Abstract
In eubacteria, the ClpS adaptor has been proposed to be essential for degradation of N-end rule substrates by the AAA+ protease ClpAP. To test this model, we assayed degradation of substrates bearing N-end rule sequences isolated in a genetic screen for efficient degradation tags. ClpS was not vital for degradation in vivo but rather stimulated turnover in a sequence-specific manner. Although ClpS substantially enhanced degradation of N-end substrates at low substrate concentrations in vitro, it suppressed the degradation rate when substrate was saturating. Thus, we conclude that ClpAP recognizes N-end rule substrates directly, whereas ClpS modulates this degradation pathway.
Keywords: Targeted proteolysis, degradation motif, PheS, SUMO
Protein degradation regulates many biological processes and participates in protein quality control in prokaryotes and eukaryotes. The ability of proteolytic enzymes to recognize their substrates with precise specificity is important in carrying out efficient degradation of desired substrates and avoiding unwanted destruction of essential cellular proteins. The 26S proteasome is the primary degradation complex in eukaryotes. In Escherichia coli, there are five ATP-dependent proteases: ClpAP, ClpXP, HslUV, Lon, and FtsH (Schirmer et al. 1996). Some proteolytic substrates such as ssrA-tagged polypeptides are degraded by multiple proteases, whereas other proteins are degraded exclusively by one protease (Gottesman 1996; Gottesman et al. 1998).
The AAA+ ATPase subunits in the 26S proteasome and bacterial proteases recognize specific degradation signals on substrates, unfold these substrates, and translocate the denatured polypeptides into a proteolytic chamber where degradation occurs (Singh et al. 2000; Benaroudj et al. 2001). Degradation signals vary in complexity and include phosphorylated residues, polyubiquitin chains, and exposed peptide sequences (Parsell et al. 1990; Pickart 1997; Craig and Tyers 1999). In bacteria, the best-characterized degradation tags are simple peptide sequences that interact directly with the protease (Gottesman et al. 1998; Kim et al. 2000), but the mechanisms governing recognition by the bacterial proteases are still not thoroughly understood.
An interesting mode of proteolytic recognition is the N-end rule, which states that the stability of a protein in vivo depends on the identity of its N-terminal residue (Bachmair et al. 1986). This pathway is present in both eukaryotes and prokaryotes (Varshavsky 1992). Natural N-end substrates have not been characterized in prokaryotes, but experiments with model substrates reveal that proteins with Leu, Tyr, Trp, or Phe at the N terminus are degraded in E. coli if the ClpAP protease is present (Tobias et al. 1991). Moreover, proteins that initially have Arg or Lys at the N terminus are converted into N-end rule substrates by addition of an N-terminal Phe or Leu in a reaction catalyzed by the Aat aminoacyl transferase (Tobias et al. 1991).
The ClpAP protease is composed of ClpA, a hexameric AAA+ ATPase/protein unfoldase, and ClpP, a 14-subunit protease (Katayama et al. 1988; Maurizi et al. 1990; Weber-Ban et al. 1999). ClpS is a monomeric adaptor protein that binds to the N-terminal domain of ClpA subunits and alters substrate recognition by ClpAP (Dougan et al. 2002; Xia et al. 2004). ClpS inhibits ClpAP degradation of ssrA-tagged substrates but enhances degradation of other substrates. A recent study reported that ClpS binds directly to the N-terminal destabilizing residues of N-end rule substrates and is essential for their degradation by ClpAP (Erbse et al. 2006). This model predicts that N-end rule substrates should not be recognized or degraded by ClpAP in the absence of ClpS.
To explore bacterial N-end rule degradation in greater detail, we devised a genetic screen for N-terminal sequences that direct degradation of a conditionally toxic E. coli protein and isolated a collection of substrates with varied degradation signals. Using these substrates, we find that ClpS is not required for N-end degradation in vivo, although proteolysis occurs more rapidly when ClpS is present. To determine if ClpAP recognizes N-end rule substrates directly, we constructed and purified proteins bearing different N-end rule tags and measured degradation rates in vitro. In the absence of ClpS, ClpAP degraded each of these substrates, indicating that ClpA has a receptor for N-end rule sequence motifs. We also find that N-end rule degradation is influenced by more than the terminal residue. Thus, our results indicate that ClpA has an intrinsic ability to recognize and degrade N-end rule substrates, whereas ClpS modulates substrate recognition and turnover.
Results and Discussion
Isolation of strong N-terminal degradation signals
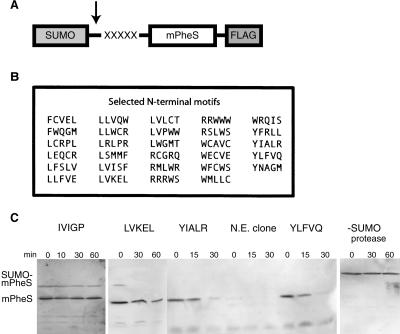
To identify sequences that could act as N-terminal degradation signals, we constructed a screening vector that expressed a fusion protein consisting of the SUMO domain from Saccharomyces cerevisiae, five randomized residues, the conditionally toxic mPheS enzyme, and a C-terminal Flag epitope (Fig. 1A). The mPheS enzyme is a mutant of E. coli phenylalanyl tRNA synthetase that allows incorporation of chloro-phenylalanine (Cl-Phe) into proteins and thus kills cells grown in the presence of this amino acid derivative (Kast and Hennecke 1991). The screening vector was transformed into cells expressing the Ulp1 protease to remove the SUMO domain and reveal the randomized sequence at the N terminus of mPheS (Li and Hochstrasser 1999).
Figure 1.
The mPheS selection assay yields N-end degradation signals of varying strengths. (A) The SUMO-mPheS protein used for the selection contains five randomized residues that become the N-terminal amino acids of mPheS following cleavage by the SUMO protease (arrow). (B) List of N-terminal tags that passed the selection and directed degradation in vivo. (C) Turnover of selected mPheS variants in wild-type cells was assayed at different times after stopping translation. The sample lacking SUMO protease expressed SUMO-YLFVQ-mPheS. N.E. clone is a nonexpressing isolate.
We found that cells expressing mPheS with ∼35 different N-terminal sequences displayed comparable growth on media with or without 15 mM Cl-Phe and slow growth on 20 mM Cl-Phe. In contrast, cells expressing most mPheS variants did not grow in the presence of either concentration of Cl-Phe. Moreover, clones that grew well in the presence of 20 mM Cl-Phe had frameshift or termination mutations in the randomized segment of the fusion protein (data not shown). For mPheS proteins with N-terminal sequences that allowed growth in the presence of Cl-Phe, we assayed intracellular stability by Western blotting after blocking protein synthesis. Six of the original 35 clones did not display detectable degradation of mPheS and were discarded. The 29 sequences listed in Figure 1B caused at least 75% turnover over the course of 1 h, with degradation mediated by some sequences being substantially faster. For example, mPheS proteins with YIALR or YLFVQ at the N terminus were completely degraded after 30 min, whereas a protein with LVKEL at the N terminus showed only modest turnover at this time point (Fig. 1C). In contrast, a sequence (IVIGP) that did not confer survival in the presence of Cl-Phe also did not cause significant degradation of mPheS (Fig. 1C).
All of the N-terminal sequences identified by the above criteria began with Phe, Leu, Arg, Trp, or Tyr (Fig. 1B). Each of these amino acids is an N-end rule residue in E. coli (Tobias et al. 1991), suggesting that our screen resulted in the isolation of N-end rule substrates. We did not recover sequences beginning with Lys, also a bacterial N-end residue, but our total library was small (∼350 clones) and contained only a tiny fraction of the possible five-residue N-terminal sequences. To determine if these sequences function as degradation signals only when present at the N terminus, we tested the intracellular stability of the YLFVQ-mPheS variant in cells with or without Ulp1 (Fig. 1C). In the absence of Ulp1, the SUMO-YLFVQ-mPheS fusion was stable over 1 h, indicating that the YLFVQ sequence needs to be exposed at the N terminus to function as a degradation signal.
ClpA but not ClpS is a required N-end rule component in vivo
To test the importance of ClpA and ClpS in intracellular degradation, mPheS proteins with WFCWS or WECVE sequences at the N terminus were introduced into strains lacking one or the other of these N-end rule components. Consistent with the observation that ClpA is essential for bacterial N-end rule degradation (Tobias et al. 1991), ΔclpA clpS+ cells expressing either substrate failed to grow on plates containing 15 mM Cl-Phe (Fig. 2A). Surprisingly, however, both the clpA+ clpS+ and clpA+ ΔclpS cells containing these substrates grew well in the presence of Cl-Phe (Fig. 2A,B). Plating serial dilutions of the two strains revealed that the ΔclpS cells had a mild plating defect on Cl-Phe, which was expressed principally as smaller colonies but also resulted in an approximately twofold to threefold reduced plating efficiency with some mPheS variants. However, the phenotype of the ΔclpS cells was clearly much milder than that of the ΔclpA strain. Thus, these data suggest that ClpS is less important than ClpAP in clearing N-end rule substrates from the cell.
Figure 2.
Effects of ClpS and ClpA on toxicity and degradation of N-end rule substrates in vivo. Wild-type, ΔclpA, or ΔclpS cells expressing SUMO protease and WFCWS-mPheS (A) or WECVE-mPheS (B) were serially diluted and spotted on plates containing 0 or 15 mM Cl-Phe. (C) Quantification of pulse-chase experiments for 35S-labeled WFCWS-mPheS in wild-type, ΔclpS, and ΔclpA strains. The half-lives were 5, 43, and 83 min, respectively. (D) Quantification of WECVE-mPheS degradation in the same three strains gave half-lives of 24, 40, and 122 min.
ClpS stimulates N-end degradation in vivo in a sequence-specific manner
To compare the degradation rates of mPheS proteins with N-terminal WFCWS and WECVE sequences, we measured the half-lives of these substrates in liquid culture. The WFCWS-mPheS protein had a half-life of 5 min in clpA+ clpS+ cells, 43 min in clpA+ ΔclpS cells, and 83 min in ΔclpA clpS+ cells (Fig. 2C). The WECVE-mPheS protein was degraded with half-lives of 24 min, 40 min, and 122 min in these strains (Fig. 2D). Because both proteins begin with the same N-end rule residue (Trp) and yet were degraded at rates differing by almost fivefold in clpA+ clpS+ cells, residues past the N terminus appear to contribute to recognition of these substrates. Moreover, ClpS enhanced ClpAP degradation of both proteins, albeit to different extents depending on the degradation signal (approximately eightfold for WFCWS and twofold for WECVE). Hence, the stimulatory effect of ClpS also depends on more than the N-terminal residue. Finally, elimination of ClpA slowed degradation more than elimination of ClpS in both cases. This result is consistent with experiments from the previous section, which indicated that ClpA is more important than ClpS for the phenotype associated with intracellular degradation of N-end rule substrates.
The results presented so far support the idea that ClpAP is the protease responsible for N-end rule degradation in E. coli (Tobias et al. 1991). Importantly, however, ClpS appears to be an auxiliary factor that enhances but is not essential for this degradation. In the following sections, we present the results of in vitro experiments that support these conclusions.
ClpAP has a receptor site for N-end motifs
For degradation experiments in vitro, we chose five of the N-terminal tags shown in Figure 1B and cloned these sequences between a His6-tagged SUMO domain and the I27 domain of the muscle protein titin (His6-SUMO-X5-titin). This fusion protein was purified by Ni++-NTA chromatography, cleaved with Ulp1, repurified by Ni++-NTA to remove uncleaved fusion protein and the His6-SUMO fragment, and subjected to a final gel-filtration step.
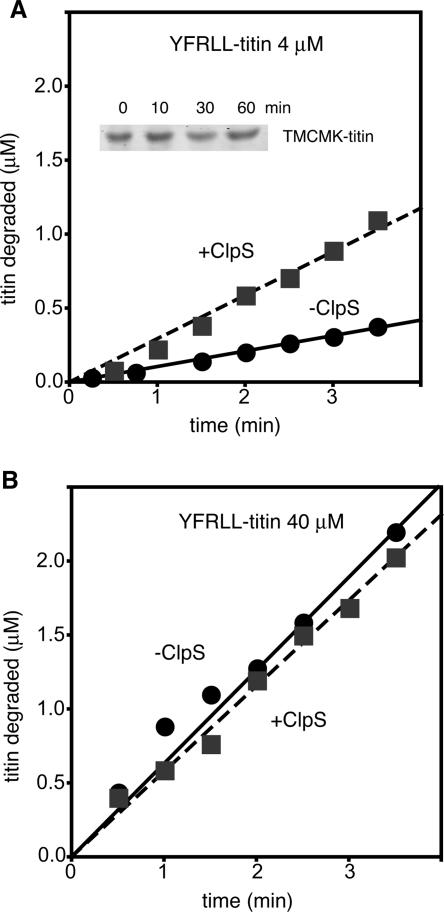
We found that purified ClpAP degraded 4 μM YFRLL-titin in the absence of ClpS and that proteolysis was accelerated threefold in the presence of ClpS (Fig. 3A). As a control for the purity of ClpA, we assayed for but did not detect any ClpS contamination by Western blotting. Moreover, degradation was N-end rule dependent because ClpAP did not degrade TMCMK-titin, which is not an N-end substrate, over the course of 1 h (Fig. 3A, inset). When degradation of 40 μM YFRLL-titin was assayed, similar rates of ClpAP proteolysis were observed whether ClpS was present or absent (Fig. 3B). These results demonstrate that ClpAP can degrade an N-end rule substrate in the absence of ClpS.
Figure 3.
ClpS improves but is not required by ClpAP for N-end degradation in vitro. Degradation of YFRLL-titin was followed by measuring the release of 35S-labeled acid-soluble peptides at different times after addition of ATP. The concentration of substrate was 4 μM (A) or 40 μM (B). (Inset) TMCMK-titin (10 μM) was not degraded by ClpAP plus ClpS. The TMCMK sequence fused to mPheS did not pass the Cl-Phe selection. Protein was detected with Sypro orange (Molecular Probes).
ClpS enhances substrate affinity but compromises enzymatic turnover
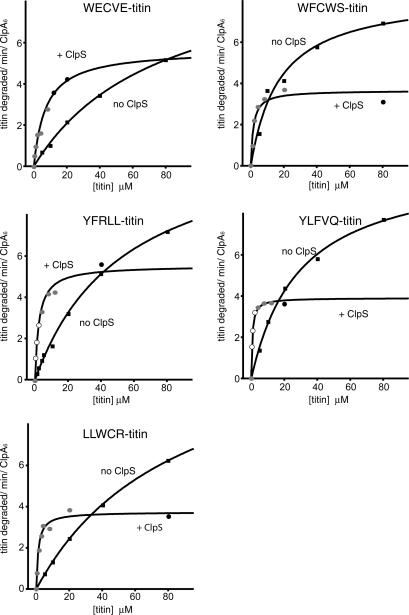
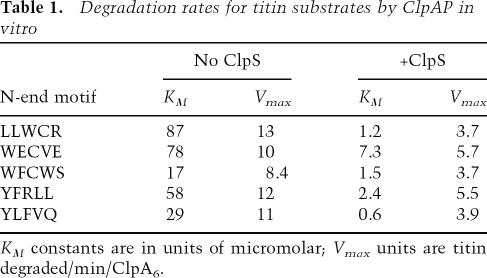
To determine whether ClpS enhances ClpAP-mediated degradation of N-end substrates by strengthening enzyme • substrate interactions, we used Michaelis-Menten analysis to determine KM and Vmax values for titin substrates with N-terminal WECVE, WFCWS, YFRLL, YLFVQ, and LLWCR signals (Fig. 4; Table 1). In each case, degradation was observed in the absence of ClpS, but the presence of the adaptor decreased KM by factors ranging from 10-fold to 70-fold. This reduction in KM allowed faster degradation at low substrate concentrations. Hence, the principal effect of the ClpS adaptor is to strengthen binding of N-end rule substrates to ClpAP when substrate is scarce. Because the identity of the degradation tag influenced the magnitude of the ClpS-dependent reduction in KM, sequence context must play a role in this process.
Figure 4.
ClpS enhances the affinity of ClpA for N-end substrates but suppresses the maximal rate of degradation. The initial rates of degradation per ClpA6 in the presence (●) or absence (◼) of ClpS were measured as a function of substrate concentration. ClpA6 concentrations were 25 nM (○), 50 nM (◼), or 100 nM (●). Each data point was the average of at least two separate experiments, and the error was between 5% and 10% of the average value. Curves were fit using the equation: Vobs = m1 × x/(m2 + x), where m1 = Vmax, m2 = KM, and x = [titin]. The R2 values varied from 0.94 to 0.99.
Table 1.
Degradation rates for titin substrates by ClpAP in vitro
KM constants are in units of micromolar; Vmax units are titin degraded/min/ClpA6.
Unexpectedly, ClpAP degraded high concentrations of each of the five N-end rule titin substrates significantly faster when ClpS was absent (Fig. 4). Indeed, values of Vmax were 1.8-fold to 3.5-fold higher in the absence of ClpS compared with its presence (Table 1). This inhibitory effect of ClpS on Vmax is not a general property of adaptor proteins. For example, the SspB adaptor decreases KM and increases Vmax for ClpXP degradation of cognate substrates (Levchenko et al. 2000). It is possible that ClpS binding to ClpA simply reduces the maximal rate at which the enzyme can unfold or translocate substrates. Alternatively, slower maximal degradation might reflect inefficient handoff of the N-end rule substrate from ClpS to ClpA. Erbse et al. (2006) have shown that ClpS interacts with the α-amino group and the side chain of the N-terminal residue of N-end rule substrates. Because our results indicate that ClpA is likely to recognize these same determinants, it is possible that dissociation of the N-end rule substrate from ClpS becomes the rate-limiting step for degradation by ClpAP at saturating substrate concentrations.
Strength of N-end rule degradation signals
In its simplest form, the N-end rule states that the half-life of a protein in vivo is determined by the identity of its N-terminal residue (Bachmair et al. 1986). Our results support the idea that specific N-terminal residues are required for N-end rule degradation in bacteria but also suggest a greater degree of signal complexity, as we find that ClpAP degraded substrates with identical N-terminal residues at substantially different rates in vivo and in vitro. A simple amendment to the N-end rule would be that the identities of the residues immediately following the N-terminal residue also influence the interaction of the degradation tag with ClpAP and ClpS. It remains to be determined how residues at positions other than the N terminus influence the degradation of N-end rule substrates by ClpAP, although Erbse et al. (2006) have suggested that a net positive charge near the N terminus may be important for ClpS binding. We note, however, that many of our N-end rule degradation tags contain no basic residues, and that tags with a strong net negative charge (like WECVE) can function as efficient degradation signals.
The bacterial N-end rule degradation pathway
The results presented here contradict the recent proposal that ClpS is an essential component of the N-end rule degradation pathway in bacteria (Erbse et al. 2006). We find that ClpAP can degrade N-end rule substrates in the absence of ClpS, both in vitro and in vivo. Moreover, although ClpS enhances ClpAP degradation of low substrate concentrations, it suppresses degradation when substrate is abundant. Thus, depending on conditions, ClpS can function either as an enhancer or inhibitor of degradation of N-end rule substrates. Although these results contradict the conclusions of Erbse et al. (2006), we note that their study contained few degradation experiments, and the in vitro degradation experiments reported used only one, low, substrate concentration, a condition we find shows the highest dependence on ClpS; thus it is not surprising that they observed a strong requirement for ClpS. Natural N-end rule bacterial substrates must be identified before it will be possible to determine whether ClpS plays a major biological role, either positively or negatively, in N-end rule degradation.
Materials and methods
Strains and plasmids
E. coli K12 strain W3110 derivatives were used for all in vivo experiments. Proteins were purified from BL2λDE3 and the appropriate expression vector.
The library plasmid was constructed from the pKSS (Kast 1994) by inserting the smt3 gene encoding SUMO using HindIII/BamHI sites. Codons 96–97 were altered from ATTGGT to ACCGGT to insert an AgeI site, causing an amino acid change that did not inhibit SUMO cleavage. A new Shine-Dalgarno sequence was inserted to replace the sequence lost during insertion of smt3 (TAAGGAGGTtaacaATG), and site-directed mutagenesis was performed to remove two Shine-Dalgarno sequences within smt3 (aaaaga → AAACGT and atggaggat → ATGGATGAT).
mPheS in the pKSS vector was replaced with a version containing a SacI site at codons 6–7 (GAACTG to GAGCTC) and a C-terminal Flag epitope tag. The randomized cassette was constructed from an oligonucleotide containing five repeats of N-N-G/C (where N is A, T, G, or C) flanked by AgeI and SacI sites. This scheme allows all 20 amino acids and one stop codon but reduces the bias between amino acids encoded by multiple codons. Nevertheless, biases in our population were evident, including an abundance of cysteine codons. Cassettes were created using the second-strand synthesis method of Reidhaar-Olson et al. (1991) and inserted into pKSS.smt3-mPheS-Flag.
For purification of the titin-Flag constructs, his6-smt3 was cloned into pET23b (Novagen) using NheI/NotI sites with the same AgeI insertion as described above. Titin-Flag proteins had N-terminal sequences as follows: X5-MSHLA-LIEVE, where X5 is the N-terminal candidate sequence followed by the first five residues of mPheS and the start of the titin domain in italics.
The catalytic domain of SUMO protease Ulp1 was cloned into pET23b using NheI/BamHI sites or purchased in purified form from Lifesensors, Inc. (Malakhov et al. 2004). For expression in vivo, pKW221, encoding Ulp1, was constructed by inserting an AvrII/XhoI fragment containing codons 403–621 of Ulp1 fused to a C-terminal Flag epitope under control of an arabinose-inducible promoter in the pACYC-based plasmid pJF122.
Media/selections/screens
YEG selection plates (Kast 1994) were made with the following modifications: 0.1% glycerol instead of glucose; 0.02% arabinose, and D,L-para-chloro-phenylalanine (Sigma).
Cells were transformed with the library of mPheS plasmids and plated on YEG plates lacking Cl-Phe. Colonies were replica-plated onto YEG plates with 0, 15, and 20 mM Cl-Phe and grown overnight at 30°C. To assess the stability of mPheS in vivo, selected clones were grown overnight at 30°C in LB containing 0.05% arabinose. Saturated cultures were diluted 1:20 into LB with 0.5% arabinose and shaken for 30 min at 37°C. Spectinomycin was added to 200 μg/mL, and 1-mL aliquots were removed at each time point. Cells were pelleted, resuspended in SDS buffer, and boiled prior to loading on a 10% Tris-glycine gel. Western blotting was performed using M2 mouse α-Flag (Sigma) at a 1:5000 dilution and an ECF detection kit (Amersham).
Assaying growth on Cl-Phe plates
Overnight cultures were diluted into M9 salts (13 mM Na2HPO4, 20 mM KH2PO4, 10 mM NaCl, 20 mM NH4Cl) and the OD595 adjusted to 0.01 (WECVE-mPheS) or 0.001 (WFCWS-mPheS). The cultures were then subjected to serial 10-fold dilutions, and 3 μL of each dilution were spotted on YEG plates containing 0 and 15 mM Cl-Phe. Plates were incubated for 18 h at 30°C and scanned on an Alpha Imager (Alpha Innotech).
Pulse-chase analysis of mPheS degradation
Overnight cultures were grown in LB with antibiotics and then diluted 1:100 in the same media with 0.01% arabinose to induce SUMO protease. At OD595 between 0.6 and 0.9, cells were pelleted, washed once in M9 media supplemented with methionine-assay medium (Difco) plus trace metals and 0.4% glucose, and then resuspended in 250 μL of this medium. After 5 min of shaking at 37°C, 100 μCi of Expre35S35S label (Perkin Elmer) was added. After 5 min, chase solution (25 mg/mL each L-Met and L-Cys) was added. At each time point, 50 μL was added to 50 μL of lysis buffer (4% SDS, 125 mM Tris at pH 6.8). Lysates were boiled for 5 min and added to 900 μL of IP buffer (50 mM Tris at pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100). Debris was pelleted, and the supernatant was added to 0.4 μL of α-Flag agarose resin (Sigma) and shaken for 1 h at 4°C. Resin was pelleted and washed twice, dried, resuspended in 20 μL of SDS buffer, and loaded onto 10% Tris-glycine gels. Dried gels were analyzed on a Typhoon 9400 imager, and quantification was performed with ImageQuant 4.0.
Purification of titin substrates
His6-SUMO-titin fusion proteins and Ulp1p were purified using Ni++-NTA according to Malakhov et al. (2004). After cleavage and removal of His6-SUMO, titin protein was chromatographed over Sephacryl-100 and concentrated in Amicon Ultra centrifugal filters. 35S-labeled substrates were purified as described (Kenniston et al. 2003).
Degradation of tagged titin proteins by ClpAP in vitro
Degradation rates of 35S-labeled titin variants were measured as a function of substrate concentration. ClpAP reaction conditions were as described in Weber-Ban et al. (1999), and degradation was initiated by addition of ATP regeneration mix containing 4 mM ATP, 50 mg/mL creatine kinase, and 5 mM creatine phosphate. Time points were removed at 30-sec intervals over 3 min, and degradation was quantified as described (Kim et al. 2000). In the experiments presented in Figure 4, degradation rates were normalized to the total ClpA6 concentration. ClpA6 concentrations of 25, 50, or 100 nM were used depending on the substrate concentration (see Fig. 4 and legend) to ensure that substrate concentrations did not change more than 25% during the time course as a result of degradation. ClpP14 was always used at twice the ClpA6 concentration. Control experiments showed that activity was linear with ClpA6 concentration over this range. The dependence of the degradation rate on the ClpS:ClpA6 ratio was also determined. Stimulation by ClpS saturated by six ClpS monomers per ClpA6 (data not shown; J. Hou, pers. comm.); when present, ClpS was used at a ratio of 9 ClpS/ClpA6.
Acknowledgments
We are grateful to J. Hou for providing strains and purified ClpS, and C. Farrell and S. Moore for development of the mPheS selection. We also thank F. Solomon, P. Chien, S. Neher, J. Kenniston, K. McGinness, and members of the Baker and Sauer laboratories for helpful discussions. This work was supported by NIH grants GM-49224 and AI-16892. K.H.W. is supported by an NSF graduate fellowship. T.A.B. is an employee of HHMI.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1511907
References
- Bachmair A., Finley D., Varshavsky A., Finley D., Varshavsky A., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Benaroudj N., Tarcsa E., Cascio P., Goldberg A.L., Tarcsa E., Cascio P., Goldberg A.L., Cascio P., Goldberg A.L., Goldberg A.L. The unfolding of substrates and ubiquitin-independent protein degradation by proteasomes. Biochimie. 2001;83:311–318. doi: 10.1016/s0300-9084(01)01244-5. [DOI] [PubMed] [Google Scholar]
- Craig K.L., Tyers M., Tyers M. The F-box: A new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog. Biophys. Mol. Biol. 1999;72:299–328. doi: 10.1016/s0079-6107(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Dougan D.A., Reid B.G., Horwich A.L., Bukau B., Reid B.G., Horwich A.L., Bukau B., Horwich A.L., Bukau B., Bukau B. ClpS, a substrate modulator of the ClpAP machine. Mol. Cell. 2002;9:673–683. doi: 10.1016/s1097-2765(02)00485-9. [DOI] [PubMed] [Google Scholar]
- Erbse A., Schmidt R., Bornemann T., Schneider-Mergener J., Mogk A., Zahn R., Dougan D.A., Bukau B., Schmidt R., Bornemann T., Schneider-Mergener J., Mogk A., Zahn R., Dougan D.A., Bukau B., Bornemann T., Schneider-Mergener J., Mogk A., Zahn R., Dougan D.A., Bukau B., Schneider-Mergener J., Mogk A., Zahn R., Dougan D.A., Bukau B., Mogk A., Zahn R., Dougan D.A., Bukau B., Zahn R., Dougan D.A., Bukau B., Dougan D.A., Bukau B., Bukau B. ClpS is an essential component of the N-end rule pathway in Escherichia coli. Nature. 2006;439:753–756. doi: 10.1038/nature04412. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Roche E., Zhou Y., Sauer R.T., Roche E., Zhou Y., Sauer R.T., Zhou Y., Sauer R.T., Sauer R.T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes & Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast P. pKSS—A second-generation general purpose cloning vector for efficient positive selection of recombinant clones. Gene. 1994;138:109–114. doi: 10.1016/0378-1119(94)90790-0. [DOI] [PubMed] [Google Scholar]
- Kast P., Hennecke H., Hennecke H. Amino acid substrate specificity of Escherichia coli phenylalanyl-tRNA synthetase altered by distinct mutations. J. Mol. Biol. 1991;222:99–124. doi: 10.1016/0022-2836(91)90740-w. [DOI] [PubMed] [Google Scholar]
- Katayama Y., Gottesman S., Pumphrey J., Rudikoff S., Clark W.P., Maurizi M.R., Gottesman S., Pumphrey J., Rudikoff S., Clark W.P., Maurizi M.R., Pumphrey J., Rudikoff S., Clark W.P., Maurizi M.R., Rudikoff S., Clark W.P., Maurizi M.R., Clark W.P., Maurizi M.R., Maurizi M.R. The two-component, ATP-dependent Clp protease of Escherichia coli: Purification, cloning, and mutational analysis of the ATP-binding component. J. Biol. Chem. 1988;263:15226–15236. [PubMed] [Google Scholar]
- Kenniston J.A., Baker T.A., Fernandez J.M., Sauer R.T., Baker T.A., Fernandez J.M., Sauer R.T., Fernandez J.M., Sauer R.T., Sauer R.T. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell. 2003;114:511–520. doi: 10.1016/s0092-8674(03)00612-3. [DOI] [PubMed] [Google Scholar]
- Kim Y.I., Burton R.E., Burton B.M., Sauer R.T., Baker T.A., Burton R.E., Burton B.M., Sauer R.T., Baker T.A., Burton B.M., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol. Cell. 2000;5:639–648. doi: 10.1016/s1097-2765(00)80243-9. [DOI] [PubMed] [Google Scholar]
- Li S.J., Hochstrasser M., Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature. 1999;398:246–251. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- Levchenko I., Seidel M., Sauer R.T., Baker T.A., Seidel M., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. A specificity-enhancing factor for the ClpXP degradation machine. Science. 2000;289:2354–2356. doi: 10.1126/science.289.5488.2354. [DOI] [PubMed] [Google Scholar]
- Malakhov M.P., Mattern M.R., Malakhova O.A., Drinker M., Weeks S.D., Butt T.R., Mattern M.R., Malakhova O.A., Drinker M., Weeks S.D., Butt T.R., Malakhova O.A., Drinker M., Weeks S.D., Butt T.R., Drinker M., Weeks S.D., Butt T.R., Weeks S.D., Butt T.R., Butt T.R. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- Maurizi M.R., Clark W.P., Katayama Y., Rudikoff S., Pumphrey J., Bowers B., Gottesman S., Clark W.P., Katayama Y., Rudikoff S., Pumphrey J., Bowers B., Gottesman S., Katayama Y., Rudikoff S., Pumphrey J., Bowers B., Gottesman S., Rudikoff S., Pumphrey J., Bowers B., Gottesman S., Pumphrey J., Bowers B., Gottesman S., Bowers B., Gottesman S., Gottesman S. Sequence and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J. Biol. Chem. 1990;265:12536–12545. [PubMed] [Google Scholar]
- Parsell D., Silber K.R., Sauer R.T., Silber K.R., Sauer R.T., Sauer R.T. Carboxy-terminal determinants of intracellular protein degradation. Genes & Dev. 1990;4:277–286. doi: 10.1101/gad.4.2.277. [DOI] [PubMed] [Google Scholar]
- Pickart C.M. Targeting of substrates to the 26S proteasome. FASEB J. 1997;11:1055–1066. doi: 10.1096/fasebj.11.13.9367341. [DOI] [PubMed] [Google Scholar]
- Reidhaar-Olson J.F., Bowie J.U., Breyer R.M., Hu J.C., Knight K.L., Lim W.A., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Bowie J.U., Breyer R.M., Hu J.C., Knight K.L., Lim W.A., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Breyer R.M., Hu J.C., Knight K.L., Lim W.A., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Hu J.C., Knight K.L., Lim W.A., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Knight K.L., Lim W.A., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Lim W.A., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Mossing M.C., Parsell D.A., Shoemaker K.R., Sauer R.T., Parsell D.A., Shoemaker K.R., Sauer R.T., Shoemaker K.R., Sauer R.T., Sauer R.T. Random mutagenesis of protein sequences using oligonucleotide cassettes. Methods Enzymol. 1991;208:564–586. doi: 10.1016/0076-6879(91)08029-h. [DOI] [PubMed] [Google Scholar]
- Schirmer E.C., Glover J.R., Singer M.A., Lindquist S., Glover J.R., Singer M.A., Lindquist S., Singer M.A., Lindquist S., Lindquist S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- Singh S.K., Grimaud R., Hoskins J.R., Wickner S., Maurizi M.R., Grimaud R., Hoskins J.R., Wickner S., Maurizi M.R., Hoskins J.R., Wickner S., Maurizi M.R., Wickner S., Maurizi M.R., Maurizi M.R. Unfolding and internalization of proteins by the ATP-dependent proteases ClpXP and ClpAP. Proc. Natl. Acad. Sci. 2000;97:8898–8903. doi: 10.1073/pnas.97.16.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias J.W., Shrader T.E., Rocap G., Varshavsky A., Shrader T.E., Rocap G., Varshavsky A., Rocap G., Varshavsky A., Varshavsky A. The N-end rule in bacteria. Science. 1991;254:1374–1377. doi: 10.1126/science.1962196. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule. Cell. 1992;69:725–735. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- Weber-Ban E.U., Reid B.G., Miranker A.D., Horwich A.L., Reid B.G., Miranker A.D., Horwich A.L., Miranker A.D., Horwich A.L., Horwich A.L. Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature. 1999;401:90–93. doi: 10.1038/43481. [DOI] [PubMed] [Google Scholar]
- Xia D., Esser L., Singh S.K., Guo F., Maurizi M.R., Esser L., Singh S.K., Guo F., Maurizi M.R., Singh S.K., Guo F., Maurizi M.R., Guo F., Maurizi M.R., Maurizi M.R. Crystallographic investigation of peptide binding sites in the N-domain of the ClpA chaperone. J. Struct. Biol. 2004;146:166–179. doi: 10.1016/j.jsb.2003.11.025. [DOI] [PubMed] [Google Scholar]