Abstract
Belowground vertical community composition and maximum rooting depth of the Edwards Plateau of central Texas were determined by using DNA sequence variation to identify roots from caves 5–65 m deep. Roots from caves were identified by comparing their DNA sequences for the internal transcribed spacer (ITS) region of the 18S–26S ribosomal DNA repeat against a reference ITS database developed for woody plants of the region. Sequencing the ITS provides, to our knowledge, the first universal method for identifying plant roots. At least six tree species in the system grew roots deeper than 5 m, but only the evergreen oak, Quercus fusiformis, was found below 10 m. The maximum rooting depth for the ecosystem was ≈25 m. 18O isotopic signatures for stem water of Q. fusiformis confirmed water uptake from 18 m underground. The availability of resources at depth, coupled with small surface pools of water and nutrients, may explain the occurrence of deep roots in this and other systems.
Plant rooting depth influences the hydrology, biogeochemistry, and primary productivity of terrestrial ecosystems (1–7). Progress in determining the maximum rooting depth of species and in identifying the resources taken up at depth is limited by several factors. Access to the soil is difficult, particularly in rocky soils and in deeper layers. In addition, no universal method exists for identifying roots obtained from the soil, especially when only fine roots are available (8–10). There is considerable variation in maximum rooting depth and root biomass distributions, which affects the functioning of ecosystems (11–15). For example, in eastern Amazonia, water uptake from 2- to 8-m soil depths contributes to more than three-fourths of the transpiration of evergreen forest in the dry season and helps maintain an evergreen canopy on >1 million km2 of tropical forest (1, 16). Characteristics of roots and the soil are also needed in models of biosphere–atmosphere interactions (17). A comparison of 14 land surface parameterizations concluded that rooting depth and vertical soil characteristics were the most important factors explaining scatter among models for simulated transpiration (18, 19), determining the amount of water available to plants and partitioning its uptake from different layers. Conclusions were similar for global soil-moisture dynamics (20, 21). Global simulations of net primary productivity and transpiration increased 16% and 18%, respectively, when optimized rooting depths incorporated soil water deeper than 1 m (22).
We developed a method for identifying roots based on DNA sequence variation and applied this method to roots collected from caves 5–65 m deep to determine the belowground community structure and maximum rooting depth of the 100,000-km2 Edwards Plateau of central Texas. The Edwards Plateau and other karst regions in Texas cover one-fifth of the state, with >3,000 caves identified to date (23, 24). Karst systems, in general, cover 7–10% of land surface area globally and supply a quarter of the earth’s population with drinking water (25). For the Edwards Plateau, we also examined the functional importance of roots at depth. We used stable isotopic signatures of water from plants, the soil, and an underground stream to examine the depth of water uptake. We also compared the concentration and potential pool of nutrients at depth and their relationship to fine-root biomass.
METHODS
The Edwards Plateau has shallow, calcareous soils overlaying fractured Cretaceous limestone (23, 24). Precipitation in the region ranges from ≈400 mm to 800 mm west to east, with a narrower range from 600 mm to 800 mm across our sites. The vegetation is primarily savanna and woodland dominated by trees in the genera Quercus, Juniperus, Ulmus, Celtis, and, farther to the west, Prosopis (26). We sampled 21 caves across the central and eastern Edwards Plateau (see Table 1 and its legend for the depths and locations of each cave). Fine roots were obtained from soil in the floor of the caves or, in a few cases, from underground streams. Most of the caves were smaller than 3 m across, and many were only 1 m in diameter or less.
Table 1.
Cave depths and plant species found at depth and the characteristics of the surface vegetation
| Cave | Depth, m | Species identified by ITS from roots | Surface vegetation by species (density of stems, m−2; average basal area, m2 per stem)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Celtis laevigata | Juniperus ashei | Quercus fusiformis | Quercus sinuata | Ulmus crassifolia | Other species | |||
| District Park Cave | 5 | Q. fusiformis | 0.05, 0.011 | 0.07, 0.068 | ||||
| Seven Room Cave | 5 | U. crassifolia | 0.032, 0.069 | 0.008, 0.078 | ||||
| Pearson’s Lost Cave | 6 | C. laevigata | 0.02, 0.008 | 0.09, 0.012 | ||||
| J. ashei | ||||||||
| Spyglass Cave | 6 | Q. fusiformis | 0.01, 0.045 | 0.06, 0.021 | 0.012, 0.02 | |||
| Cotterell Cave | 7 | J. ashei | 0.01, 0.008 | 0.08, 0.025 | 0.04, 0.015 | |||
| Q. sinuata | ||||||||
| Mystery Hole | 7 | U. crassifolia | 0.07, 0.008 | 0.01, 0.05 | ||||
| Sour Cave | 7 | Ulmus americana | 0.06, 0.007 | 0.01, 0.49 U. americana | ||||
| Turtle Shell Cave | 8 | J. ashei | 0.09, 0.009 | 0.02, 0.11 | 0.005, 0.47 | |||
| Q. fusiformis | ||||||||
| Cave of Many Names | 9 | U. crassifolia | 0.087, 0.008 | 0.027, 0.053 | ||||
| Natural Bridge (south cave) | 9 | Q. fusiformis | 0.005, 0.024 | 0.025, 0.061 | 0.02, 0.022 | |||
| Sweet Cave | 11 | Q. fusiformis | 0.053, 0.004 | 0.013, 0.051 | 0.007, 0.11 | |||
| Cicurina Cave | 14 | Q. fusiformis | 0.001, 0.010 | 0.013, 0.005 | 0.008, 0.048 | 0.004, 0.021 | 0.001, 0.088 | 0.001, 0.15 Carya illinoiensis |
| 0.002, 0.17 Quercus stellata | ||||||||
| Powell’s Cave | 18 | Q. fusiformis | 0.005, 0.053 | 0.006, 0.17 | 0.002, 0.019 | 0.006, 0.017 Prosopis glandulosa | ||
| 0.006, 0.010 Bumelia lanuginosa | ||||||||
| HoneyCreek Cave | 22 | Q. fusiformis | unknown | |||||
| Cave Without A Name | 28 | None | 0.04, 0.005 | 0.06, 0.067 | ||||
| Bracken Bat Cave | 31 | None | 0.018, 0.028 | 0.005, 0.18 | 0.008, 0.016 | |||
| Gorman Cave | 41 | None | 0.01, 0.03 | 0.04, 0.01 | 0.01, 0.05 | 0.005, 0.02 | 0.025, 0.06 | |
| HoneyCreek Cave | 44 | None | 0.02, 0.06 | |||||
| Natural Bridge (north cave) | 65 | None | 0.036, 0.022 | 0.042, 0.02 | 0.005, 0.003 | |||
The table lists the name and depth of caves sampled in Texas, the plant species found in them, and the surface vegetation above the caves [density of stems (m−2) and average basal area for each species (m2 per stem)]. The methods for identifying roots by using internal transcriber spacer (ITS) analysis and for describing the surface vegetation are found in Methods. Cave depths were obtained from maps of the Texas Speleological Survey. The locations of the caves are District Park, 30°12′82" N, 97°51′78"W; Seven Room, 30°40′24" N, 97°141′44" W; Pearson’s Lost, 31°02′66" N, 98°29′37" W; Spyglass, 30°15′40 N, 97°47′46" W; Cotterrell, 30°22′16" N; 97°45′79" W; Mystery Hole, 31°01′79" N; 98°30′38" W; Sour, 31°02′36" N, 98°28′80" W; Turtle Shell, 31°02′53" N, 98°28′83" W; Cave of Many Names, 31°02′52" N; 98°30′31" W; Natural Bridge (south), 29°41′52" N, 98°20′50" W; Sweet, 31°02′37" N, 98°28′85" W; Cicurina, 31°02′83" N, 98°30′25" W; Powell’s, 30°55′69" N, 99°54′93" W; Cave Without A Name, 29°53′16" N, 98°37′02" W; Bracken, 29°41′20" N, 98°21′15" W; Gorman, 31°03′00" N, 98°28′19" W; Honey Creek, 29°50′12" N, 98°31′15" W; and Natural Bridge (north), 29°41′52" N; 98°20′50" W. Honey Creek Cave appears twice in the table, because it was sampled twice, approximately 1 km in distance apart.
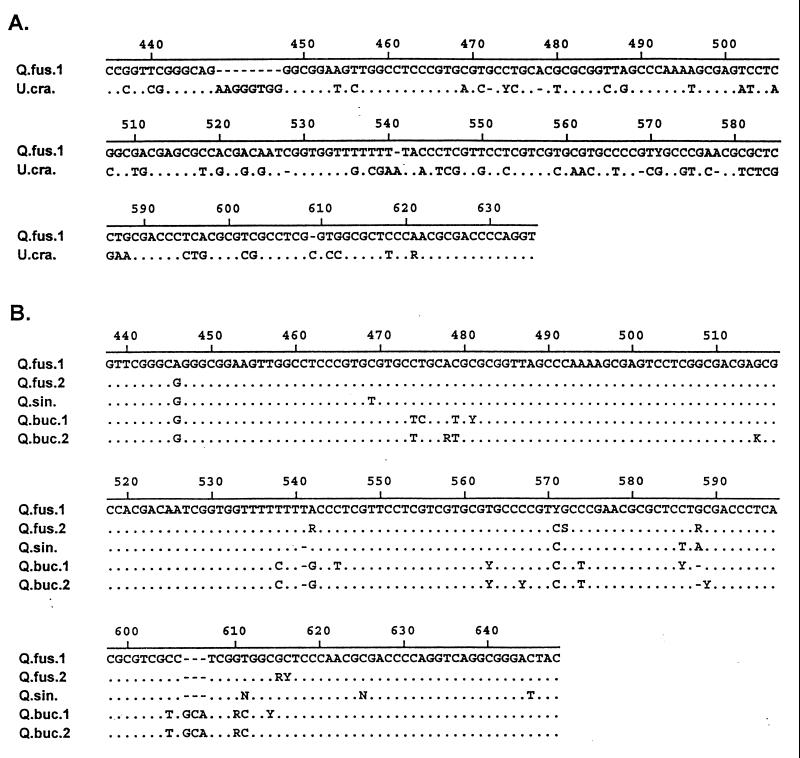
To identify the roots obtained from the caves, we compared root DNA sequences of the ITS region of the 18S–26S nuclear ribosomal DNA repeat with an ITS reference database we developed for woody plants of the Edwards Plateau. The ITS evolves rapidly and is easily amplified in plants, allowing transgeneric and usually congeneric species to be readily identified (refs. 27–29; Fig. 1). Root and leaf DNA were extracted by using the techniques of Doyle and Doyle (30), except for a modified extraction buffer [3% (wt/vol) cetyltrimethylammonium bromide/1.4 M NaCl/20 mM EDTA/1.0 M Tris⋅HCl, pH 8.0/0.2% (vol/vol) 2-mercaptoethanol; see ref. 31] and the use of chloroform-phenol for removing proteins (32). Extracted DNA was gel purified (QIAquick Gel Extraction Kit, Qiagen, Chatsworth, CA) and amplified (27–29) for the entire ITS region (ITS1, ITS2, and the 5.8S ribosomal RNA gene). PCR products were purified (QIAquick PCR Purification Kit, Qiagen) and sequenced by using an ABI 377 Prism automated sequencer with dichloro-rhodamine dye terminators and other standard reagents and protocols (Perkin–Elmer). Electropherograms were proofread by eye. Both strands of the complete ITS were sequenced for all reference sequences; for root samples, the ITS was sequenced only enough to identify unambiguously the species to which it should be assigned. Unknown sequences were aligned with reference sequences to determine the species to which they belonged (33). We confirmed species identified from ITS sequences with vegetation surveys aboveground. Vegetation surveys were taken with plots centered over each cave. Species identity, density, and the basal area of each individual >10 cm in circumference at breast height were measured in a series of plots 100–1,000 m2 in size.
Figure 1.
Alignments of reference ITS sequences illustrating the ability to distinguish between genera (A) and species within a genus (B). (A and B) For both alignments, the top sequence is used as a reference for the sequences below it. Agreement with the reference sequence is indicated by a period, and differences are indicated by a nucleotide symbol. Gaps introduced to improve the alignment are indicated by dashes. Standard International Union of Biochemistry nucleotide symbols are used: A = adenosine, G = guanosine, C = cytidine, T = thymidine, R = purine (A or G), Y = pyrimidine (C or T), S = G or C, W = A or T, K = G or T, M = A or C, and N = any nucleotide (unreadable). All polymorphisms were confirmed on both strands. (A) Alignment of a portion of the ITS sequences for Q. fusiformis and U. crassifolia, the two most closely related genera in the reference database. All other intergeneric alignments showed even less similarity. (B) Alignment of three oak species: Q. fusiformis, Q. sinuata, and Quercus buckleyi. Note that there are two representatives for Q. fusiformis and Q. buckleyi. Although there are fewer diagnostic characteristics within the oaks, they can be distinguished readily by consistent differences across the ITS region (Q. fusiformis and Q. sinuata are the two most difficult species we distinguish). Vegetation surveys at the surface of each cave also constrain the species potentially found in each cave (and act as a check on the ITS results).
To test the functional importance of deep roots in this system, we used the natural abundance of stable isotopes of 18O in plants and the soil to examine water uptake from an 18-m-deep underground stream at Powell’s Cave. Branch samples from shrub and tree species and root/crown samples of grass and forb species were taken on October 25, 1997 and June 27, 1998. Powell’s Cave, like most of the Edwards Plateau, is privately owned; it is only accessible on the last weekends of February, June, and October, making a more complete time series difficult. The species sampled were Abutilon fructicosum Guill and Perrotet (a forb), Aristida purpurea Nutt. var. wrightii (Nash) Allred (a grass), Guttierezia dracunculoides (DC.) Blake (a subshrub), and Q. fusiformis Small (live oak). Samples of soil randomly located above the cave were taken from the soil surface to bedrock (never more than 20 cm deep) on the same dates, as were stream samples from the cave. It was not possible to obtain water samples from the intervening bedrock. All samples were sealed immediately and analyzed relative to standard mean ocean water at the Stable Isotope Research Facility for Environmental Research at the University of Utah (Salt Lake City, UT).
To estimate total soil N and organic C concentrations in cave soil as well as fine-root biomass and densities, we placed random 0.25-m2 plots on the floors of the caves. The plots were excavated to depths of 20 cm. Fine roots (<2 mm in diameter) were separated from the soil, and the roots were dried at 60°C and weighed. Total N and organic C determinations of the soil were made with a CE Elantech NC 2100 Soil Analyzer (Lakewood, NJ). The six caves sampled were Spyglass, Cotterell, Turtle Shell, Sweet, Cicurina, and Powell’s (see Table 1 for the location, depth, and species present in each cave). Surface-soil nutrient concentrations were estimated from random samples to bedrock (between 10 and 20 cm deep) above the caves at the same sites.
RESULTS AND DISCUSSION
Six tree species common to the Edwards Plateau were found with roots below 5 m (Table 1). These six species in the genera Celtis, Juniperus, Quercus, and Ulmus represent three-fourths of woody plants on the plateau, as determined by importance values, with J. ashei and Q. fusiformis comprising over half that total (26). Roots were observed above 20 m in every cave sampled with one exception, a 13-m-deep cave above which the surface vegetation had been cleared previously (data not shown). Despite the diversity of species found between 5 and 10 m, only one species was found below 10 m: the live oak Q. fusiformis from four caves between 10 and 25 m in depth (Table 1). Five caves deeper than 25 m had abundant Q. fusiformis populations at the surface, and three had permanent underground streams. Nonetheless, no roots of any species were found below 25 m, which we estimate to be the maximum rooting depth for the ecosystem.
A hierarchy of rooting depth for woody species emerges from the data (Table 1). Of the species present at the surface above five or more caves, C. laevigata was apparently the shallowest rooted. It was found at seven sites but was detected in only one cave at a depth of 6 m. J. ashei, the most common tree, occurred at 15 sites overall; it was found in three of nine caves shallower than 10 m (at 6, 7, and 8 m). U. crassifolia, though less abundant than J. ashei, was more likely to be found in caves when present at the surface (roots at 5, 7, and 9 m in three of five shallow caves). Q. fusiformis, the deepest of the species, was observed dependably in eight caves to depths of ≈20-m when present at the surface (Table 1).
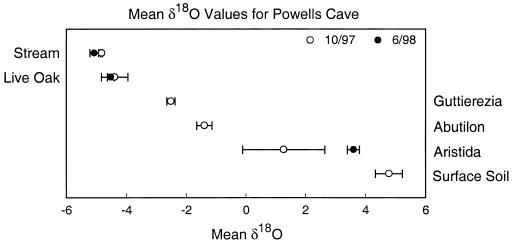
We examined the functional importance of deep roots in the system by using 18O signatures of water in plants, soil, and a permanent underground stream at the Powell’s Cave site. (We also used the 18O data as a check of the ITS results.) Live oaks at the site had 18O signatures that closely matched the signature of the underground stream water (Fig. 2), indicating that the trees apparently were using this water as their primary source. Stem water in a subshrub (Guttierezia dracunculoides), a forb (Abutilon fructicosum), and a grass (Aristida purpurea) had progressively heavier 18O signatures that reflected the uptake of evaporatively enriched water from the surface soil layer (and, most likely, increasingly shallow rooting depths from subshrub to grass; Fig. 2).
Figure 2.
Comparison of 18O isotopic signatures on two dates for stem water of plants at the surface of the Powell’s Cave site with the signatures of water from surface soil (surface to bedrock) and from an 18-m-deep underground stream (mean ± SEM; n = 2–5). The species sampled were Abutilon fructicosum (a forb), Aristida purpurea (a grass), Guttierezia dracunculoides (a subshrub), and Q. fusiformis Small (live oak tree), and the two sampling dates were October 25, 1997 and June 27, 1998 (the cave is open only on the last weekends of February, June, and October). Isotopic analyses were run at the Stable Isotope Research Facility of the University of Utah. Water in the stems of live oaks at the surface had an 18O signature that closely matched the underground stream water, evidence that the trees are likely using this water as a primary source.
Although root biomass generally decreases with depth in the soil (34–36), plants show great flexibility in allocating roots and adjusting resource uptake in layers with high resource availability (37–40). Such flexibility may help capture belowground resources that vary with depth through time. For example, the availability of water in surface and deeper soil layers may vary depending on a site’s hydrology and the seasonal pattern of precipitation and plant water use. We estimated a rough lower bound on the pool of water available to deep roots in our system based on data from the 11,400-km2 drainage area of the Edwards aquifer. Average annual recharge from 1970–1997 was 1.08 km3 in the drainage area (41). Aquifer recharge across the zone is therefore 90 mm/year, the average amount of water that percolates beyond the rooting depth of plants and more than 10% of annual precipitation. Basing the calculation on the 3,900-km2 recharge zone increases the estimated percolation from 90 mm/year to 260 mm/year in that smaller area. Five of our caves were in the aquifer drainage area, and three were in the recharge zone. Evidence for the presence of deep soil water also comes from the slow but observable trickle of water from the ceilings of caves we visited during the severe drought of 1998. Such slowly percolating water is the resource deep roots most likely exploit.
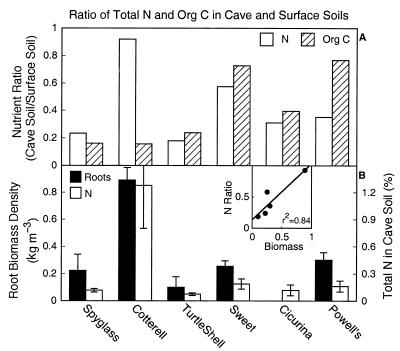
In addition to taking up water, the plants may also take up nutrients at depth. The relatively low nutrient concentrations and the shallow soils of the Edwards Plateau result in surface nutrient pools that are small. Organic matter in cracks and fissures and in cave soils may be the source of additional nutrients for plants across the region. Cave soils at six sites had total N concentrations ranging from 0.1% to 1.3% N compared with 0.3% to 1.4% in surface soils at the same sites (Fig. 3). Expressed as a ratio, N concentrations in cave soils were 17% to 92% of those in surface soils, and organic C contents were 16% to 77% of those at the surface (Fig. 3). The N in cave soils may come partly from bat guano [total N concentrations below bat roosts in Powell’s Cave were 19.4% ± 0.81% (SEM)]. However, only a few caves had bat colonies, and Cotterell Cave, with the highest N concentrations, had none. Root biomass densities were between 0.1 and 0.9 kg/m3 (Fig. 3B), with area-based biomass estimates of 0.02–0.2 kg/m2 (a mean of 0.072 kg/m2 or 72 g/m2); these area-based estimates were ≈10–20% of values from the soil surface to bedrock. There was no relationship between root biomass and cave depth (r2 = 0.03; P = 0.77), but root biomass increased significantly as the ratio of N in cave soil to surface soil increased (Fig. 3 Inset; r2 = 0.84; P = 0.03). No analogous relationship existed for soil organic C (r2 = 0.08). The presence of deep resources, coupled with the low water-holding capacity and small N pools in surface soils of the Edwards Plateau, may have selected for deep roots in this ecosystem.
Figure 3.
(A) The ratio of total N and C concentrations of cave soils relative to surface soils at six cave sites. (B) Root biomass density (kg m−3) and total N (%) in cave soil. The caves are arranged in order of increasing depth from left to right; see Table 1 for the location and depth of each cave. (Inset) The relationship between root biomass density in cave soils and the proportion of total N in cave soil relative to surface soil (r2 = 0.84; P = 0.03).
The ITS analysis described here is, to our knowledge, the first universal method for identifying plant roots. The primers used to amplify the ITS region are known to perform throughout the plant kingdom (42), and it is well established from phylogenetic studies that the ITS region reliably distinguishes among genera and, very often, among species (28). The widespread use of ITS for phylogenetic reconstructions also means that many of the species needed for a reference database may already be available in GenBank, saving researchers time and expense (though confirming ITS sequences locally is preferable). With modern PCR and DNA-sequencing technologies, roots can be identified from as little as 10 ng of DNA; a typical extraction from 0.1 g of tissue will often yield several hundred micrograms of DNA. Hence, fine roots from any soil core or profile in field or greenhouse experiments can be positively identified. Although other techniques, such as fluorescence of chemical abstracts (8), gas chromatography (9), and stable isotope analyses (10) of roots, have been used to distinguish species in isolated cases (e.g., species pairs), none of these approaches are either unequivocal or universal. Our technique should be broadly applicable in biological and agricultural studies.
The ability of some plants to grow into bedrock has been recognized for decades (43–46). Bedrock can hold up to 15% water by mass (47), and the uptake of this water has been proposed to help plants during drought (48, 49). This observation is certainly true in our system, as determined from the 18O data and the combination of low rainfall (<800 mm) and shallow surface soil (<20 cm) across the Edwards Plateau. We cannot yet determine how much water is taken up from particular depths for all species in the system. Future studies may address this question with long-term stable-isotope analyses of plant and soil water and with sap-flow studies that measure the total amount of water used by individual trees and the flow of water through their tap roots at specific depths.
There is no credible evidence that plants can “sense” underground resources before contacting them (i.e., roots do not grow preferentially toward water and nutrients, though they often proliferate after reaching them; refs. 14, 38, and 39). Thus, the deep-rooted plants in this system most likely explore cracks randomly throughout the bedrock of the Edwards Plateau, extracting the water the cracks hold. The environment in the caves we sampled may not be perfectly representative of such cracks. Rather the caves provide access to different depths underground and to the roots found there. The caves may also provide some plants with a source of additional nutrients and water, but the resources held in the ubiquitous cracks are probably much more important in this system. Resources in the cave soils do provide a clear example of the ability of plants to take advantage of locally abundant resources (37–40), whether those resources are present at the soil surface or are tens of meters underground.
As shown here, the presence of relatively deep roots and the ability of plants to use deep soil resources may be much more widespread than currently recognized. An increasing number of systems, both xeric and mesic, have been found with deep roots (e.g., forests in Australia, eastern Amazonia, and eastern North America; savannas of western North America and Africa; and the deserts of Africa, North America, and the Middle East; refs. 1, 12, and 13). The presence of such roots in many systems and their functional importance is overlooked in current theories of ecosystem functioning and in most models (e.g., land-surface parameterizations and also regional biogeography and gap models, in which species-based community information is appropriate). A relatively small biomass of roots at depth may be disproportionately important for the functioning of some ecosystems. For example, a study with winter wheat showed that 3% of the root system below 1 m supplied ≈20% of water uptake during a summer dry period (50). Another example is the potential importance of hydraulic lift, the passive movement of water through roots from relatively wet, deeper soil layers to relatively dry, shallower ones (51, 52). Although plants often compete for water, the presence of deep-rooted species may enhance ecosystem productivity by leaking water into surface layers where it is available to relatively shallow species such as grasses or forbs. Decomposition and nutrient diffusion may also be enhanced in surface layers by the extra water, further influencing system productivity. Data on community composition at depth should help our understanding of such processes.
Rooting depth influences a host of factors, including plant productivity, the pool of water available to plants, subsurface hydrology, ground-water recharge, and the seasonal exchange of material with the atmosphere (1–7, 15). In our system, that exchange extends more deeply into the ground than the plants are tall. With our ability to identify plant species underground, the application of this and other molecular tools to community and ecosystem questions, and the opportunity to study root functioning and physiology in situ, the global processes and key species that control the exchange of matter and energy can be understood more clearly.
Acknowledgments
We thank members of the Texas Speleological Survey, particularly T. Holsinger and J. Kennedy, for their help in locating caves and for providing depth surveys; the many private landowners who gave us access to their property; and L. J. Anderson, M. M. Caldwell, F. S. Chapin, R. E. Dickinson, A. H. Fitter, E. G. Jobbágy, W. H. Schlesinger, D. Tilman, and two anonymous reviewers for making helpful suggestions about the manuscript. This research was supported by a National Science Foundation Presidential Early Career Award in Science and Engineering, the Andrew W. Mellon Foundation, National Institute for Global Environmental Change/Department of Energy, and the U.S. Department of Agriculture.
ABBREVIATION
- ITS
internal transcribed spacer
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Nepstad D C, de Carvalho C R, Davidson E A, Jipp P H, Lefebvre P A, Negreiros G H, da Silva E D, Stone T A, Trumbore S E, Vieira S. Nature (London) 1994;372:666–669. [Google Scholar]
- 2.Brimhall G H, Chadwick O A, Lewis C J, Compston W, Williams I S, Danti K J, Dietrich W E, Power M E, Hendricks D, Brah J. Science. 1991;255:695–702. doi: 10.1126/science.255.5045.695. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M J, Rao I M, Ayarza M A, Lascano C E, Sanz J I, Thomas R J, Vera R R. Nature (London) 1994;371:236–238. [Google Scholar]
- 4.Greenwood E A N. Adv Bioclimatol. 1992;1:89–154. [Google Scholar]
- 5.Jackson R B, Mooney H A, Schulze E-D. Proc Natl Acad Sci USA. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrick R L, Pregitzer K S. Nature (London) 1993;361:59–61. [Google Scholar]
- 7.Webb R S, Rozenzweig C E, Levine E R. Global Biogeochem Cycles. 1993;7:97–108. [Google Scholar]
- 8.Caldwell M M, Richards J H, Manwaring J H, Eissenstat D M. Nature (London) 1987;327:615–616. [Google Scholar]
- 9.Lamont B B, Lange B J. New Phytol. 1976;76:353–360. [Google Scholar]
- 10.Polley H W, Johnson H B, Mayeux H S. Plant Soil. 1992;142:97–106. [Google Scholar]
- 11.Lewis D C, Burgy R H. J Geophys Res. 1964;69:2579–2588. [Google Scholar]
- 12.Stone E, Kalisz P J. For Ecol Manage. 1990;46:59–102. [Google Scholar]
- 13.Canadell J, Jackson R B, Ehleringer J R, Mooney H A, Sala O E, Schulze E-D. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- 14.Casper B B, Jackson R B. Annu Rev Ecol Syst. 1997;28:545–570. [Google Scholar]
- 15.Jackson R B. In: Integrating Hydrology, Ecosystem Dynamics, and Biogeochemistry in Complex Landscapes. Tenhunen J, Kabat P, editors. New York: Wiley; 1999. pp. 219–240. [Google Scholar]
- 16.Hodnett M G, da Silva L P, da Rocha H P, Cruz Senna R C. J Hydrol (Amsterdam) 1995;170:233–254. [Google Scholar]
- 17.Zeng X, Dai Y, Dickinson R E, Shaikh M. Geophys Res Lett. 1998;25:4533–4536. [Google Scholar]
- 18.Henderson-Sellers A. Global Planet Change. 1996;13:99–115. [Google Scholar]
- 19.Mahfouf J-F, Ciret C, Ducharne A, Irannejad P, Noilhan J, Shao Y, Thornton P, Xue Y, Yang Z-L. Global Planet Change. 1996;13:73–88. [Google Scholar]
- 20.Dunne K A, Willmott C J. Int J Climatol. 1996;16:841–859. [Google Scholar]
- 21.Douville H. Clim Dyn. 1998;14:151–171. [Google Scholar]
- 22.Kleidon A, Heimann M. Global Change Biol. 1998;4:275–286. [Google Scholar]
- 23.Elliot W R, Veni G, editors. The Caves and Karst of Texas. Huntsville, AL: Natl. Speleolog. Soc.; 1994. [Google Scholar]
- 24.Wermund E G, Cepeda J C, Luttrell P E. Regional Distribution of Fractures in the Southern Edwards Plateau and Their Relationship to Tectonics and Caves. Vol. 78. San Antonio, TX: Bureau Econ. Geol.; 1978. (2). [Google Scholar]
- 25.Ford D C, Williams P W. Karst Geomorphology and Hydrology. London: Unwin Hyman; 1989. p. 6. [Google Scholar]
- 26.Auken O W, Ford A L, Stein A, Stein A G. Tex J Sci. 1980;32:23–35. [Google Scholar]
- 27.Baldwin B. Mol Phylogenet Evol. 1992;1:3–16. doi: 10.1016/1055-7903(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin B G, Sanderson M J, Porter J M, Wojciechowski M F, Campbell C S, Donoghue M J. Ann Mo Bot Gard. 1995;82:247–277. [Google Scholar]
- 29.Schilling E E, Linder C R, Noyes R D, Rieseberg L H. Syst Bot. 1998;23:177–187. [Google Scholar]
- 30.Doyle J J, Doyle J L. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 31.Steenkamp J, Wiid I, Lourens A, van Helden P. Am J Enol Viti. 1994;45:102–106. [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. , Appendix B.5. [Google Scholar]
- 33.Thompson J D, Higgins D G, Gibson T J. Nucleic Acid Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Böhm W. Methods of Studying Root Systems. Berlin: Springer; 1979. [Google Scholar]
- 35.Gale M R, Grigal D F. Can J For Res. 1987;17:829–834. [Google Scholar]
- 36.Jackson R B, Canadell J, Ehleringer J R, Mooney H A, Sala O E, Schulze E-D. Oecologia. 1996;108:389–411. doi: 10.1007/BF00333714. [DOI] [PubMed] [Google Scholar]
- 37.Jackson R B, Manwaring J H, Caldwell M M. Nature (London) 1990;344:58–60. doi: 10.1038/344058a0. [DOI] [PubMed] [Google Scholar]
- 38.Hutchings M J, de Kroon H. Adv Ecol Res. 1994;25:159–238. [Google Scholar]
- 39.Fitter A H. In: Exploitation of Environmental Heterogeneity by Plants. Caldwell M M, Pearcy R W, editors. San Diego: Academic; 1994. pp. 305–323. [Google Scholar]
- 40.Robinson D. Ann Bot. 1996;77:179–185. [Google Scholar]
- 41.United States Geological Survey Annual Bulletins (1970–1998) (U.S. Geol. Surv., Water Resour. Div., San Antonio, TX).
- 42.White T J, Bruns T, Lee S, Taylor J. In: PCR Protocols: A Guide to Methods and Applications. Innis M A, Gelfand D H, Sninsky J J, White T J, editors. San Diego: Academic; 1990. pp. 315–324. [Google Scholar]
- 43.Cannon, W. A. (1911) Carnegie Inst. Washington Publ.131.
- 44.Zohary M, Orhansky G. Palest J Bot Jerusalem Ser. 1951;5:119–128. [Google Scholar]
- 45.Oppenheimer H R. Bull Res Counc Isr Sect D. 1956;5:219–222. [Google Scholar]
- 46.Zwieniecki M A, Newton M. Plant Soil. 1995;172:181–187. [Google Scholar]
- 47.Jones D P, Graham R C. Soil Sci Soc Am J. 1993;57:256–261. [Google Scholar]
- 48.Cooper, W. S. (1922) Carnegie Inst. Washington Publ.319.
- 49.Wang Z Q, Newton M, Tappeiner J C, II. For Sci. 1995;41:744–757. [Google Scholar]
- 50.Gregory P J, McGowan M, Biscoe P V. J Agric Sci. 1978;91:103–116. [Google Scholar]
- 51.Breazeale J F. Ariz Agric Exp Stn Tech Bull. 1930;29:137–177. [Google Scholar]
- 52.Caldwell M M, Dawson T E, Richards J H. Oecologia. 1998;113:151–161. doi: 10.1007/s004420050363. [DOI] [PubMed] [Google Scholar]