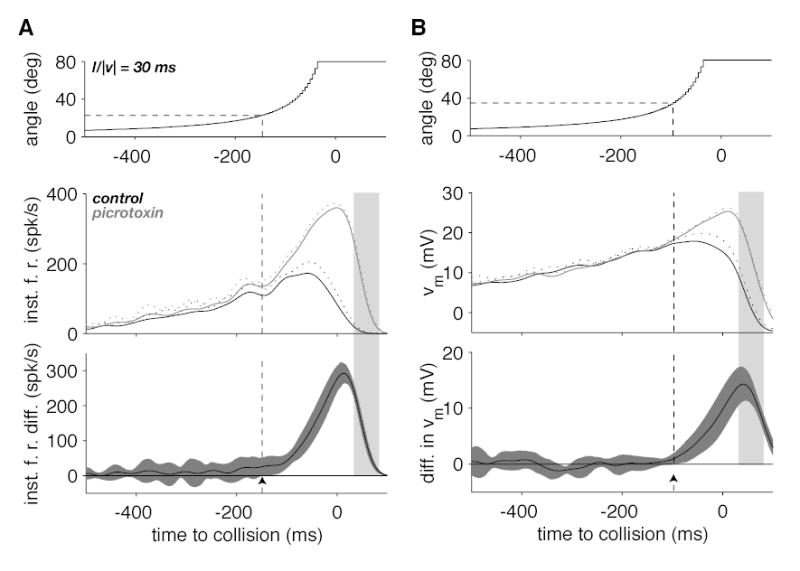
Effect of picrotoxin on the time-course of the LGMD instantaneous firing rate and membrane potential during a looming stimulus. A. The top panel illustrates the time course of the stimulus angular size as a function of time to collision. The middle panel shows the time course of the firing rate (mean and mean + SD, solid and dotted lines) in control (black) and picrotoxin conditions (gray). The bottom panel illustrates the firing rate difference between the picrotoxin and control conditions (black line) as well as its 95% confidence interval (gray). The arrowhead shows the location where the lower confidence interval boundary crosses the zero difference line corresponding to a statistically significant change in firing rate. The time when statistical significance is reached and the corresponding angular size are given by the dashed vertical and horizontal lines, respectively. B. Time-course of the stimulus (top), median, low-pass filtered membrane potential (middle) and the difference in median, low-pass filtered membrane potential (bottom panel). Same plotting conventions as in A. Data illustrated in A and B correspond to the data illustrated in Fig. 1A. The light grey shaded area delimits the same time interval as in Fig. 1A and B.
P2. Next, we studied the intracellular membrane potential of the LGMD to presentation of the fast flow stimulus before and after picrotoxin ejection. Once intracellular recordings from the LGMD were obtained and confirmed by paired hook electrode recordings of the DCMD, 10 presentations of the fast-flow stimulus were alternated pseudo-randomly with 10 presentations of a white background of equal duration (inter-trial interval: 20 s; white background between trials). Another set of 20 presentations was carried out after picrotoxin ejection. The protocol was applied on four animals (duration: ~ 20 mins).
P3. A series of experiments aimed at estimating the effect of a possible diffusion of picrotoxin from the lobula onto medulla neurons upstream of the LGMD. Medulla multi-unit responses were recorded with the 16-channels Michigan probe while the LGMD/DCMD firing was monitored with hook electrodes placed on the contralateral connective. In these experiments, 30 looming stimuli (l/|v|=25ms) were presented spaced by time intervals of 40–60s, in control condition and after pressure ejection of picrotoxin on the dorsal lobula. The activity was sometimes measured after successive ejections of picrotoxin. This protocol was carried out on five locusts (duration : ~ 1 h and 15 mins).
P4. In the next set of experiments, the time-course of the LGMD/DCMD firing rate was monitored extracellularly while presenting looming stimuli at three different l/|v| values (10, 30 and 50 ms). The approach sequence stopped at one out of four final angular sizes: 20, 40, 60 or 80º. Each of the 12 possible stimuli (3 l/|v| values × 4 final sizes) was presented eight times in pseudo-random order with an inter-trial interval of 40s. This protocol lasted approx. 1h and 15 min and was applied to 14 animals. To assess a possible effect of habituation on the responses (Rowell, 1974), the same protocol was applied to an additional set of six animals with a longer inter-trial interval (160s) and a decreased number of trials (two per stimulus) to keep the duration of the experiment approximately unchanged.
P5. A similar protocol was used to study the effect of picrotoxin on the LGMD/DCMD responses during looming. In these experiments, the approaches with the two smallest final angular sizes (20 and 40º) were presented first at three l/|v| values (10, 30 or 50 ms) selected pseudo-randomly, followed by the two largest final angular sizes (60 and 80º; same l/|v| values selected pseudo-randomly). Each of these 12 stimuli (3 l/|v| values × 4 final sizes) was presented three times with a 60s inter-trial interval both before and after picrotoxin application (expt. duration: ~1h and 30 min). The protocol was applied to 10 animals.
P6. To study quantitatively the effect of picrotoxin on looming responses obtained with different final approach angles, we performed experiments similar to those described under protocol 5, but at a fixed value of l/|v|. The four final angles (20, 40, 60 and 80º) were presented pseudo-randomly and the number of stimulus presentations was increased to 10 by applying inter-trial intervals of 60s. The same stimuli were presented after picrotoxin application (expt. duration: ~1h and 30 min). We tested 5 animals at l/|v| = 10 ms, 5 additional animals at l/|v| =30 ms and 5 others at l/|v| = 50 ms.
P7. To investigate the effect of picrotoxin on the LGMD/DCMD responses to receding stimuli we started by using four initial angles (20, 40, 60 and 80º) and three l/|v| values (10, 30 and 50 ms). Each of these 12 stimuli was presented three times pseudo-randomly before and after picrotoxin application at inter-trial intervals of 60 s. This experiment was performed on five animals and lasted ~1h 15 min.
P8. Next, we performed experiments at a single l/|v| value (30 ms) and four start angles (20, 40, 60 and 80º) to study quantitatively the effect of picrotoxin on the responses to receding objects. This allowed us to present each stimulus eight times pseudorandomly for a similar experiment duration as in protocol 7 (inter-trial interval: 60s). This protocol was used on five animals.
P9. Finally, we studied the effect of picrotoxin on the responses to receding objects at three l/|v| values (10, 30 and 50 ms) for a single initial angle of 40º. Each stimulus was presented pseudo-randomly 10 times (60s inter-trial interval; duration of experiment ~1h15 min). This protocol was applied on five animals.
Data Analysis
During data analysis, we routinely screened for possible habituation of the LGMD/DCMD responses. LGMD/DCMD spikes were extracted from the extra- or intracellular potential traces by thresholding (Gabbiani et al., 1999). Estimates of the instantaneous firing rate were then obtained by convolution of the resulting spike trains with a Gaussian kernel (σ= 20 ms). The time shifts introduced during convolution by the discrete Fourier transform were compensated for. The resulting spike density function was normalized to yield the total number of spikes after integration over the duration of the trial. For the experiments of protocol 3, extracellular spike time detection was performed as described in Cohen et al. (2000) using routines written in the Lab VIEW programming environment (National Instruments, Austin, TX; available at http://glab.bcm.tmc.edu/Spikoscope). Multi-unit activity was quantified by counting the total number of spikes during a trial pooled across the 16 recording sites of the Michigan probe. In addition, estimates of peak multi-unit instantaneous firing frequency were obtained after convolution of the pooled spike trains with a Gaussian kernel as described above (σ= 50 ms). Both the total number of spikes and peak firing rates were averaged over 30 consecutive looming trials.
The time-course of the subthreshold membrane potential during looming was obtained from the intracellular membrane potential of the LGMD (protocol 1) by median filtering (15 ms sliding window). To facilitate comparison with the time-course of the instantaneous firing rate, the resulting traces were subsequently convolved with a Gaussian filter (σ = 20 ms). Differences in the mean instantaneous firing rate or the time course of the subthreshold membrane potential (Fig. 3) were assessed by a t-test taking into account unequal variances (Smith-Satterthwaite method, Milton and Arnold, 1995).
During the presentation of a looming stimulus, the response of the LGMD/DCMD typically increases, peaks and decays to spontaneous levels at the end of stimulation (Fig. 4; Gabbiani et al., 1999, 2001, 2002; Matheson et al., 2004). When analyzing the data obtained with protocols 4–6, we quantified the time at which the responses of the LGMD/DCMD to looming stimuli stopped using two different methods. In the first method, we computed the time at which the instantaneous firing decayed to 20% of its mean peak value (Fig. 4, ▴). In the second method, we computed for each trial the time at which the last spike occurred, excluding those spikes occurring after the mean instantaneous firing rate had decayed to less than 15% of its peak value. This 15% criterion allowed to exclude outliers occurring well after the end of stimulation, such as those marked in Fig. 4A (♦, right panel in first row). The last spikes computed by this method are marked by a leftward pointing arrowhead (◂) for each spike raster in Figs. 4 and 5.
Figure 4.
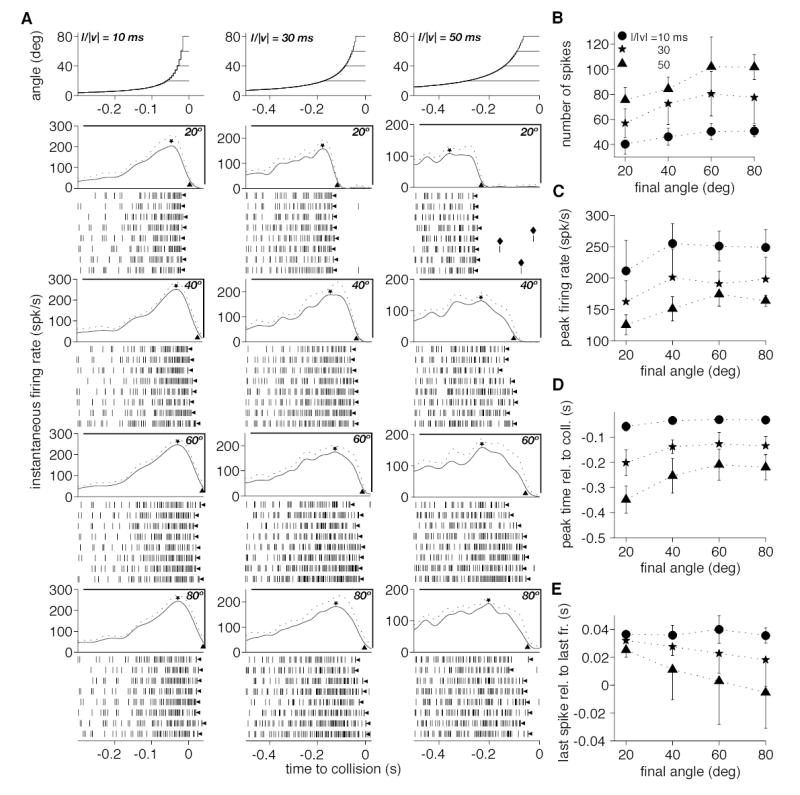
Responses of the DCMD to approaching squares at three l/|v| values and four final approach angles. A. The angle subtended at the retina by the approaching square is plotted in the top three panels as a function of time to collision. Each panel corresponds to a different l/|v| value (10, 30 and 50 ms) and depicts the time course of angular size for four final angles (20, 40, 60 and 80º). The four panels below the stimuli plot the time course of the instantaneous firing rate (mean and mean + SD, solid and dotted lines) as a function of time to collision for the four final angular sizes. In each panel, a ⋆ indicates the mean peak firing rate and a ▴ points to the firing rate corresponding to 20% of the peak. Rasters of spikes below each panel are responses for 8 individual trials. The leftward pointing arrowheads (◂) indicate the last spike of the trials (as defined in the Methods). The spikes marked by a ♦ are examples of outliers excluded by the 15% criterion (Methods). B. Number of spikes (mean, SD) as a function of final angular size of the approaching square. Each symbol denotes a different l/|v| value. C. Peak firing rate (mean, SD) as a function of final angular size. D. Time of peak firing (mean, SD) as a function of final angular size. E. Time of last spike (leftward pointing arrowheads in A; mean, SD) as a function of final angular size. The significance of symbols in C, D and E is the same as in B.
Figure 5.
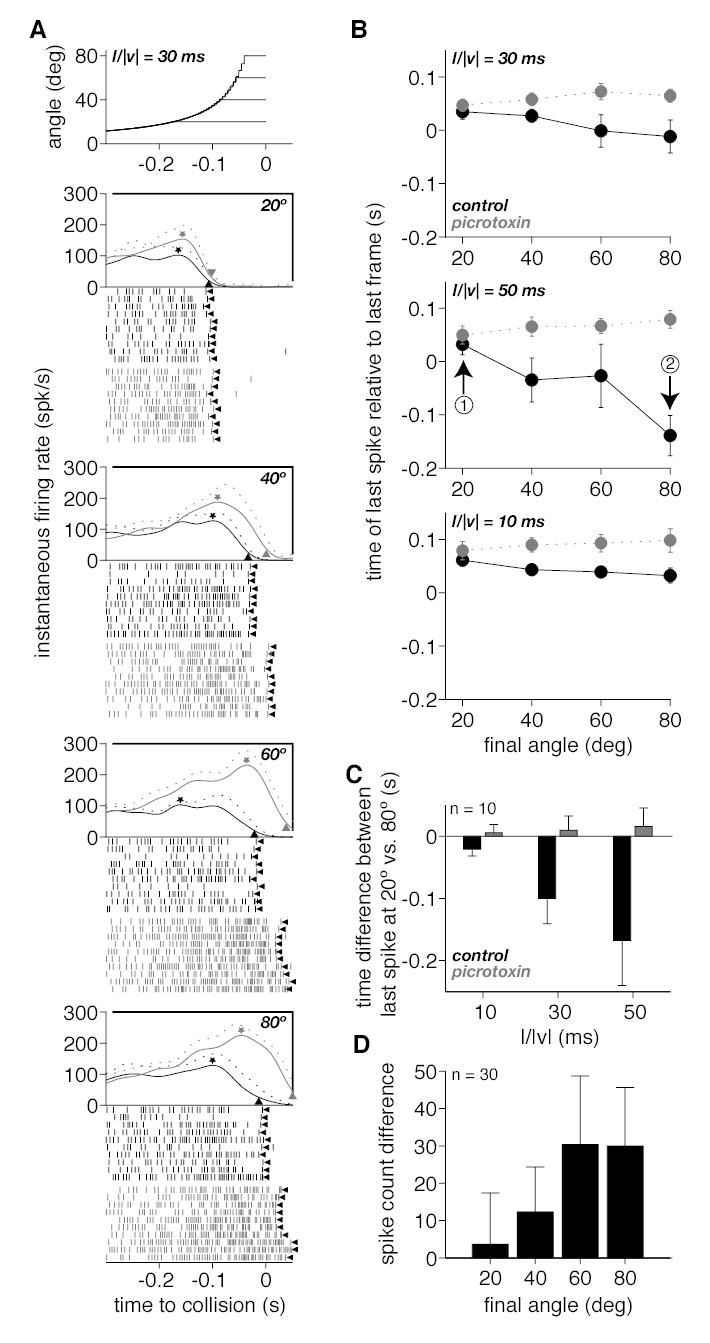
Effect of picrotoxin on the responses of the DCMD to approaching squares. A. The angle subtended at the retina by the approaching squares is plotted in the top panel as a function of time to collision. Each of the four curves corresponds to an approach ending with a different final angular size (20, 40, 60 and 80º) at a single l/|v| value (30 ms). The next four panels plot the time course of the instantaneous firing rate (mean and mean + SD, solid and dotted lines) as a function of time to collision for the four final angular sizes before and after picrotoxin ejection (black and gray traces, respectively). In each panel, a ⋆ indicates the mean peak firing rate and a ▴ points to the firing rate value corresponding to 20% of the peak. Rasters of spikes below each panel are responses for 10 individual trials before and after picrotoxin ejection (black and gray rasters, respectively). The leftward pointing arrowhead (◂) indicates the last spike of the trial (defined in Methods). B. The time of last spike relative to the last frame of stimulation is plotted as a function of the final angle subtended by the stimulus at the retina before and after picrotoxin ejection (black and gray symbols). Each of the three panels correspond to squares approaching with a different l/|v| value (10, 30 and 50 ms) obtained in three different experiments (top panel is derived from data in A). The arrows labelled ➁ and ➂ in the middle panel point to an example of the two values used to derive the plot in C. C. Plot of the mean time difference between the last spike relative to the last stimulus frame at a final approach angle of 20º and 80º (i.e., data point indicated by arrow ➂ minus that indicated by arrow ➁ in B) as a function of the approach parameter l/|v| (mean and SD across 10 expts.). Black and gray bars denote experiments before and after picrotoxin ejection, respectively. D. Mean difference in spike counts before and after picrotoxin ejection as a function of the final angle subtended at the retina by the approaching object (mean and SD across 10 expts. and 3 l/|v| values).
All data analysis was performed using Matlab (Mathworks, Natick, MA). The measures of variability illustrated in all the Figures are standard deviations (SD). In Figures 4–7, spike rasters are arranged in ascending chronological order: for each stimulus the first presentation corresponds to the bottom raster and the last one to the top raster. Because stimuli were chosen pseudo-randomly, the time interval between two successive presentations of the same stimulus is variable. The non-parametrical statistical tests used in the Results are described in Lehmann (1998). The analysis of covariance applied to protocols 4–6 is described in Mickey et al. (2004).
Figure 7.
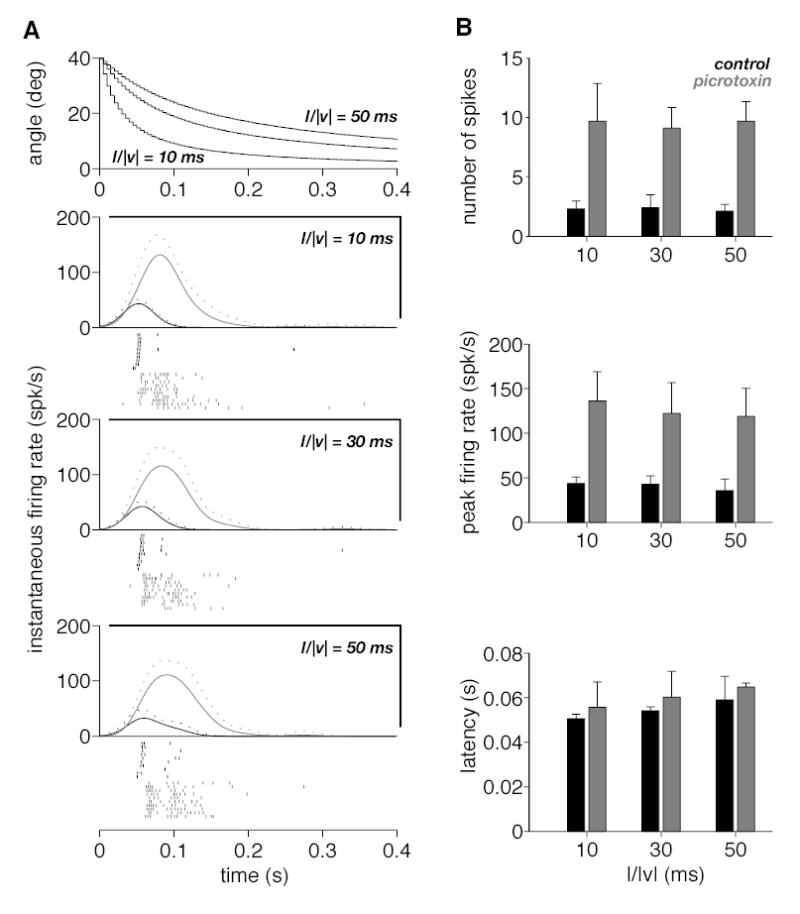
Responses of the DCMD to a black square receding from a fixed initial angular size (40º) at three half-size to speed ratios (l/|v| = 10, 30 and 50 ms). A. The angle subtended at the retina by the receding squares is plotted as a function of time on top. The next three panels give the instantaneous firing rate (mean and mean + SD, solid and dotted lines) before and after picrotoxin ejection (black and gray). Spike rasters below each panel correspond to the 10 trials before and after picrotoxin ejection (black and gray). B. Each panel from top to bottom gives summary statistics for the number of spikes, the peak firing rate and the latency to the first spike following the onset of recession, respectively (obtained from A). In each case mean values over 10 trials are plotted as a function of the kinematical parameter l/|v|. Black and gray bars correspond to trials before and after picrotoxin ejection, respectively. Error bars denote SD.
RESULTS
This report is based on recordings performed on 79 different animals. Data collected in 14 of those animals has been briefly reported earlier (Gabbiani et al., 2002).
Block of feed-forward inhibition onto the LGMD by picrotoxin
Both the LGMD and its postsynaptic target, the DCMD, respond vigorously to objects approaching on a collision course with the animal. This is illustrated in Fig. 2A, where the top panel depicts the angle subtended at the retina by an approaching black square. The half-size to speed ratio of the square was l/|v| = 30 ms (Fig. 1B, l = half-size, |v| = approach speed; Methods, P1). The bottom five intracellular traces are successive recordings from a single LGMD neuron in response to this looming stimulus. The top extracellular trace is a simultaneous recording of the DCMD activity in 1-1 correspondence with the top intracellular trace. In this example, the LGMD/DCMD response increased gradually during the approach sequence and stopped ~50 ms after the last frame of object motion. The LGMD receives both excitation and feed forward inhibition onto its dendritic tree. Thus, the termination of the response may be due to activation of feed forward inhibition, or termination of excitation, or a combination of both. An active role of feed forward inhibition may be inferred from the steep decrease in membrane potential (Fig. 2A, shaded area) following the last LGMD spikes, suggesting that IPSPs contribute to terminate the response. To address this question more directly, we ejected picrotoxin, a blocker of GABAA channels, locally onto the dorsal lobula where the LGMD receives its inhibitory inputs (Rowell et al, 1977; Strausfeld and Naessel, 1981). The effect of picrotoxin was noticeable almost immediately, i.e., within a few minutes of application. It lasted over the entire duration of an experiment, for typically as long as 45 mins (see Experimental protocols in Methods). The right panels of Fig. 2A illustrate the responses obtained in the same neuron after picrotoxin application. The number of spikes elicited over the course of looming was considerably increased (on average from 49 to 78, n=5 trials), as was the duration of the response, with the time of the last spike occurring on average 36 ms later in picrotoxin (last spike times in control and picrotoxin were on average 11 and 47 ms after collision, respectively). Furthermore, the decay of the membrane potential following picrotoxin application was less rapid than it was in controls (compare the membrane potential time-course in the shaded areas of Fig. 2A). These observations repeated in a total of six animals suggest a role of feed forward inhibition in terminating the response to object approach (Rind, 1996).
To verify that picrotoxin was acting postsynaptically on feed forward inhibition onto the LGMD, we performed a series of experiments using a visual stimulus consisting of alternating white and grey stripes rapidly translating across the visual field (fast optic flow stimulus; Methods, P2). Similar stimuli have been reported earlier to effectively activate feed-forward inhibition (O’Shea and Rowell, 1975; Rowell et al, 1977). The top two panels of Fig. 2B confirm this observation: when the fast optic flow stimulus was presented (middle panel, arrowhead), an EPSP was elicited, rapidly followed by a strong IPSP recorded after a latency of ~40 ms and lasting approximately 100 ms. In contrast, background illumination did not elicit any detectable responses (Fig. 2B, top). After picrotoxin ejection, the fast flow stimulus elicited strong excitation in the LGMD (Fig. 2B, bottom panel), consistent with a block of GABA-ergic inhibition and suggesting that the fast flow stimulus is also effective at activating excitation onto the LGMD. Under control conditions, however, this excitation appears to be tightly controlled by feed forward inhibition.
In addition to its effect on the LGMD, ejection of picrotoxin onto the dorsal lobula may also affect the activity of presynaptic neurons in the medulla or lamina. This appears unlikely as these two neuropils are located several hundreds of microns away from the lobula. Nonetheless, to directly address this possibility, we performed extracellular multielectrode recordings in the medulla while monitoring simultaneously the activity of the LGMD/DCMD during looming (Methods, P3). The multielectrode probes were placed approx. 200 microns distal to the lobula and sampled a region where retinotopic motion-sensitive units are routinely recorded (Cohen and Gabbiani, 2003). The mean number of spikes and the mean peak firing rate elicited by the looming stimulus were computed for both the recorded medulla neuron populations and the LGMD. Values obtained after picrotoxin ejection were expressed relative to control levels, prior to picrotoxin ejection. Figure 2C shows the mean peak firing rates and numbers of spikes (open and filled symbols, respectively) after picrotoxin application relative to control for medullary neuron populations as a function of those recorded in the LGMD. There was little increase in medullary population activity under picrotoxin (average of 3 and 19% above control for spike counts and peak firing rates, n = 5 animals) compared to that observed in the LGMD (average of 118 and 125% above control), suggesting that the effect of picrotoxin was localized and predominantly postsynaptic. The magnitude of LGMD mean and peak firing rate changes following picrotoxin application was variable across experiments. Despite this variability, the resulting changes in the characteristics of the responses described in the following were consistent across animals.
Time-dependent activation of feedforward inhibition during looming
The data illustrated in Fig. 2A show that the firing rate of the LGMD is considerably higher in picrotoxin than in control conditions over a large fraction of the looming approach. This suggests a substantial role of feed forward inhibition in controlling excitation before the termination of the response, in the time window that corresponds to the shaded area in Fig. 2A. Thus, in control conditions excitation appears to mask a concurrent activation of inhibition. To investigate quantitatively the time-course of activation of feed forward inhibition during looming, we computed from the data obtained from protocol 1 an estimate of the instantaneous firing rate of the LGMD/DCMD before and after picrotoxin ejection. Figure 3A (middle panel) illustrates these instantaneous firing rates for the same neuron as in Fig. 2. The difference between the two traces and its 95% confidence interval are illustrated in the bottom panel (black line and dark gray surrounding area, respectively). This difference between control and picrotoxin conditions is a measure of the activation of feed forward inhibition during looming independent of how excitation and feed forward inhibition interact within the LGMD and can be seen to gradually increase during object approach. Activation of inhibition reaches its peak after the peak in firing rate during control conditions (Fig. 3A middle, black line) and subsequently starts to decay in parallel to the instantaneous firing rate during the termination of the response (light gray shaded area) that follows the cessation of object motion. A significant activation of feed-forward inhibition was detected when the object exceeded an angular size of 22.5º (arrowhead and dashed lines in Fig. 3A, bottom panel). On average, this detection angle amounted to 23.3º (SD: 19º, n=6 animals) when pooled across the two l/|v| values tested. The range of detection angles observed at l/|v| = 30 ms (mean: 28.6º, SD: 14.2º) was significantly larger than that observed at l/|v| = 10 ms (mean: 18.1º, SD: 22.4º; Wilcoxon rank-sum test, p = 0.019). Fig. 3B illustrates for the same cell the mean subthreshold membrane potential before and after picrotoxin application (middle panel) and the mean and 95% confidence interval for their difference (bottom panel). The difference in membrane potentials has qualitatively the same shape as the difference in firing rates in panel 2A. However, the membrane potential difference was a less sensitive measure of feed forward inhibition activation as significant differences (arrowhead, 52.5º) were consistently detected later than at the level of the instantaneous firing rate (mean significant membrane potential difference for 6 animals at two l/|v| values: 67.6º, SD: 24.3º). In this experiment, the peak in membrane potential difference (shaded area in Fig. 3B) coincided with the onset of response termination in control conditions (Fig. 2A). The weaker sensitivity of membrane potential differences as compared to instantaneous firing rate differences will be considered in the Discussion. Since we wanted to characterize the dynamical properties of feed forward inhibition, we focused on analyzing the LGMD/DCMD firing rate rather than its membrane potential in subsequent experiments.
Responses to looming stimuli of different final angles in control and picrotoxin conditions
The experiments illustrated in Fig. 2 and 3 suggest that as an object approaches, both excitation and feed forward inhibition onto the LGMD increase in parallel. To gain further insight on their relative time course of activation, we performed experiments in which a looming stimulus stopped its approach at four different final angular sizes (20, 40, 60 or 80º; Methods, P4). According to the results presented above, this protocol is expected to lead to increasing levels of excitation and feed forward inhibition, correlated with the increasing final angular size of the stimulus. The dynamics of visual stimulation might also affect the relative balance of excitation and feed forward inhibition within the LGMD. To assess this point, we used three different values of the approach parameter l/|v| (10, 30 and 50 ms). Fig. 4A illustrates the data obtained in one out of 14 experiments in which both the final angle and the kinematical parameter l/|v| were systematically varied. Qualitatively similar results were obtained in the remaining experiments and in another 6 animals when a longer inter-trial interval was used (Methods, P4). The top row illustrates the stimuli and each row underneath corresponds to a different final angle. Each column corresponds to a single value of l/|v|, leading to a total of 12 possible combinations of final angles and l/|v| values. The spike rasters in each condition were used to compute estimates of the instantaneous firing rate (illustrated above the rasters) and to extract further quantitative measures of the responses. Both the number of spikes (Fig. 4B) and the peak firing rate (Fig. 4C; ⋆ in Fig. 4A) increased and plateaued with final angle, irrespective of l/|v|. The increase was most pronounced from 20 to 40º (35% for the number spikes and 25% for the peak firing rate; average across 14 animals for 3 l/|v| values; SD: 64% and 39%, respectively). Both variables remained relatively stable thereafter and over the same sample, there was only a 7% (SD: 26%) increase in mean spike number from 40 to 80º and on average no increase in peak firing rate (SD: 16%). Fig. 4D illustrates the timing of the peak firing rate relative to collision. Consistent with the previous observations, the peak occurred closer to collision as angular size increased from 20 to 40º (34% decrease on average over 14 animals for 3 l/|v| values; SD: 12%), but there was much less change beyond a final angular size of 40º (on average a 10% decrease from 40 to 80º; SD: 29%). These results suggest that, once activated, feed forward inhibition rapidly controls the level of excitation. This control of excitation proceeds independent of time or of the stimulus dynamics up to the peak firing rate.
Next, we investigated two measures of the termination of the response: i) the time at which the mean instantaneous firing rate decreased to 20% of its peak value (▴ in the instantaneous firing rate plots of Fig. 4A; see Data analysis in Methods); and ii) the time of the last spike evoked by the stimulus (◂ Fig. 4A), excluding potential outliers (see Data Analysis in Methods). Both measures yielded similar results and only the second one will be illustrated. Fig. 4E shows the time of the last spike relative to the last frame of stimulus motion as a function of the final angular size of the stimulus for the three l/|v| values. At 20º the last spike occurred ~30 ms after the last frame of stimulation, independent of l/|v|. For l/|v| = 10 ms, this value changed little with final angle. However, the last spike occurred increasingly earlier as the final angle increased for l/|v| = 30 ms and this trend was most pronounced for l/|v| = 50 ms. When fitted separately with straight lines, the slopes for the three l/|v| values were significantly different (analysis of covariance, F2,90 = 4.48, p = 0.014). These results suggest that as final angular size and, consequently, feed forward inhibition increase, the latter is able to overcome excitation sooner after peak firing occurred, particularly for slow approach sequences. To confirm this hypothesis, we compared the timing of the last spike relative to the last motion frame before and after picrotoxin application. Fig. 5A illustrates the spike rasters and instantaneous firing rates obtained in such an experiment where l/|v| was fixed at 30 ms (Methods, P6). The time of the last spike relative to the last motion frame before and after picrotoxin application is illustrated in the top panel of Fig. 5B. Its value decreased with increasing final angle in control conditions, a tendency that was abolished by picrotoxin application. The middle panel of Fig. 5B illustrates the outcome of a similar experiment for which the value of l/|v| was set to 50 ms. In control conditions, the change in last spike time as a function of final angle was more pronounced than it was for l/|v| = 30 ms, consistent with the data of Fig. 4E. After picrotoxin application this dependence was completely abolished. Similar results are illustrated in one example for l/|v| = 10 ms in the bottom panel of Fig. 5B. The other experiments performed using this protocol (P6, Methods) yielded similar results for all three l/|v| values. Qualitatively identical observations were also made in ten additional experiments were the three l/|v| values were presented in the same animal at the expense of a lower number of stimulus repetitions (Methods, P5). Fig. 5C shows for these ten experiments the mean difference between the time of the last spike relative to the last frame at final angular sizes of 80º and 20º both in picrotoxin and control conditions. This corresponds to subtracting the ordinate of point ➁ from that of point ➀ in the middle panel of Fig. 5B. In control conditions, as l/|v| increases, the difference increases, reflecting the fact that for a final size of 80º, the last spike occurs increasingly earlier relative to the last frame (e.g., Fig 4E). This trend is abolished by picrotoxin application and the last spike occurs a fixed time after the last frame, irrespective of l/|v|. Fig. 5D shows the mean difference in number of spikes in the same 10 experiments, pooled across the three l/|v| values as a function of final angular size. On average, picrotoxin application caused a mean response increase with a plateau at about 30 spikes per trial when the final angular size exceeded 40º. The plateau observed above 40º was also obtained when analyzing each l/|v| value separately.
Effect of picrotoxin on LGMD/DCMD responses to receding stimuli
Next, we investigated the responses of the LGMD/DCMD to objects appearing and then receding away from the animal. In these protocols (P7-P9, Methods), the black square suddenly appeared on the screen, stayed stationary for 2.5 s and subsequently receded in a sequence that was reversed in time compared to that of approaching stimuli. In Fig. 6A, the responses to object appearance at four different angular sizes (20, 40, 60 and 80º; top panel) is illustrated for one experiment. Four additional experiments yielded similar results (P8, Methods). As may be seen by inspection of the spike rasters elicited in control conditions (Fig. 6A, black), the smallest object size elicits the strongest response. Accordingly, both the mean number of spikes and the peak in firing rate were highest for an angle of 20º (Fig. 6A, instantaneous firing rate plots above rasters; Fig. 6C top and middle panel, respectively, black bars). The mean number of spikes, for example, declined by 73% from 20 to 80º (89.2% on average across 5 animals, SD: 10.0%). This is consistent with the strength of inhibition increasing in parallel with the area of the visual field affected by the rapid change in luminance caused by the object. Interestingly, the latency of the response systematically decreased with object size, in spite of the decrease in firing rate. The mean latency value observed at 20º was around 200 ms; it was reduced to less than 100 ms at 80º (Fig. 6C, bottom panel, black bars). On average, the latency decreased by 80 ms (SD: 69 ms) or 32% from 20 to 80º (n = 5 animals). In this experiment and in four additional ones, the dependence of latency on object size was only slightly affected by block of feed forward inhibition through picrotoxin ejection (Fig. 6A, gray rasters; Fig. 6C, bottom panel, gray bars). In contrast, both the number of spikes and the peak firing rates increased and plateaued at values independent of object size after picrotoxin ejection (Fig. 6C, top and middle panels, gray bars). At a size of 20º the mean number of spikes was multiplied on average by a factor 2.7 (SD: 1.2) and the peak firing rate by a factor 2.4 (SD: 0.7) after picrotoxin application (n = 5 animals).
Fig. 6B shows the responses to receding objects of increasing initial size both before and after picrotoxin ejection. In control conditions, the LGMD/DCMD reproducibly fired 1–2 spikes following the onset of motion (see also Fig. 7A). A few additional spikes were also fired later during the trials. Both the mean number of spikes and the peak firing rates increased following block of feed forward inhibition (Fig. 6B black vs. gray rasters; Fig 6D, top and middle panels). On average, at a starting size of 80º the mean number of spikes was multiplied by a factor 3.5 (SD: 1.6) and the peak firing rate by a factor 3.4 (SD: 1.0) after picrotoxin application (n = 5 animals). However, both in control and after picrotoxin ejection, the total number of spikes and peak firing rates elicited were smaller than those elicited by time-reversed, approach sequences (e.g., Fig. 4B, C). This suggests both excitation and feed forward inhibition to be less strongly activated by receding than approaching objects. The changes in latency as a function of initial angular size were very similar to those observed for abruptly appearing squares across all 5 animals (compare, e.g., Fig. 6A and B). We also performed experiments in which the start size of the receding object was fixed and the kinematical parameter l/|v| was varied between 10, 30 and 50 ms (Methods, P9). The rasters and instantaneous firing rate before and after picrotoxin ejection in such an experiment are illustrated in Fig. 7A. There was little dependence of the mean spike numbers, peak firing rate or response latency on the kinematical parameter l/|v| (Fig. 7B, C and D). In this particular neuron, response latency (~50 ms) was shorter than in the example illustrated in Fig. 6. The range of latencies to receding stimuli ranged from 54 to 235 ms (mean: 120 ms, SD: 63 ms) for l/|v| = 30 ms and a 40º initial angle in ten animals. Similar results were obtained in an additional set of four animals tested with the same protocol and in another five animals where both l/|v| and start size were varied together (P7, Methods).
DISCUSSION
This study investigated what effect localized picrotoxin injection to the dorsal lobula had on the responses of the LGMD/DCMD to three types of visual stimuli presented in the center of its receptive field: approaching, suddenly appearing and receding squares. Picrotoxin had a marked effect on the responses of the LGMD in all three cases, suggesting a preponderant role of feed forward inhibition in shaping the neuron’s responses under normal conditions. By using approaching and receding stimuli with different kinematical properties, we were able to shed light on the dynamic interaction between excitation and feed forward inhibition within the LGMD's dendritic arborizations. Our results provide strong evidence for a precise timing and parallel time course of their relative activation. These results set new constraints on the biophysical factors shaping the receptive field properties of the LGMD to looming and receding stimuli.
Effects of picrotoxin on the membrane potential and firing rate of the LGMD
In control conditions, the membrane potential of the LGMD usually showed an abrupt repolarization after the cessation of object motion at the end of approach (Fig. 2A, shaded area). The temporal dynamics of repolarization was similar to that observed after activation of feed forward inhibition by the fast flow stimulus during looming in the same neurons (Fig. 3a from Gabbiani et al., 2002). It is also consistent with the time course of IPSPs measured in the LGMD in response to the fast flow stimulus alone (Fig. 2B). These observations suggest that IPSPs contribute to the repolarization of the membrane potential at the end of stimulation. Similar observations have been made in previous intracellular recordings of the LGMD in response to looming (Figs. 1 and 3 in Rind, 1996). Consistent with these observations, the repolarization of the LGMD membrane potential slowed down after ejection of picrotoxin into the lobula (Fig. 2B).
Local ejection of picrotoxin on the dorsal lobula, where the LGMD's inhibitory dendritic subfields are located, had significant effects both on the time course of the membrane potential and the instantaneous firing rate during looming. In contrast, picrotoxin application elicited only weak changes in the activity of units recorded in the medulla, the neuropil immediately presynaptic to the lobula (Fig. 2C). These results suggest that the effect of picrotoxin was sufficiently localized and that the changes observed with the LGMD were predominantly due to post-synaptic block of GABAA receptors. The excitatory dendritic subfield of the LGMD receives cholinergic inputs from retinotopic afferents and the presynaptic contacts thought to mediate lateral inhibition between such neighboring afferents in the lobula are also cholinergic (Rind and Simmons, 1998). Therefore we conclude that picrotoxin specifically blocked inhibitory feed forward inputs to the two inhibitory dendritic subfields of the LGMD.
The difference in membrane potential between picrotoxin and control conditions turned out to be a less sensitive measure of the activation of feed forward inhibition than the difference in instantaneous firing rate (Fig. 3). Our intracellular recordings were always performed in the dendritic tree of the LGMD at a significant electrotonic distance from the spike initiation zone, as may be inferred from the reduced size of action potentials in Fig. 2A (compare, e.g., with Fig. 4a of Gabbiani et al., 2002). At these dendritic locations, the membrane potential is less tightly coupled to the instantaneous firing rate and may be significantly influenced by synaptic inputs. Thus, during the early phases of looming, the membrane potential typically fluctuated rapidly between single spikes or spike bursts, both in control and picrotoxin conditions as is evident for instance in Fig. 2. The variability of these fluctuations across trials is therefore a likely cause for the decrease in sensitivity observed for membrane potential differences compared to instantaneous firing rate differences. Intracellular recordings close to the spike initiation zone may yield a sensitivity comparable to that observed at the level of firing rates. These recordings are technically more difficult because of the smaller size of the axon relative to that of the main dendritic compartments of the LGMD. Alternatively, voltage clamp techniques similar to those developed by Borg-Graham et al. (1998) might prove useful to monitor directly changes in membrane conductance caused by feed forward inhibition.
Activation of feed forward inhibition during looming
A comparison of the instantaneous firing rate in control and picrotoxin conditions revealed that activation of feed forward inhibition is delayed with respect to excitation, but otherwise follows a similar time course (Fig. 3). On average, a significant activation of feed forward inhibition was detected early during the trials, when the objects approached ~23º in size and well in advance of response termination (Figs. 2 and 3). This finding is consistent with observations by Rowell et al. (1977), showing that feed forward inhibition can be activated by small field stimuli. In contrast, a different presynaptic, lateral inhibitory process to the LGMD becomes ineffective beyond those angles (Gabbiani et al., 2002, Fig. 3). Thus our experiments reveal that in control conditions excitation actually masks an increasingly large concurrent recruitment of feed forward inhibitory inputs, as summarized in Fig. 8A. This finding resembles observations made in directionally selective V1 simple cells, where motion in the preferred direction is often reported to cause a large activation of both excitation and delayed inhibition when compared to the null direction (Monier et al., 2003; Priebe and Ferster, 2005). Concurrent activation of excitation and inhibition has also been reported for preferred and null direction motion in fly lobula plate motion sensitive tangential neurons (Single et al., 1997).
Figure 8.

Relative time course of activation of excitation and feed forward inhibition for approaching, receding and suddenly appearing objects as inferred from the experimental data. A. Excitation and inhibition are activated in parallel during approach, with excitation leading inhibition by approximately 50 ms. In control conditions, inhibition becomes only apparent during response termination as excitation decays earlier and faster than inhibition (shaded area). The relative strength of inhibition relative to excitation is expected to increase faster with angular size at high l/|v| values, to account for faster response termination (Figs. 3, 4). B. In response to receding stimuli, excitation is sharply activated, followed by delayed inhibition. This leads to a short summation time window (~10 ms) during which 1–2 spikes are reliably emitted. Thereafter, inhibition is expected to hover roughly at the same level as excitation, since additional spikes are sporadically emitted in response to the stimulus. A similar activation pattern is expected for suddenly appearing objects. However, excitation is likely to be activated less sharply, as the probability of spiking at the onset of the stimulus is decreased. The absolute activation level is expected to be smaller than for approaching objects.
The experiments in which looming stimuli stopped at different final angles, showed that the increase in excitation caused by larger final sizes was very effectively controlled by feed forward inhibition. Indeed, the number of spikes, peak firing rates and peak firing times changed only slightly for angular sizes in excess of 20º, i.e., beyond the effective activation threshold for feed forward inhibition (Fig. 4). Thus, the concurrent increase in excitation and feed forward inhibition is remarkably well balanced over the course of object approach. Further, these experiments show that the termination of the response is affected by the kinematical parameter of the stimuli. For high values of l/|v| the angular size of the approaching object increases more slowly, presumably causing a slower and weaker activation of excitation that is overcome earlier by inhibition as final angular size increases from 20 to 80º. This hypothesis was confirmed by subsequent picrotoxin ejection experiments, since after block of feed forward inhibition the l/|v| dependence of response termination on final angle was abolished (Fig. 5).
Preferential tuning of excitation and feed forward inhibition for approaching objects
The responses of the LGMD/DCMD to a square suddenly appearing on the screen were typically weak, except at the smallest object size (20º) which, according to our previous experiments, was below the threshold for significant activation of feed forward inhibition. Interestingly, the latency of the response decreased with object size in exactly the same way both before and after picrotoxin application. This demonstrates directly the tight relation between the activation of excitation and feed forward inhibition with varying object size under these experimental conditions. Similar observations were made for the response onset to object recession (Fig. 6B).
In control conditions, the responses to object recession had similar characteristics to those reported by Rind (1996). The precise timing of spikes observed at the onset of recession was presumably due to the short delay between the activation of excitation and feed forward inhibition similar to that observed in Fig. 2B for the fast flow stimulus (see also Fig. 8B). Thus, feed forward inhibition decreases the temporal summation window for excitatory inputs (Pouille and Scanziani, 2001; Perez-Orive et al., 2002; Mittman et al., 2004). After picrotoxin ejection, both peak firing rates and mean spike counts increased, confirming the role of feed forward inhibition in terminating the responses to receding stimuli (Rind, 1996). Thus feed forward inhibition plays a role in shaping the preferential tuning of the LGMD to approaching vs. receding stimuli. However, even after picrotoxin ejection, peak firing rates and mean spike counts were well below those obtained for inverted sequences of approaching stimuli with the same l/|v| value. Further, no obvious changes in the dynamical interplay of excitation and inhibition were revealed by varying l/|v| (Fig. 7), in contrast to approaching sequences. These results suggest that both excitation and feed forward inhibition are weaker for receding than approaching stimuli and that they show little dependence on the kinematics of recession. The weaker activation of excitation for receding stimuli might be due to lateral inhibition between adjacent excitatory afferents mediated by muscarinic acethylcholine receptors (Rind and Bramwell, 1996). The mechanism responsible for weaker activation of feed forward inhibition is presently unknown.
Implications for biophysical modeling of LGMD responses to looming stimuli
These results have implications for modeling the responses of the LGMD to looming and receding stimuli. In an earlier biophysical model of the LGMD for example, Rind and Bramwell (1996) assumed that activation of feed forward inhibition occurs only at the last stages of object approach (e.g., their Fig. 4). Their model assumed instead a significant effect of lateral inhibition during most of the approach sequence. On the contrary, as described in Gabbiani et al. (2002), activation of lateral inhibition is ineffective at controlling excitation beyond ~23º and, according to the present results, feed forward inhibition is significantly activated for angular sizes in excess of 23º, that is, over a large fraction of a looming trial. The response properties of the LGMD over a wide range of l/|v| values and final angles, as gathered here, should provide an effective way to constrain the relative activation of excitation and feed forward inhibition onto the LGMD in a biophysical model. Thus, future biophysical models of the LGMD will need to take into account current constraints on the dynamics of excitation and feed forward inhibition, as well as recently derived constraints on the spatial receptive field properties of the LGMD (Krapp and Gabbiani, 2005). These models are in turn likely to provide important constraints on the biophysical mechanisms underlying the sensitivity of the LGMD to looming stimuli and the multiplicative interaction between excitation and feed forward inhibition characterized in earlier experiments (Gabbiani et al., 2002 and 2004).
Acknowledgments
Use of the QNX 6 operating system was made possible through the Educational Program of QSSL. Multichannel silicon probes were provided by the University of Michigan Center for Neural Communication Technology sponsored by NIH NCRR grant P41-RR09754. Thanks to Dr. H.G. Krapp for comments on the manuscript.
Footnotes
GRANTS Supported by funds of the National Institute of Health and an Alfred P. Sloan Fellowship (FG).
References
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 1998. 1998;393:369–73. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Cohen I and Gabbiani F. Multielectrode extracellular recordings from the locust optic lobe. Program No. 491.4 Abstract Viewer/Intinerary Planner Washington, DC: Soc Neurosc. Online, 2003.
- Cohen I, Miles R. Contributions of intrinsic and synaptic activities to the generation of neuronal discharges in in vitro hippocampus. J Physiol. 2000;524:485–502. doi: 10.1111/j.1469-7793.2000.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Graziano MS. Super-flinchers and nerves of steel: defensive movements altered by chemical manipulation of a cortical area. Neuron. 2004;43:585–593. doi: 10.1016/j.neuron.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Elphick M, Williams L, O’Shea M. New features of the locust optic lobe: evidence of a role for nitric oxide in insect vision. J Exp Biol. 1996;199:4139–4143. doi: 10.1242/jeb.199.11.2395. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) J Neurophysiol. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci. 1999;19:1122–1141. doi: 10.1523/JNEUROSCI.19-03-01122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Mo C, Laurent G. Invariance of angular threshold computation in a wide-field looming sensitive neuron. J Neurosci. 2001;21:314–329. doi: 10.1523/JNEUROSCI.21-01-00314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature. 2002;420:320–324. doi: 10.1038/nature01190. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Hatsopoulos N, Mo CH, Koch C, Laurent G. Multiplication and stimulus invariance in a looming-sensitive neuron. J Physiol Paris. 2004;98:19–34. doi: 10.1016/j.jphysparis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos N, Gabbiani F, Laurent G. Elementary computation of object approach by widefield visual neuron. Science. 1995;270:1000–1003. doi: 10.1126/science.270.5238.1000. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Alonso JM, Reid RC, Martinez LM. Synaptic integration in striate cortical simple cells. J Neurosci. 1998;18:9517–9528. doi: 10.1523/JNEUROSCI.18-22-09517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GR, Koch C. Shunting inhibition does not have a divisive effect on firing rates. Neural Comput. 1997;9:1001–1013. doi: 10.1162/neco.1997.9.5.1001. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Aertsen A, Rotter S. Neuronal integration of synaptic input in the fluctuation-driven regime. J Neurosci. 2004;24:2345–2356. doi: 10.1523/JNEUROSCI.3349-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp HG, Gabbiani F. Spatial distribution of inputs and local receptive field properties of a wide-field looming-sensitive neuron. J Neurophysiol. 2005;93:2240–2253. doi: 10.1152/jn.00965.2004. [DOI] [PubMed] [Google Scholar]
- Matheson T, Krapp HG, Rogers SM. Plasticity in the visual system is correlated with a change in lifestyle of solitarious and gregarious locusts. J Neurophysiol. 2004;91:1–12. doi: 10.1152/jn.00795.2003. [DOI] [PubMed] [Google Scholar]
- Mickey RM, Dunn OJ and Clark V. Applied Statistics: Analysis of Variance and Regression. Wiley: San Diego, CA, 2004.
- Milton JS and Arnold JC. Introduction to probability and statistics. 3rd ed. McGraw-Hill: New York, 1995.
- Mittmann W, Koch U and Hausser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol in press, 2005. [DOI] [PMC free article] [PubMed]
- Monier C, Chavane F, Baudot P, Graham LJ, Frégnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Rowell CH. Protection from habituation by lateral inhibition. Nature. 1975;254:53–55. doi: 10.1038/254053a0. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Williams JLD. The anatomy and output connection of a locust visual interneurone; the Lobular Giant Movement Detector (LGMD) Neurone. J Comp Physiol A. 1974;91:257–266. [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RL, Laurent G. Oscillations and sparsening of odor representation in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Mazor O, Laurent G. Using noise signature to optimize spike-sorting and to assess neuronal classification quality. J Neurosci Methods. 2002;122:43–57. doi: 10.1016/s0165-0270(02)00276-5. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Rauh JJ, Lummis SC, Sattelle DB. Pharmacological and biochemical properties of insect GABA receptors. Trends Pharmacol Sci. 1990;11:325–329. doi: 10.1016/0165-6147(90)90236-2. [DOI] [PubMed] [Google Scholar]
- Rind FC. Intracellular characterization of neurons in the locust brain signalling impending collision. J Neurophysiol. 1996;75:986–995. doi: 10.1152/jn.1996.75.3.986. [DOI] [PubMed] [Google Scholar]
- Rind FC, Simmons PJ. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. I. Selective responses to approaching objects. J Neurophysiol. 1992;68:1654–1666. doi: 10.1152/jn.1992.68.5.1654. [DOI] [PubMed] [Google Scholar]
- Rind FC, Simmons PJ. Local circuit for the computation of object approach by an identified visual neuron in the locust. J Comp Neurol. 1998;395:405–415. [PubMed] [Google Scholar]
- Rowell CH, O’Shea M. The neuronal basis of a sensory analyzer, the acridid movement detector system. III. Control of response amplitude by tonic lateral inhibition. J Exp Biol. 1976;65:617–625. doi: 10.1242/jeb.65.3.617. [DOI] [PubMed] [Google Scholar]
- Rowell CH, O’Shea M, Williams JLD. The neuronal basis of a sensory analyzer, the acridid movement detector system. IV. The preference for small field stimuli. J Exp Biol. 1977;68:157–185. doi: 10.1242/jeb.68.1.157. [DOI] [PubMed] [Google Scholar]
- Rind FC, Bramwell DI. Neural network based on the input organization of an identified neuron signalling impending collision. J Neurophysiol. 1996;75:967–985. doi: 10.1152/jn.1996.75.3.967. [DOI] [PubMed] [Google Scholar]
- Rowell CHF. The orthopteran descending movement detector (DMD) neurones: a characterization and review. Z vergl Physiol. 1971;73:167–194. [Google Scholar]
- Rowell CHF. Boredom and attention in acell in the locust visual system. In: Experimental analysis of insect behavior, edited by Browne L.B. New-York: Springer Verlag, pp 87–99, 1974.
- Schlotterer GR. Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Can J Zool. 1977;55:1372–1376. [Google Scholar]
- Sheperd G.The synaptic organization of the brain, 5th edition. Oxford: Oxford University Press, 2003.
- Single S, Haag J, Borst A. Dendritic computation of direction selectivity and gain control in visual interneurons. J Neurosci. 1997;17:6023–6030. doi: 10.1523/JNEUROSCI.17-16-06023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ and Naessel DR. Neuroarchitectures serving compound eyes of crustacea and insects. In: Handbook of Sensory Physiology, vol. VII/7B, edited by Autrum H. Berlin: Springer, pp. 1–132, 1981.
- Sun H, Frost BJ. Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci. 1998;1:296–303. doi: 10.1038/1110. [DOI] [PubMed] [Google Scholar]
- Warzecha AK, Egelhaaf M, Borst A. Neural circuit tuning fly visual interneurons to motion of small objects. 1. Dissection of the circuit by pharmacological and photoinactivation methods. J Neurophysiol. 1993;69:329–339. doi: 10.1152/jn.1993.69.2.329. [DOI] [PubMed] [Google Scholar]
- Wicklein M, Strausfeld NJ. Organization and significance of neurons that detect change of visual depth in the hawk moth Manduca sexta. J Comp Neurol. 2000;424:356–376. [PubMed] [Google Scholar]
- Wilson M. Angular sensitivity of light and dark adapted locust retinula cells. J Comp Physiol. 1975;97:323–328. [Google Scholar]


