Abstract
Protein accumulation is a hallmark of many neurodegenerative disorders. In Alzheimer’s disease (AD), a hyperphosphorylated form of the protein tau (p-tau) forms intracellular inclusions known as neurofibrillary tangles. Deposits of p-tau have also been found in the brains of patients with Down’s syndrome, supranuclear palsy, and prion disease. Mutations in tau have been causally associated with at least one inherited neurologic disorder, frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), implying that tau abnormalities by themselves can be a primary cause of degenerative diseases of the CNS. Removal of these p-tau species may occur by both chaperone-mediated refolding and degradation. In this issue of the JCI, Dickey and colleagues show that a cochaperone protein, carboxyl terminus of Hsp70-interacting protein (CHIP), in a complex with Hsp90 plays an important role in the removal of p-tau (see the related article beginning on page 648). Pharmacologic manipulation of Hsp90 may be used to alleviate p-tau accumulation in disease.
Role of chaperones in degradation and refolding of abnormal tau
Chaperones are molecular machines designed to maintain proteins in a properly folded state (1). Misfolded proteins that cannot be refolded by chaperones are ubiquitinated and thereby targeted for degradation by the proteasome (2). It has been proposed that the chaperone and proteasome systems may act in concert in clearing abnormal tau and other proteins that accumulate in neurologic diseases (3). Indeed, in Alzheimer’s disease (AD) brains, neurons with a higher content of the molecular chaperones Hsp70 or Hsp90 have fewer neurofibrillary tangles (NFTs; ref. 4). In a transgenic mouse model expressing a mutant version of human tau that is associated with the neurologic disorder frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), reduced levels of Hsp90 were detected in the hippocampi of affected animals (4). Conversely, inhibition of the proteasome in cells overexpressing parkin, a protein found in aggregates in Parkinson’s disease patients, leads to parkin inclusion formation (5).
The chaperone cofactor proteins Hsp70-interacting protein (Hip) and Hsp70/Hsp90-organizing protein (Hop) both interact with Hsp70 via their tetratricopeptide repeat (TPR) domains. In a screen for additional TPR-encoding cDNAs, a protein named carboxyl terminus of Hsp70-interacting protein (CHIP) was identified (6). As its name suggests, CHIP interacts with Hsp70 and inhibits its ATPase activity, indicating that CHIP might be involved in an Hsp70-mediated process distinct from refolding (6). Hsp90, which binds CHIP via its TPR domain (7), is different from Hsp70 and other chaperones in that it does not act in nascent protein folding and most of its known substrates are signal transduction proteins. It is generally believed that Hsp90 binding follows the formation of an Hsp70/substrate complex (8). CHIP can interact with either Hsp70 or Hsp90 through the same TPR, displacing them from Hop or other TPR-containing proteins (7, 8).
CHIP has been shown to be an E3 ubiquitin ligase whose activity is dependent on Hsp90 or an Hsp70/40 complex (9). Petrucelli and colleagues previously showed that CHIP ubiquitinates tau in transfected cells and stimulates tau aggregation (10). Hsp70 upregulation attenuated tau aggregation concomitantly with a decrease in total tau levels, suggesting a role for CHIP/Hsp70 in promoting tau degradation (10). Independently, Shimura and colleagues showed that a CHIP/Hsp70 complex specifically ubiquitinates AD-type hyperphosphorylated tau (p-tau) and that soluble p-tau is toxic. Surprisingly, they also found that insoluble p-tau is not toxic, even when both hyperphosphorylated and ubiquitinated (11). CHIP levels were increased in AD brains and corresponded directly to levels of Hsp90, but not Hsp70, in both diseased and normal brains. Furthermore, CHIP levels were inversely proportional to p-tau, suggesting that CHIP in conjunction with Hsp90 may play a protective role (12). Consistent with this idea, both homo- and heterozygous CHIP knockout mice were shown to have increased levels of insoluble tau in the brain (12). An independently created CHIP-null mouse also had elevated levels of both total tau and p-tau that were concomitant with a decrease in Hsp70 levels (13). The accumulated tau did not form NFT-like lesions. When the CHIP-null mice were crossed with transgenic mice expressing a pathogenic human tau (P301L), p-tau levels increased without any increase in aggregation, suggesting that CHIP is required for both degradation and aggregation (13).
Hsp90 inhibition may enhance degradation of abnormal p-tau
The study in this issue of the JCI by Dickey, Petrucelli, and colleagues provides evidence that an Hsp90 inhibitor reduced total tau and p-tau levels both in cultured human HeLa cells and in mice (14). EC102, which is reported to inhibit Hsp90 ATPase activity, was previously shown to enhance degradation of abnormal p-tau in P301L-tau–expressing cells (15, 16). In the current study, treatment of tau-transfected cells with EC102 caused a reduction of both total tau and p-tau levels by up to 40%. Knocking down Hsp90, but not Hsp70, by means of siRNA abolished the EC102 effect, indicating that Hsp90 is required as a structural component of a tau-refolding/degradation complex. siRNAs of several other factors, including Hop, Hsp40, and CHIP, also abrogated the effect of EC102. In addition, downregulation of these factors increased total tau levels. In contrast, siRNA of heat shock factor 1 (HSF1) had no effect on EC102-mediated p-tau reduction. Because HSF1 activates the Hsp genes but is normally inhibited by Hsp90, these data show that the involvement of Hsp90 was direct and that de novo synthesis of chaperones was not responsible for the EC102-induced decrease in tau levels. It also makes it unlikely that general activation of Hsp genes plays a role in the EC102 mechanism of tau downregulation. In fact, Hsp70 knockdown had virtually no effect on total tau or p-tau levels or the EC102-induced effect.
The present findings (14) point to a pivotal role for Hsp90 in aberrant tau degradation and potentially in tau refolding, although the exact function of Hsp90 in this process is unclear. Treatment with siRNA of Hsp90 without EC102 resulted in an increase in total tau levels and also in p-tau. The effect of Hsp90 knockdown on p-tau levels was overcome by overexpressing CHIP. Moreover, CHIP formed complexes with tau in immunoprecipitation experiments with or without Hsp90 knockdown. Taken together, these data suggest a model (Figure 1) in which the chaperones Hsp70, Hsp40, and possibly Hsp90, when presented with abnormal p-tau, first attempt to refold it and return to the “normal,” microtubule-associated tau pool. If that fails, CHIP comes into play and ubiquitinates the abnormal p-tau protein, thereby targeting it for degradation. Consistent with this hypothesis, siRNA treatment of refolding-stimulating factors P23 and Pin1 enhanced tau degradation. Should this degradation fail, aggregation may present the safety net by which the toxic protein will be retained and isolated from the intracellular environment.
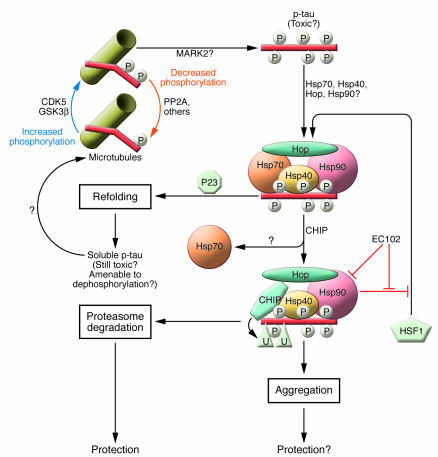
Figure 1. Cellular fates of abnormal tau.
The tau protein can be phosphorylated on a large number of serine and threonine residues along its length, resulting in an enormously complex pattern of potential phospho-isoforms. Under normal cellular conditions, each tau molecule contains only 2–3 phosphate groups and binds microtubules (18). Tau hyperphosphorylation to form p-tau is believed to be the trigger event in the tau aggregation cascade. The kinases responsible for this event are not known but may include CDK5, GSK3β, and MARK2. Soluble p-tau is believed to be the toxic moiety, while p-tau aggregation may represent a protective mechanism of sequestration. p-tau is misfolded, which results in recruitment of hsps such as Hsp70, Hsp40, and Hsp90. These chaperones attempt to refold the abnormal p-tau, which could ultimately result in p-tau reincorporation into microtubules. This reincorporation is most likely contingent upon dephosphorylation. P23 is found in mature Hsp90-substrate complexes and stimulates Hsp90 ATPase and substrate dissociation, promoting substrate refolding. If refolding fails, the p-tau–chaperone complex is reprogrammed to eliminate the defective p-tau protein. If substrate (p-tau) release is blocked by an inhibitor of Hsp90 ATPase, such as EC102, CHIP proceeds to ubiquitinate (U) p-tau and target it for degradation by the proteasome. EC102 also induces an HSF1 feedback loop that leads to increased chaperone expression. In this issue, Dickey et al. (14) now show that Hsp90 inhibition with EC102 leads to enhanced CHIP-mediated degradation of p-tau both in human culture cells and in mice.
Reducing p-tau levels in transgenic mice
Unlike geldanamycin and other ansamycin-class antibiotics previously used to inhibit Hsp90 (4, 17), EC102 can cross the blood-brain barrier and may therefore be used to treat CNS disorders. In an exciting follow-up to their in vitro results, Dickey et al. administered EC102 to transgenic mice overexpressing human tau (14). Mice undergoing a treatment regimen as short as a once-daily dose of EC102 for 7 days showed an approximately 50% reduction in aberrant p-tau levels, but not in levels of normal tau. Most remarkably, the affinity of EC102 for Hsp90 from affected regions of human AD brains was found to be approximately 1,000-fold higher compared with that for Hsp90 from unaffected regions of the brains of the same patients or from control patients. This finding could explain the specificity of the EC102 effect on abnormal p-tau in transgenic mice and makes it a highly promising candidate for anti-tauopathy therapeutics.
While the current study (14) provides an exciting advance in our understanding of the relationship between chaperones, proteasome-mediated degradation, and abnormal tau accumulation, it fails to address several important questions. The main question concerns the mechanism of Hsp90 involvement in tau degradation. One possibility is that when Hsp90 is inhibited, CHIP, tau, and Hsp90 are more likely to stay in a complex, giving CHIP more time to tag tau for degradation by ubiquitination (8). However, the present study does not support this idea, since more CHIP/tau complexes formed in cells treated with Hsp90 siRNAs than in control-treated cells. Moreover, EC102-treated mice had much lower Hsp90 levels than did controls, in an apparent contradiction with the abrogation of the EC102 effect by Hsp90 siRNA in cultured cells.
The relative contributions of protein refolding, degradation, and aggregation to the neutralization of toxic tau are therefore far from being elucidated. Given that Hsp90 is involved in multiple signaling events unrelated to tau metabolism, it will be important to establish what potential side effects EC102 might have in long-term treatment. The key postulate of the study (EC102 specificity for Hsp90) may also need revisiting. EC102 has been reported to inhibit Hsp90 and not Hsp70 (16), but these studies do not address whether EC102 is specific for Hsp90. Downregulation of other components of the chaperone system also blocked the EC102-induced decrease in p-tau, raising the question whether this is due to the specific action of EC102 on Hsp90. These concerns notwithstanding, the notion that the chaperone machinery is a promising target for pharmacologic intervention in AD and other tauopathies has just received a robust boost.
Footnotes
Nonstandard abbreviations used: AD, Alzheimer’s disease; CHIP, carboxyl terminus of Hsp70–interacting protein; FTDP-17, frontotemporal dementia with parkinsonism on chromosome 17; Hop, Hsp70/Hsp90-organizing protein; HSF1, heat shock factor 1; NFT, neurofibrillary tangle; p-tau, hyperphosphorylated tau; TPR, tetratricopeptide repeat.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:590–592 (2007). doi:10.1172/JCI31505.
See the related article beginning on page 648.
References
- 1.Young J.C., Barral J.M., Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem. Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Ardley H.C., Robinson P.A. The role of ubiquitin-protein ligases in neurodegenerative disease. . Neurodegener. Dis. 2004;1:71–87. doi: 10.1159/000080048. [DOI] [PubMed] [Google Scholar]
- 3.Muchowski P.J., Wacker J.L. Modulation of neurodegeneration by molecular chaperones. . Nat. Rev. Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 4.Dou F., et al. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. U. S. A. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardley H.C., et al. Inhibition of proteasomal activity causes inclusion formation in neuronal and non-neuronal cells overexpressing Parkin. Mol. Biol. Cell. 2003;14:4541–4556. doi: 10.1091/mbc.E03-02-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballinger C.A., et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell P., et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 8.Young J.C., Moarefi I., Hartl F.U. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata S., Minami Y., Minami M., Chiba T., Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrucelli L., et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 11.Shimura H., Schwartz D., Gygi S.P., Kosik K.S. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. . J. Biol. Chem. 2004;279:4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 12.Sahara N., et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J. Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 13.Dickey C.A., et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J. Neurosci. 2006;26:6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickey C.A., et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biamonte M.A., et al. Orally active purine-based inhibitors of the heat shock protein 90. . J. Med. Chem. 2006;49:817–828. doi: 10.1021/jm0503087. [DOI] [PubMed] [Google Scholar]
- 16.Dickey C.A., et al. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J. 2006;20:753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 17.Panaretou B., et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong C.X., Liu F., Grundke-Iqbal I., Iqbal K. Post-translational modifications of tau protein in Alzheimer’s disease. J. Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]