Abstract
Angiogenesis requires the deposition of type IV collagen by endothelial cells into the basement membrane of new blood vessels. Stabilization of type IV collagen triple helix depends on the hydroxylation of proline, which is catalyzed by the iron-containing enzyme prolyl hydroxylase. This enzyme, in turn, requires ascorbic acid to maintain the enzyme-bound iron in its reduced state. We hypothesized that dietary ascorbic acid might be required for tumor angiogenesis and, therefore, tumor growth. Here, we show that, not surprisingly, ascorbic acid is necessary for the synthesis of collagen type IV by human endothelial cells and for their effective migration and tube formation on a basement membrane matrix. Furthermore, ascorbic acid depletion in mice incapable of synthesizing ascorbic acid (Gulo-/-) dramatically restricts the in vivo growth of implanted Lewis lung carcinoma tumors. Histopathological analyses of these tumors reveal poorly formed blood vessels, extensive hemorrhagic foci, and decreased collagen and von Willebrand factor expression. Our data indicate that ascorbic acid plays an essential role in tumor angiogenesis and growth, and that restriction of ascorbic acid or pharmacological inhibition of prolyl hydroxylase may prove to be novel therapeutic approaches to the treatment of cancer.
Keywords: Ascorbic acid, tumor, angiogenesis, collagen, prolyl hydroxylase
Introduction
Ascorbic acid (vitamin C) is an essential dietary requirement in humans and other primates due to the genetic absence of a key enzyme in ascorbic acid synthesis, l-gulono-γ-lactone oxidase [1,2]. Humans deficient in ascorbic acid develop scurvy—a disease that came to prominence in the era of long sea voyages and was first systematically described by Lind [3] in 1753. Bleeding gums, hemorrhage, and ecchymoses, which are the major manifestations of this deficiency, are due to impaired collagen deposition leading to defective blood vessel formation and repair [4].
The clinical manifestations of scurvy reflect the crucial importance of ascorbic acid in angiogenesis and blood vessel repair. Angiogenesis, in particular, is a prerequisite for tumor growth in vivo. Tumors are unable to grow beyond ∼1 to 2 mm3 without the formation of new blood vessels, which will deliver metabolic substrates and oxygen [5]. Effective angiogenesis requires the deposition of mature type IV collagen in the basement membrane of blood vessels by endothelial cells [6]. Type IV collagen folding, in turn, is dependent on the hydroxylation of proline by the iron-dependent enzyme prolyl hydroxylase, which requires ascorbic acid to reduce iron to its ferrous state for the reactivation of the enzyme [7]. These considerations provide general support for the idea that depletion of ascorbic acid might limit tumor growth by impairing blood vessel formation and repair.
However, there are reasons to suppose that ascorbic acid deficiency might have the opposite effect on tumor growth. For example, ascorbic acid is also important in maintaining normal immune function and in augmenting host immunity against cancer [8–10]. Although early studies attributed ascorbic acid-dependent augmentation of tumor immunity to improvement in cytolytic functions of lymphocytes and phagocytes, the precise mechanisms have not been clearly defined [11,12]. However, in the particular case of lymphocyte function, more recent data suggest that ascorbic acid may improve host immunity by inhibiting T-cell apoptosis [13].
Ascorbic acid deficiency may also affect the expression of hypoxia-inducible factor-1α (HIF-1α) in such a way as to promote tumor growth. Neoplastic cells exposed to low oxygen respond by stabilizing HIF-1α, which acts to induce the transcription of several mRNA transcripts that are essential for tumor growth, including glycolytic enzymes and vascular endothelial growth factor (VEGF). Ascorbic acid is a cofactor for the proline hydroxylation and subsequent proteosomal degradation of HIF-1α. Depletion of ascorbic acid reportedly interrupts this degradation, causing high HIF-1α expression and upregulation of several protumorigenic transcriptional targets [14]. Accordingly, dietary ascorbic acid restriction might enhance tumor growth by allowing increased stabilization of HIF-1α and/or by impairing tumor immunity.
Thus, it is not clear whether ascorbic acid deficiency might increase or decrease tumor growth. A major impediment to testing the importance of ascorbic acid in tumor growth has been that mice, the most facile model for such studies, are able to synthesize ascorbic acid and do not require dietary vitamin C. Recently, mice bearing a homozygous deletion of the l-gulono-γ-lactone oxidase gene (Gulo-/-) have been generated; like humans, they require ascorbic acid supplementation to avoid manifestations of vitamin C deficiency [15]. Plasma ascorbic acid concentrations fall to approximately 10 µM (∼ 1.7 ± 0.2 µg/ml) in Gulo-/- mice kept on restricted dietary ascorbic acid for 3 to 5 weeks and, after ∼ 6 weeks of restriction, these animals demonstrate weight loss and hemarthroses [15].
Using this new animal model, we examined the requirement of dietary ascorbic acid for the growth of highly angiogenic Lewis lung carcinoma. Our results indicate that tumor growth is greatly diminished in vitamin C-deficient Gulo-/- mice. Tumors grown in these mice also show defective angiogenesis and intratumoral hemorrhagic foci. Contrary to what might be expected, however, there are no significant effects on HIF-1α expression by tumor cells. The marked suppression of tumor growth in these vitamin C-deficient mice may provide a rationale for clinical trials in humans to evaluate the effects of dietary ascorbic acid restriction on cancer progression.
Materials and Methods
Cell Lines and Culture Conditions
Human umbilical vein endothelial cells (HUVECs) were obtained from Cambrex (Walkersville, MD) and were grown in culture with basal medium (EGM; Cambrex) supplemented with bovine brain extract, human endothelial cell growth factor, hydrocortisone, gentamicin, amphotericin B, and 2% fetal bovine serum (FBS) (EGM BulletKit; Cambrex). HUVECs used for experiments were between six and eight passages. Lewis lung carcinoma cells were obtained from ATCC (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (HyClone Laboratories, Logan, UT) and gentamicin (Invitrogen). All cell lines were grown at 37°C in 5% CO2. For some experiments, 0 to 200 µM l-ascorbic acid (Sigma, St. Louis, MO) was used to supplement the growth medium. Lewis lung carcinoma cells were exposed to hypoxia by incubation for 16 hours in 1% oxygen balanced with nitrogen and 5% CO2 in a modular incubator chamber (Billups-Rothenburg, Del Mar, CA).
Collagen Detection by Enzyme-Linked Immunosorbent Assay (ELISA)
HUVECs were plated at a density of 4 x 103 cells/well on 96-well plates (Costar Corning, Corning, NY) in EGM containing 0 to 200 µM l-ascorbic acid (Sigma). For these ELISA determinations, human placental collagen type IV (Rockland Immunochemicals, Gilbertsville, PA) was used as a positive control. After 1 to 10 days in culture, HUVECs were detached with 0.25% trypsin-EDTA (Invitrogen), and the plates were washed with Tris-buffered saline (Tris/NaCl; 50 mM Tris, 150 mM NaCl, pH 8.5). The wells were then blocked with 1% bovine serum albumin (Sigma) in phosphate-buffered saline (PBS) for 1 hour at 37°C and washed with Tris/NaCl. The plates were incubated with rabbit anti-human type IV collagen (1:4000; Rockland Immunochemicals) for 2 hours at 37°C, washed thrice with Tris/NaCl/0.05% Tween-20, and then incubated with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin (1:8000; Rockland Immunochemicals) for 1 hour. After three washes with Tris/NaCl/Tween-20, color development in the presence of 2,2′-azino-bis [3-ethylbenzthiazoline-6-sulfonic acid] (ABTS; Rockland Immunochemicals) was read with a spectrophotometer at 420 nm. The assay was performed in quadruplicate and was repeated twice.
Endothelial Tube Formation Assay
Twenty-four-well plates (Costar Corning) were coated with Matrigel basement membrane matrix (250 µl/well; BD Biosciences, Bedford, MA) according to the manufacturer's instructions and allowed to polymerize at 37°C for 60 minutes. HUVECs grown in culture were detached, resuspended in EGM, and seeded on Matrigel-coated wells (8 x 104 well-1) in 0 to 200 µM l-ascorbic acid. The plates were incubated at 37°C in 5% CO2 and examined for tube formation through an inverted photomicroscope every 6 hours for 18 hours. At 18 hours, intact tubes formed were enumerated in three random fields, and representative photomicrographs were taken (original magnification, x40). The assay was performed in triplicate and repeated twice.
In Vivo Experiments
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee at the University of Louisville (Louisville, KY). Gulo+/- heterozygous breeding pairs (C57Bl6/J mice B6.129P2-Gulotm1Unc/Ucd) were obtained from the Mutant Mouse Regional Resource Center, and homozygous (Gulo-/-) mice were bred. Genotyping was performed using tail biopsy specimens with the REDExtract-N-Amp Tissue PCR Kit (Sigma) and primers as previously described [15]. All mice used in the experiments were < 3 months of age.
Mice were weaned at 3 weeks of age onto irradiated mouse chow (PicoLab Mouse Diet 5058; PMI Feeds, St. Louis, MO) containing 110 mg/kg ascorbic acid and were maintained on this diet during all experiments. Because this amount is not sufficient to maintain a physiological concentration of vitamin C in Gulo-/- animals [15], ascorbic acid was supplemented in drinking water (330 mg/l l-ascorbic acid + 0.01 mM EDTA), which was changed weekly.
For initial outgrowth experiments, Gulo-/- mice were depleted of ascorbic acid by withholding supplementation in drinking water for 4 weeks immediately after weaning and then were randomized to continued depletion or repletion by supplementation with 330 mg/l ascorbic acid in drinking water. A third group of Gulo-/- mice was maintained on full supplementation with 330 mg/l ascorbic acid before and after tumor implantation. Each group contained 10 animals.
For studying the growth of established tumors, the Gulo-/- mice used were maintained on 165 mg/l ascorbic acid supplementation in water for 2 weeks before starting the study. They were then randomized either to depletion (by withholding supplementation) or to continued supplementation at 165 mg/l. Each group contained 10 animals.
For experiments on minimal supplementation, Gulo-/- mice were depleted by withholding ascorbic acid supplementation for 4 weeks immediately after weaning and then were randomized to either continued depletion, 10% of full supplementation (33 mg/l ascorbic acid), or full supplementation (330 mg/l ascorbic acid). Each group contained 10 animals.
Lewis lung carcinomacells were collected fromexponential growth phase culture, washed twice, and then resuspended in PBS (1 x 107 cells/ml). Groups of Gulo-/- mice were injected subcutaneously in the right flank with 0.1 ml of cell suspension (1 x 106 cells). Tumor measurements were started with the appearance of established tumors measuring 150 mg (between 5 and 7 days after implantation). After the initial appearance, tumors were measured with microcalipers every 2 days, and tumor masses were calculated using the formula: [(width in mm)2 x (length in mm)] / 2 [16]. When tumor volume had reached 10% of body weight, animals were euthanized using carbon dioxide narcosis and tumors were removed and fixed in 10% formaldehyde for further analyses.
All data are expressed as the mean ± SD of three experiments. Statistical significance was assessed by two-sample t-test (independent variable).
Ascorbic Acid Measurements
Ascorbic acid concentrations in plasma samples were measured by the 2,2′-dipyridyl method [17].
Protein Extraction and Western Blot Analysis
Cells were treated with 0.25% trypsin-EDTA, washed in PBS, and lysed in 2x RIPA buffer. Protein samples were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. Membranes were blocked in TBS-Tween-20 (1%) containing 5% milk. Mouse monoclonal anti-HIF-1 antibody (1:500; Novus Biologicals, Littleton, CO) and mouse anti-β-actin (1:5000; Sigma) were resuspended in 10 ml of TBS-Tween-20 (5% milk) and incubated with the membrane for 1 hour. The secondary antibodies used were rabbit anti-mouse or goat anti-mouse HRP-conjugated (1:8000; Pierce Biotechnology, Rockford, IL).
Histology and Immunohistochemistry
Five-micrometer sections of formalin-fixed and paraffin-embedded tumor tissues were treated with xylene to remove paraffin and then rehydrated. Hematoxylin-eosin staining and Masson's trichrome staining for collagen were performed using standard procedures. For immunohistochemical staining, deparaffinized and rehydrated sections were blocked by incubation with serum blocking buffer for 30 minutes at room temperature. Tissue sections were incubated for 1 hour with rabbit anti-mouse von Willebrand factor (vWF) antibody (1:500; Dakocytomation, Carpinteria, CA) or mouse monoclonal anti-HIF-1α (1:250; Novus Biologicals) for the detection of vWF and HIF-1α, respectively. The sections were then incubated with biotinylated goat anti-rabbit (for vWF) or rabbit anti-mouse IgG (for HIF-1α) for 30 minutes (1:500; Vector Laboratories, Burlingame, CA) and developed with an avidin-biotin peroxidase reaction using 3,3′-diaminobenzidine tetrahydrochloride as chromogen. After counterstaining with Mayer's hematoxylin (Sigma), the sections were dehydrated and coverslips were attached with Permount (Fisher Scientific, Pittsburgh, PA). Appropriate negative controls (by omission of the primary antibody) were used. Anti-HIF-1α antibody functionality and specificity were confirmed by Western blot analysis using Lewis lung carcinoma cells exposed to hypoxia for 16 hours as positive control.
Quantitative immunohistochemistry imaging analyses were performed using TIFFalyzer on images acquired by a digital RGB camera (DXM 1200F; Nikon, Tokyo, Japan) and saved in TIFF format [18,19]. All data are expressed as the mean ± SD of three experiments. Statistical significance was assessed by two-sample t-test (independent variable).
Results
Type IV Collagen Secretion By Endothelial Cells Requires Ascorbic Acid
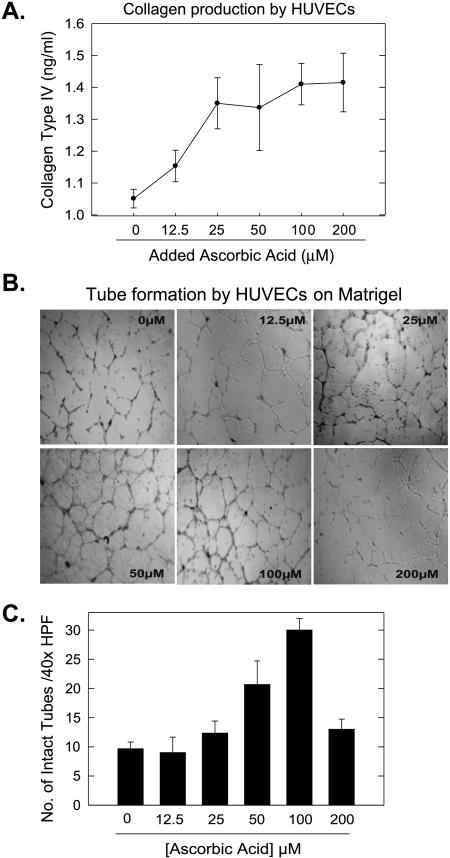
To test whether type IV collagen secretion by endothelial cells requires ascorbic acid, we pulsed cultured HUVECs with 0 to 200 µM ascorbic acid and then examined the plates for the presence of immunoreactive type IV collagen using an ultrasensitive ELISA. We found that HUVECs produced type IV collagen in the absence of added ascorbic acid, but that the addition of ascorbic acid increased type IV collagen secretion. The culture medium (EGM) contains approximately 5 µM ascorbic acid, which may be sufficient to allow for the observed type IV collagen secretion in the absence of added vitamin C. The concentration of added ascorbic acid that maximized collagen type IV accumulation (25 µM) is approximately 50% below the physiological concentration of ascorbic acid in healthy humans and mice (Figure 1A). Finally, we should note that the collagen detected by this ELISA was not necessarily stabilized type IV collagen triple helix.
Figure 1.
Immunoreactive type IV collagen and intact tube formation by endothelial cells require the presence of a physiological concentration of ascorbic acid. Type IV collagen production by HUVECs in 0 to 200 µM ascorbic acid was measured by ELISA. (A) Representative photomicrographs of tube formation on Matrigel basement membrane matrix in 0 to 200 µM ascorbic acid (original magnification, x40). (B) Intact tubes formed on Matrigel in 0 to 200 µM ascorbic acid were enumerated (original magnification, x40) (C).
Endothelial Cell Migration and Tube Formation Require Ascorbic Acid
Deposition of mature collagen type IV is essential for endothelial cell migration and forms a scaffold for new blood vessel formation. The importance of collagen type IV in angiogenesis is further emphasized by evidence that inhibitors of proline hydroxylation impair angiogenesis in vitro [20]. Migration and tube formation on the basement membrane matrix mimic blood vessel formation, and we speculated that restriction of ascorbic acid would limit migration and tube formation. We cultured HUVECs on Matrigel in EGM containing 0 to 200 µM added ascorbic acid and examined the cells for tube formation by light microscopy (Figure 1B). Alignment of HUVECs into tube-like structures began within 2 to 4 hours and was completed by 18 hours. Very few intact tubes were formed in 0 to 12.5 µM added ascorbic acid (despite the estimated 5-µM background ascorbic acid in the medium). As the concentration of ascorbic acid was increased, greater numbers of intact tubes were observed, reaching a maximum at 50 to 100 µM (23 ± 1.5 and 27.6 ± 2 tubes, respectively, per x40 high-power field). Interestingly and for unknown reasons, we observed decreased numbers of intact tubes at supraphysiologic concentrations of ascorbic acid (Figure 1C).
Restriction of Dietary Ascorbic Acid Depletes Plasma Ascorbic Acid in Gulo-/- Mice
Restriction of vitamin C in the diet of Gulo-/- mice has previously been demonstrated to deplete plasma ascorbic acid [15]. We sought to confirm this finding in our animals before embarking on experiments with tumor growth. We measured plasma ascorbic acid concentrations in Gulo-/- mice before limiting dietary ascorbic acid and at weekly intervals after starting restriction until the animals were euthanized. We found a gradual decrease in the concentration of plasma ascorbic acid over the 4 weeks of restriction from a baseline concentration of 7.35 ± 0.6 µg/ml (approximately 42 µM) to 0.73 ± 0.1 µg/ml by 4 weeks when the animals were fed only a diet containing 110 mg/kg ascorbic acid without further supplementation. We observed only a small further decline in the concentration of plasma ascorbic acid in restricted Gulo-/- mice over the course of tumor growth (0.47 ± 0.12 µg/ml by 6 weeks) (Figure 2A).
Figure 2.
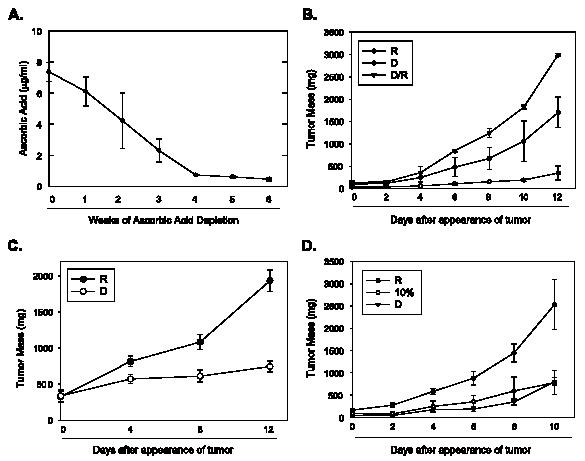
Tumor growth is significantly reduced in Gulo-/- mice on restricted ascorbic acid. Plasma ascorbic acid concentrations in Gulo-/- mice were measured before and at weekly intervals following depletion of ascorbic acid and during tumor growth for 6 weeks (n = 7 in all groups). (A) Lewis lung carcinoma cells were injected subcutaneously into Gulo-/- mice depleted of ascorbic acid for 4 weeks following which animals were either continued on a depleted diet (D) or a replete diet (D/R). A control group of Gulo-/- mice was fully supplemented with ascorbic acid before and after injection with Lewis lung carcinoma cells (R). Tumors were measured with microcalipers on indicated days (n = 10 per group). (B) Gulo-/- mice implanted with Lewis lung carcinoma cells were randomized to ascorbic acid depletion (D) or full repletion (R) when tumors were visible, and tumors were measured with microcalipers on indicated days (n = 10 per group). (C) Gulo-/- mice depleted of ascorbic acid were implanted with Lewis lung carcinoma cells and, when tumors were palpable, were randomized to depletion (D), 10% repletion (10%), or 100% (R) repletion, and tumors were followed for 10 days (n = 10 per group) (D).
Restriction of Dietary Ascorbic Acid Suppresses Tumor Growth in Gulo-/- Mice In Vivo
Our findings that endothelial cell migration and tube formation require the presence of physiological concentrations of ascorbic acid supported the generally accepted idea that vitamin C is required for effective blood vessel formation in vivo. To determine whether suppression of angiogenesis by a scorbutic state would limit tumor growth, we used the highly angiogenic tumor Lewis lung carcinoma in Gulo-/- mice. Before starting our experiments, we first sought to examine the survival and proliferation of Lewis lung carcinoma cells in vitro in the absence of added ascorbic acid in the medium. We found that the cells survived and demonstrated no significant differences in growth from cells cultured in the presence of added ascorbic acid (data not shown).
To study the effect of ascorbic acid restriction on initial tumor outgrowth, animals were depleted of ascorbate for 4 weeks and then implanted subcutaneously with Lewis lung carcinoma cells. After implantation, depleted animals were either: 1) continued on a depleted diet (D), or 2) made replete with ascorbic acid (D/R). We also included a group of Gulo-/- animals maintained on full supplementation before and after tumor implantation (R) (n = 10 in all groups). We followed tumor volumes from appearance until the point at which tumor burden necessitated euthanization. At 12 days of growth after tumor appearance, tumors in the depleted animals were significantly smaller than either in depleted/replete groups or in fully supplemented groups (D, 332.4 ± 67.3 mm3; D/R, 2992.6 ± 248 mm3; R, 1703 ± 316.4 mm3; P < .005 in D versus either D/R or R at 12 days; n = 10 for all groups) (Figure 2B). Interestingly, tumors in the depleted/replete mice were significantly larger than those in animals maintained on ascorbic acid before and after tumor implantation.
We then examined the effects of ascorbic acid depletion on the growth of previously established tumors. For this, we implanted Lewis lung carcinoma cells in Gulo-/- animals maintained on 165 mg/l ascorbic acid in drinking water for 2 weeks before the start of the study. When tumors were palpable, we randomized animals to ascorbic acid depletion (D) or continued ascorbic acid supplementation of 165 mg/l (R) (n = 10 in both groups). At 12 days of growth, we found that tumors in the depleted group were significantly smaller than those in the replete group (D, 744.3 ± 198.4 mm3; R, 1934.7 ± 384 mm3; P < .005; n = 10) (Figure 2C).
As observed in previous studies, depletion of ascorbic acid beyond approximately 6 weeks in homozygous Gulo-/- mice led to weight loss and decreased activity with the onset of hemarthroses and petechial bleeding. We therefore sought to test whether minimal supplementation of ascorbic acid would delay the onset of scorbutic symptoms while still retarding tumor growth. Following depletion of ascorbic acid for 4 weeks in Gulo-/- mice, we randomized animals to either continued depletion (D), 10% of the full supplementation dose (33 mg/l ascorbic acid) (10%), or full supplementation (330 mg/l ascorbic acid) (R), and implanted Lewis lung carcinoma cells subcutaneously in all the animals. We followed tumor growth from the time of appearance up to 10 days of growth and found that tumors in the D group and in the 10% supplemented group were both significantly smaller than those in the fully supplemented group (D, 800 ± 92.9 mm3; 10% of full supplementation, 776 ± 270 mm3; R, 2529 ± 562 mm3; P < .05 for D and 10% of full supplementation versus R; n = 10) (Figure 2D). Importantly, the mice on the 10% ascorbic acid diet did not exhibit weight loss or bleeding (data not shown).
Tumors from Animals on Low-Ascorbic-Acid Diets Are Hemorrhagic and Show Decreased Capillary Density
We speculated that the differences we observed in tumor size between ascorbic acid-restricted and supplemented animals were due to inadequate delivery of essential nutrients as a result of poor blood vessel formation. To examine this, we first stained sections of representative tumors depleted and replete mice with Masson's trichrome to determine the character of the tumor vasculature. The vasculature in tumors excised from the depleted/replete (D/R) group was well defined (Figure 3, A and C). In contrast, tumors in depleted animals showed multiple areas of hemorrhage and poorly formed blood vessels (Figure 3, B and D). Furthermore, the absolute number of blood vessels was markedly reduced in depleted tumors in comparison with that in tumors from fully supplemented animals (blood vessels per x100 high-power field: D, 5 ± 2; D/R, 18.6 ± 3; R, 25 ± 5; P < .005).
Figure 3.
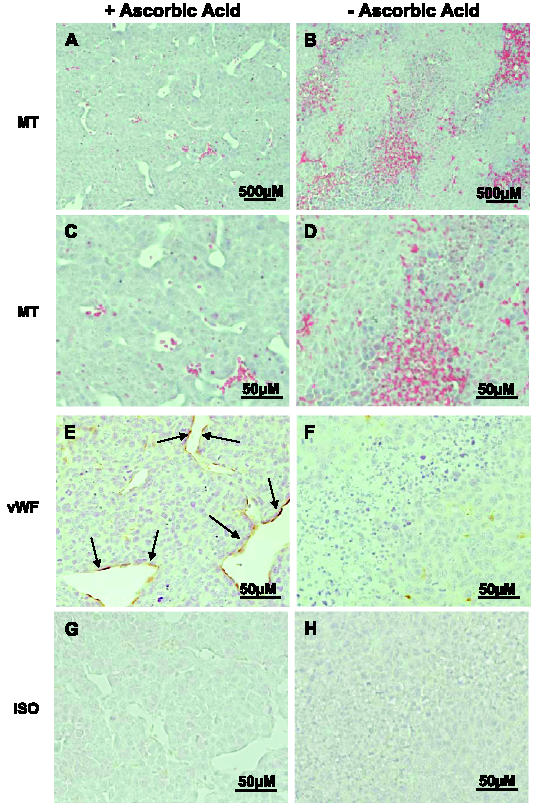
Tumors in ascorbic acid-restricted Gulo-/- mice exhibit poorly formed vasculature with multiple hemorrhagic foci. Tissue sections from representative tumors in replete (A, C, E, and G) and depleted (B, D, F, and H) Gulo-/- mice were examined for vasculature after staining with Masson's trichrome (MT) stain (A and C), anti-vWF polyclonal antibody (vWF; E and F), or preimmune rabbit serum (ISO; G and H). Endothelial cells stained with α-vWF are in brown and shown by arrows (A and B: original magnification, x200; C–H: original magnification, x400).
To further evaluate blood vessel development and to enumerate capillaries, we stained tumors from depleted and replete animals with an antibody specific for vWF—a marker of mature endothelial cells [21,22]. We observed increased vWF staining indicative of endothelial cells in tumor sections from the depleted/replete group of mice (Figure 3E) in comparison to minimal staining in depleted tumors (Figure 3F).
Ascorbic Acid Restriction Limits Collagen Synthesis in Tumors
Because ascorbic acid is required for the action of prolyl hydroxylase and, hence, collagen stabilization, we expected that collagen staining in tumors excised from mice on restricted ascorbic acid would be decreased. We stained representative tissue sections from all groups with Masson's trichrome and found decreased collagen staining in the fibrotic sheath of the depleted group in comparison with that in the replete group (Figure 4).
Figure 4.
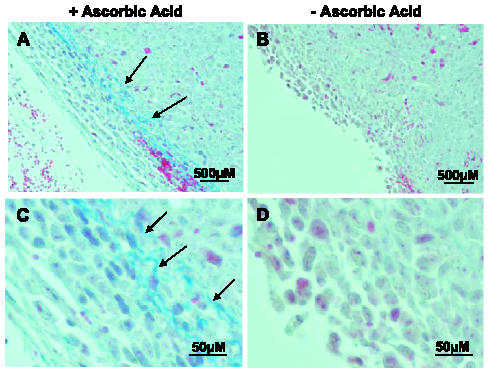
Collagen synthesis is decreased in subcutaneous tumors formed in Gulo-/- mice on an ascorbic acid-restricted diet. Sections from representative tumors harvested from replete (A and C) and depleted (B and D) Gulo-/- mice were evaluated for collagen after staining with Masson's trichrome. Collagen appears as blue-stained fibrillar material indicated by arrows (A and B: original magnification, x200; C and D: original magnification, x400).
Ascorbic Acid Restriction Does Not Affect HIF-1α Protein Expression
As mentioned in the Introduction, ascorbic acid acts as a cofactor in HIF proline hydroxylation by prolyl hydroxylase, which promotes proteosomal destruction of HIF. In vitro studies have demonstrated that physiological concentrations of ascorbic acid suppress HIF-1α levels in transformed cells [14]. Extrapolating from these results, it would be possible that lack of HIF hydroxylation (secondary to ascorbic acid deficiency) might slow its proteosomal destruction and increase its intracellular levels. However, staining of tissue sections of tumors with an anti-HIF-1α antibody did not show differences in HIF-1α expression. The function and specificity of this antibody were confirmed by a band at 120 kDa by Western blot analysis in Lewis lung carcinoma cells exposed to hypoxia (data not shown). In addition, quantitative immunohistochemical analyses of tumor sections in depleted and replete groups, in comparison with isotype control sections, did not reveal a significant difference in HIF-1α expression (HIF-1α in replete tumors, 490 ± 118.0 vs 487 ± 28.3 energy units/pixel in ascorbic acid-depleted tumors). Therefore, at least in the in vivo environment, ascorbic acid depletion may selectively suppress tumor blood vessel formation/stability without affecting HIF levels (Figure 5).
Figure 5.
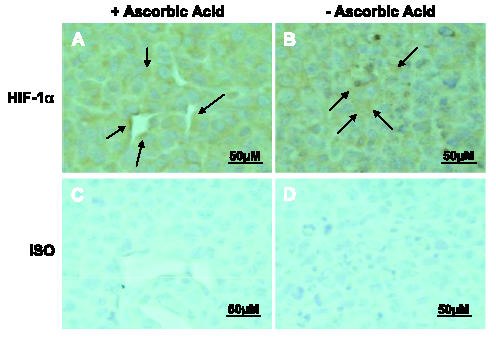
HIF-1α protein expression is similar in tumors from replete Gulo-/- animals and tumors from depleted Gulo-/- animals. Tissue sections from representative tumors in replete (A and C) and depleted (B and D) Gulo-/- mice were examined by immunohistochemistry using an anti-HIF-1α mouse monoclonal antibody (A and B) or preimmune mouse serum (C and D) (original magnification, x400). Arrows indicate representative areas of HIF-1α expression.
Discussion
The importance of vitamin C in carcinogenesis and tumor growth has been the subject of controversy for years. Early observational clinical studies demonstrated a 10% regression rate [23] and up to a 20-fold increase in survival in patients with advanced cancer treated with high-dose ascorbic acid (10 g/day intravenously for 10 days, followed by oral administration of the same dose) in comparison to controls retrospectively analyzed from a population of similar terminal cancer patients [24,25]. The results of these studies were widely disputed, in part due to lack of randomization. Two subsequent randomized placebo-controlled double-blinded trials using oral vitamin C [26,27] demonstrated no improvement in survival in patients treated with high doses of ascorbic acid. Perhaps as a result, the use of megadoses of vitamin C as a possible therapy for cancer fell by the wayside and has not been revisited until recently [28,29].
The present work was based on precisely the opposite hypothesis: that restriction of the availability of ascorbic acid would suppress tumor growth, perhaps through diminishing angiogenesis. Ascorbic acid plays an essential role in angiogenesis as a cofactor for prolyl hydroxylase in catalyzing hydroxyproline synthesis [7]. Hydroxyproline is required for effective type IV collagen production by endothelial cells. Collagen type IV forms a critical part of the vascular basement membrane and is vital for new blood vessel formation.
Tumor growth and metastasis are dependent on angiogenesis. One early study demonstrated that tumor growth in the avascular anterior chamber of the rabbit eye was arrested at a small size (∼ 1 mm3) due to the absence of neovascularization, whereas tumors grew readily on the surface of the iris where new vasculature could develop [30]. Tumors secrete angiogenic growth factors such as VEGF, which promote endothelial cell proliferation and new blood vessel development [31]. That anti-VEGF antibodies and a dominant interfering form of the VEGF receptor 2 (flk-1) have proven to be successful in impairing neovascularization and in causing tumor regression demonstrates the importance of angiogenesis in tumor growth [32,33].
An early study on the importance of ascorbic acid in angiogenesis was carried out in guinea pigs, which, like primates and Gulo-/- mice, are incapable of vitamin C synthesis. In subcutaneously implanted polyvinyl sponges (which promote angiogenic activity), investigators found dilated blood vessels, interstitial hemorrhage, and disintegration of connective tissues in scorbutic guinea pigs [34,35]. A later investigation demonstrated decreased type IV collagen mRNA in blood vessels in the presence of restricted ascorbic acid [35]. In addition, inhibitors of prolyl hydroxylase (e.g., ciclopirox and 2,2′-dipyridyl) cause inhibition of both tube formation on Matrigel and aortic arch sprouting in vitro [20]. Similarly, proline analogs such as cis-hydroxyproline, which impair a helix formation and collagen secretion, lead to the formation of large and dilated blood vessels in a plasma clot culture model [36] and inhibit angiogenesis in chick chorioallantoic membranes [37]. These earlier observations are in accord with our in vitro data from cultured HUVECs showing a requirement for ascorbic acid for both type IV collagen synthesis and endothelial tube formation on a basement membrane matrix. Our data indicate that although tube formation is maximal at concentrations of ascorbic acid that correspond to physiological concentrations, supraphysiologic concentrations appear to retard endothelial cell migration and tube formation. In contrast, type IV collagen was not affected by supraphysiologic ascorbic acid.
Our experiments in homozygous Gulo-/- mice indicate that dietary ascorbic acid restriction in these mice retards both initial tumor outgrowth and the growth of previously established tumors. Histopathological data from tumors in ascorbic acid-restricted mice demonstrate a paucity of blood vessels (from both microscopic examination and staining for vWF) and areas of hemorrhage. In contrast, tumors grown in mice supplemented with ascorbic acid have numerous and well-defined blood vessels and few areas of hemorrhage. Collagen staining of tumors in ascorbic acid-deficient and ascorbic acid-replete mice also suggests that collagen synthesis in these tumors is affected by dietary restriction of vitamin C. The possibility of using ascorbic acid restriction clinically is supported by our observation that restricting ascorbic acid to 10% of the full supplementation dose delays the onset of scorbutic symptoms in Gulo-/- mice while still significantly retarding tumor growth. Interestingly, we observed a trend toward increased tumor growth in mice that had been depleted of and then made replete with ascorbic acid. We postulate that host support for tumor growth may be increased secondary to overcompensation after ascorbic acid depletion. For example, tumor-associated endothelial cells may have upregulated surface transporters in response to low extracellular ascorbic acid, and this overexpression may confer the capacity for increased angiogenesis. However, the precise mechanism(s) for this observation is not obvious, and we intend to examine this phenomenon in a future study.
This is, by no means, the first attempt to modify tumor growth through depletion of ascorbic acid. One early study reported the effects of ascorbic acid depletion on the growth and biology of sarcomas initiated by 20-methylcholanthrene in guinea pigs [38]. These investigators found that animals given high doses of ascorbic acid showed accelerated tumor growth, whereas tumors grew slowly (or even regressed) in guinea pigs fed a scorbutic diet. However, other studies in guinea pigs have provided conflicting results regarding the requirements for ascorbic acid in collagen synthesis [35,39], making interpretation of the effects of ascorbic acid depletion on tumor growth in guinea pigs difficult.
A recent study by Parsons et al. [40] evaluated tumor growth in a mammary tumor model in Gulo-/- mice bred in a Balb/c background. In these experiments, mice were ascorbic acid-depleted for 2 weeks before tumor implantation and did not show any differences in tumor growth in comparison with control animals on full ascorbic acid supplementation. We cannot, with certainty, explain why these results are so divergent from those reported here, but differences in tumor type and/or regimen used to deplete ascorbic acid might be involved. In our experiments, we restricted dietary ascorbic acid for 4 weeks so that the serum ascorbic acid concentration decreased to approximately five-fold below the normal physiological range. Furthermore, we chose to study Lewis lung carcinoma because this tumor relies heavily on angiogenesis [41,42]. Finally, strain differences in mice used in each study may also have affected the outcomes reported.
Tumors have been found to have an unusual metabolism of ascorbic acid, actively concentrating ascorbic acid relative to surrounding normal fluids and tissues [43,44]. This occurs across a concentration gradient, and uptake appears to involve the oxidized form dehydroascorbic acid, which enters through facilitative glucose transporters (GLUTs) [45]. In certain malignant cell types (e.g., melanoma), dehydroascorbic acid uptake may be up to 10 times greater than that exhibited by normal melanocytes [44]. However, despite evidence demonstrating that ascorbic acid is concentrated by cancer cells, the precise function performed by vitamin C in tumor cells is far from clear. In U937 lymphoma cells, ascorbic acid was reported to decrease Fas pathway-mediated apoptosis [46], and similar findings have been made on apoptosis engendered by the NFκB pathway in several other tumor cell lines [47]. Furthermore, pretreatment of cells with the readily transported form of ascorbic acid, dehydroascorbic acid, has been shown to decrease radiation-induced apoptosis in HL60 promyelocytic leukemia cells [48]. These data indicate that ascorbic acid, in addition to supporting angiogenic activities, may also promote tumor cell survival and protect against oxidative stress.
Ascorbic acid depletion may lead to stabilization of HIF-1α and thus contribute to tumor growth through factors downstream of HIF such as VEGF and GLUT-1, thus increasing angiogenesis and glycolysis [14]. Surprisingly, our immunohistochemical analyses of tumors demonstrated that HIF-1α expression was not significantly altered by the absence of ascorbic acid. These data indicate that ascorbic acid depletion may preferentially affect collagen formation—and, thus, new vessel formation—without affecting HIF-1α stability.
Our results, although obtained with a single mouse model and a single type of implantable tumor, support the idea that ascorbic acid depletion powerfully restricts tumor growth in vivo, likely through interference with angiogenesis. Recent reports continue to advocate an increase in ascorbic acid intake over the recommended daily allowance for optimal reduction of the risk of cancer and chronic disease [49]. At the least, our results suggest that ascorbic acid supplementation in patients with cancer should be reevaluated. Additionally, we believe that decreasing dietary ascorbic acid or disrupting prolyl hydroxylases may provide novel therapeutic approaches to the treatment of cancer.
Acknowledgements
We gratefully acknowledge helpful discussions with Richard Bucala and Otto Grubraw.
Abbreviations
- HIF-1α
hypoxia-inducible factor-1α
- HUVECs
human umbilical vein endothelial cells
- VEGF
vascular endothelial growth factor
- Gulo
l-gulono-γ-lactone oxidase gene
References
- 1.Chatterjee I. Biosynthesis of ascorbic acid in animals. Methods Enzymol. 1970;18A:28–34. [Google Scholar]
- 2.Grollman AP, Lehninger AL. Enzymic synthesis of l-ascorbic acid in different animal species. Arch Biochem Biophys. 1957;69:458–467. doi: 10.1016/0003-9861(57)90510-6. [DOI] [PubMed] [Google Scholar]
- 3.Lind J. A Treatise on the Scurvy. 1753. [DOI] [PMC free article] [PubMed]
- 4.Hodges RE, Hood J, Canham JE, Sauberlich HE, Baker EM. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432–443. doi: 10.1093/ajcn/24.4.432. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 6.Bonanno E, Iurlaro M, Madri JA, Nicosia RF. Type IV collagen modulates angiogenesis and neovessel survival in the rat aorta model. In Vitro Cell Dev Biol Anim. 2000;36:336–340. doi: 10.1290/1071-2690(2000)036<0336:TICMAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Myllylä Raili E-RK-SaKIK. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978;83:441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- 8.De la Fuente M, Victor VM. Ascorbic acid and N-acetylcysteine improve in vitro the function of lymphocytes from mice with endotoxin-induced oxidative stress. Free Radic Res. 2001;35:73–84. doi: 10.1080/10715760100300611. [DOI] [PubMed] [Google Scholar]
- 9.Jeng KC, Yang CS, Siu WY, Tsai YS, Liao WJ, Kuo JS. Supplementation with vitamins C and E enhances cytokine production by peripheral blood mononuclear cells in healthy adults. Am J Clin Nutr. 1996;64:960–965. doi: 10.1093/ajcn/64.6.960. [DOI] [PubMed] [Google Scholar]
- 10.Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003;2:7. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron E, Pauling L. The orthomolecular treatment of cancer: I. The role of ascorbic acid in host resistance. Chem Biol Interact. 1974;9:273–283. doi: 10.1016/0009-2797(74)90018-0. [DOI] [PubMed] [Google Scholar]
- 12.Cameron E, Pauling L, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979;39:663–681. [PubMed] [Google Scholar]
- 13.Campbell JD, Cole M, Bunditrutavorn B, Vella AT. Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis. Cell Immunol. 1999;194:1–5. doi: 10.1006/cimm.1999.1485. [DOI] [PubMed] [Google Scholar]
- 14.Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- 15.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000;97:841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taetle R, Rosen F, Abramson I, Venditti J, Howell S. Use of nude mouse xenografts as preclinical drug screens: in vivo activity of established chemotherapeutic agents against melanoma and ovarian carcinoma xenografts. Cancer Treat Rep. 1987;71:297–304. [PubMed] [Google Scholar]
- 17.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 18.Matkowskyj KA, Cox R, Jensen RT, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem. 2003;51:205–214. doi: 10.1177/002215540305100209. [DOI] [PubMed] [Google Scholar]
- 19.Matkowskyj KA, Schonfeld D, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software Photoshop and MATLAB. J Histochem Cytochem. 2000;48:303–312. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]
- 20.Clement PM, Hanauske-Abel HM, Wolff EC, Kleinman HK, Park MH. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer. 2002;100:491–498. doi: 10.1002/ijc.10515. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe EA, Hoyer LW, Nachman RL. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973;52:2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadler JE. von Willebrand factor. J Biol Chem. 1991;266:22777–22780. [PubMed] [Google Scholar]
- 23.Cameron E, Campbell A. The orthomolecular treatment of cancer: II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974;9:285–315. doi: 10.1016/0009-2797(74)90019-2. [DOI] [PubMed] [Google Scholar]
- 24.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978;75:4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creagan ET, Moertel CG, O'Fallon JR, Schutt AJ, O'Connell MJ, Rubin J, Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301:687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 27.Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 28.Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr. 2000;19:423–425. doi: 10.1080/07315724.2000.10718941. [DOI] [PubMed] [Google Scholar]
- 29.Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. CMAJ. 2001;164:353–355. [PMC free article] [PubMed] [Google Scholar]
- 30.Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 32.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 33.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 34.Stolman JMGHM, Gould BS. Ascorbic acid and blood vessels. Arch Pathol. 1961;72:59–69. [PubMed] [Google Scholar]
- 35.Mahmoodian F, Peterkofsky B. Vitamin C deficiency in guinea pigs differentially affects the expression of type IV collagen, laminin, and elastin in blood vessels. J Nutr. 1999;129:83–91. doi: 10.1093/jn/129.1.83. [DOI] [PubMed] [Google Scholar]
- 36.Nicosia RF, Belser P, Bonanno E, Diven J. Regulation of angiogenesis in vitro by collagen metabolism. In Vitro Cell Dev Biol. 1991;27A:961–966. doi: 10.1007/BF02631124. [DOI] [PubMed] [Google Scholar]
- 37.Ingber D, Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988;59:44–51. [PubMed] [Google Scholar]
- 38.Migliozzi JA. Effect of ascorbic acid on tumour growth. Br J Cancer. 1977;35:448–453. doi: 10.1038/bjc.1977.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spanheimer RG, Peterkofsky B. A specific decrease in collagen synthesis in acutely fasted, vitamin C-supplemented, guinea pigs. J Biol Chem. 1985;260:3955–3962. [PubMed] [Google Scholar]
- 40.Parsons KK, Maeda N, Yamauchi M, Banes AJ, Koller BH. Ascorbic acid-independent synthesis of collagen in mice. Am J Physiol Endocrinol Metab. 2006;290:E1131–E1139. doi: 10.1152/ajpendo.00339.2005. [DOI] [PubMed] [Google Scholar]
- 41.Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–5710. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langemann H, Torhorst J, Kabiersch A, Krenger W, Honegger CG. Quantitative determination of water- and lipid-soluble antioxidants in neoplastic and non-neoplastic human breast tissue. Int J Cancer. 1989;43:1169–1173. doi: 10.1002/ijc.2910430634. [DOI] [PubMed] [Google Scholar]
- 44.Spielholz C, Golde DW, Houghton AN, Nualart F, Vera JC. Increased facilitated transport of dehydroascorbic acid without changes in sodium-dependent ascorbate transport in human melanoma cells. Cancer Res. 1997;57:2529–2537. [PubMed] [Google Scholar]
- 45.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Cruz I, Carcamo JM, Golde DW. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood. 2003;102:336–343. doi: 10.1182/blood-2002-11-3559. [DOI] [PubMed] [Google Scholar]
- 47.Carcamo JM, Pedraza A, Borquez-Ojeda O, Golde DW. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 48.Witenberg B, Kletter Y, Kalir HH, Raviv Z, Fenig E, Nagler A, Halperin D, Fabian I. Ascorbic acid inhibits apoptosis induced by X irradiation in HL60 myeloid leukemia cells. Radiat Res. 1999;152:468–478. [PubMed] [Google Scholar]
- 49.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]