Abstract
The hormone aldosterone increases extracellular fluid volume and blood pressure by activating epithelial Na+ channels (ENaCs). Serum- and glucocorticoid-induced kinase 1 (SGK1) is an aldosterone-stimulated signaling molecule that enhances distal nephron Na+ transport, in part by preventing the internalization of ENaCs from the plasma membrane. In this issue of the JCI, Zhang et al. demonstrate that SGK1 enhances transcription of the α subunit of ENaC by preventing histone methylation, providing an additional mechanism by which SGK1 increases ENaC-mediated Na+ transport in the distal nephron (see the related article beginning on page 773).
There are two general classes of Na+-selective ion channels in humans. The first, voltage-gated Na+ channels, are transiently activated in response to modest degrees of membrane depolarization. Activation of these channels leads to a large but transient depolarization of the plasma membrane. In contrast, the second class, epithelial Na+ channels (ENaCs), facilitate the bulk transit of Na+ across an epithelial monolayer. These channels are not strictly regulated by membrane voltage, and other mechanisms have been found to modulate their activity. The extent to which an ENaC is open and conducting Na+, a process referred to as open probability, is regulated by a number of factors, including phosphorylation, the presence of anionic phospholipids, and processing of ENaC extracellular domains by specific proteases (1–3). The number of channels present at the plasma membrane is also highly regulated, either by altering rates of channel biosynthesis and delivery to the plasma membrane or by channel retrieval from the plasma membrane (4–6).
ENaCs have key roles in the reabsorption of filtered Na+ in the distal nephron of the kidney as well as in the regulation of Na+ content in the extracellular fluids, volume of extracellular fluids, and blood pressure. The hormone aldosterone increases extracellular fluid volume, in large part via activation of ENaCs. Over the past decade, a variety of convergent mechanisms involved in the activation of ENaCs by aldosterone have been described.
SGK1 is an aldosterone-regulated stimulator of ENaC
Originally identified as a glucocorticoid-regulated gene in a breast cancer cell line (7), serum- and glucocorticoid-induced kinase 1 (SGK1) is closely related to the Akt family of kinases and, like its cousins, has antiapoptotic effects in cultured cells. Later, SGK1 was identified in kidney collecting duct cells as an aldosterone-stimulated regulator of ENaC-mediated Na+ transport (5). This latter function has somewhat surprisingly emerged as its most crucial function in vivo. Sgk1-null mice have no apparent defect in growth or development, but they do demonstrate aldosterone resistance as well as resistance to salt-sensitive hypertension (8). One of the more interesting features of SGK1 regulation is that both its activity and its expression are controlled by a variety of hormonal and nonhormonal signals. Regulators of this expression include glucocorticoids and mineralocorticoids, osmotic shock, follicle-stimulating hormone, and TGF-β. Control of SGK1 activity is achieved primarily through a PI3K-dependent phosphorylation cascade; however, some regulators that act through this pathway, such as insulin, appear to have more of a pathophysiologic than a physiologic role.
SGK1 regulates ENaC activity, trafficking, and gene transcription
Some aspects of SGK1’s mechanism of action are well defined, most notably its modulation of ENaC trafficking via inhibition of Nedd4-2, an ENaC inhibitor (reviewed in ref. 9). Well known for its role in Liddle’s syndrome, Nedd4-2 is a Hect domain–containing ubiquitin ligase that interacts with the C-terminal tail of ENaC subunits and ubiquitinylates a set of N-terminal lysines within channel subunits. Because Nedd4-2 triggers channel internalization and degradation, the effect of SGK1 in this context is one of disinhibition. Liddle’s syndrome, which is one form of pseudohyperaldosteronism, results from an ENaC mutation that disrupts the interaction between channel and ligase and hence preempts SGK1-mediated inhibition of Nedd4-2. There is also some evidence to support the idea that SGK1 can regulate ENaC single-channel activities through direct phosphorylation of its α subunit, ENaCα (3). In this issue of the JCI, Zhang et al. suggest what we believe to be a new way for SGK1 to regulate ENaCα: through control of its gene transcription by altering local chromatin structure (10).
Mineralocorticoid receptor–mediated gene regulation: early and late
Like the gene encoding ENaCα itself, SGK1 is a classically regulated mineralocorticoid receptor (MR) target gene, with canonical mineralocorticoid response elements (MREs) driving its expression in response to MRs or glucocorticoid receptors (GRs) (11). However, SGK1 and ENaCα differ strikingly in the nature of their responses: SGK1 is an immediate early gene, the expression of which increases within minutes after cell exposure to corticosteroids and peaks within 1–2 hours. In contrast, ENaCα expression increases slowly, with no change for the first several hours after exposure to aldosterone. Peak expression is not achieved for at least 12 hours. Moreover, prior evidence suggested that although direct MR regulation of ENaCα gene transcription through its MREs was important, it was not the only mechanism involved, and that SGK1 might indeed be implicated (6). The basis for the differences in time course between immediate early genes such as SGK1 and late genes such as ENaCα has been mysterious. The current study by Zhang et al. (10) is exciting in that establishing a mechanistic connection between SGK1 and the activity of a histone methyl transferase complex provides one possible solution to this enigma.
Histone methylation: an important locus of regulated gene expression
Over the last several years, histone methylation has emerged as a central mechanism in the regulation of gene transcription (12). Through modification of arginines and lysines, primarily in the tails of histones H3 and H4, methylation can have complex stimulatory and inhibitory effects on gene transcription. Disruptor of telomeric silencing alternative splice variant a (Dot1a), the relevant methyl transferase in the control of ENaC expression, suppresses gene transcription by methylating Lys79 within the globular domain of histone H3 (i.e., histone H3 Lys79 methylation). This results (at least in yeast) in the recruitment of silencing proteins such as Sir2 and Sir3 and in chromatin stabilization (13–15).
Zhang, Kone, and colleagues demonstrated in earlier studies that aldosterone decreases histone H3 Lys79 methylation as it stimulates ENaCα mRNA levels and that the complex of Dot1a with ALL1-fused gene from chromosome 9 (AF9) mediates this effect (16, 17). This set the stage for their current work, which demonstrates the role of SGK1 in mediating this process and establishes a link between MR regulation of SGK1 on the one hand and of ENaCα on the other (10). The authors demonstrate that both SGK1 and AF9 are associated with the ENaCα promoter and that Ser435 within AF9 is a target of SGK1. SGK1-dependent phosphorylation of AF9 reduced the interaction between Dot1a and AF9, causing impaired association of Dot1a with the ENaCα promoter and concomitant histone H3 hypomethylation within specific subregions of the ENaCα promoter. Furthermore, repression of the ENaCα promoter by the Dot1a-AF9 complex was impaired with an AF9 Ser435Ala mutant. As expected based on these observations, mice maintained on a low-salt diet exhibited both low levels of renal ENaCα protein expression and enhanced levels of AF9 phosphorylation. Mice lacking SGK1 expression exhibited reduced levels of AF9 phosphorylation and ENaCα mRNA levels when placed on a low-salt diet compared with wild-type mice fed the same diet.
The present data (10) provide strong evidence for the role of SGK1 in enhancing ENaCα transcription by inducing AF9 phosphorylation and reducing the association of Dot1a with the ENaCα promoter, with an associated decrease in histone H3 Lys79 methylation. This effect of SGK1 is enhanced by aldosterone-dependent repression of both Dot1a and AF9 expression (16, 17). The SGK1-dependent increase in ENaCα expression provides an additional mechanism by which SGK1 activates ENaC (Figure 1).
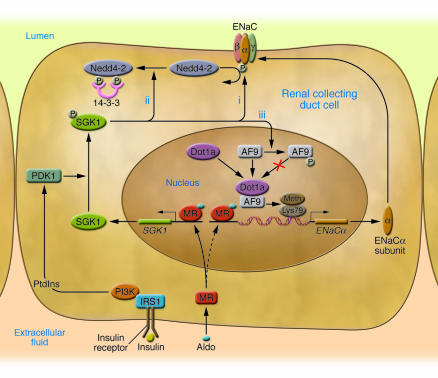
Figure 1. SGK1 acts at multiple levels to regulate ENaC-mediated Na+ transport in renal collecting duct cells.
At low aldosterone (Aldo) levels, ENaCα gene transcription is low because of tonic inhibition by a complex of Dot1a and AF9, which methylates Lys79 on histone H3. At the same time, Nedd4-2 is active and maintains plasma membrane ENaCs at low levels through ubiquitinylation, which results in channel internalization and degradation. With an elevation in aldosterone levels, activated MR translocates to the nucleus, binds to MREs in the SGK1 5′ regulatory region, and rapidly stimulates SGK1 transcription (solid arrow). ENaCα is not initially responsive to aldosterone-activated MR because the Dot1a-AF9 complex maintains surrounding chromatin in a hypermethylated, stable state, which blocks entry of transcription factors, including MR itself, to their DNA-binding sites (dashed arrow). SGK1 catalytic activity is stimulated by PI3K-dependent generation of 3-phosphorylated phosphoinositides (PtdIns), which activate phosphoinositide-dependent protein kinase 1 (PDK1) and ultimately SGK1 itself. SGK1 activates ENaC through phosphorylation of several key substrates: the channel itself (i), resulting in increased open probability; Nedd4-2 (ii), resulting in inhibition of Nedd4-2 through recruitment of inhibitory 14-3-3 chaperone proteins and subsequent decreased channel internalization and degradation; and AF9 (iii), resulting in inhibition of the Dot1a-AF9 complex and diminished H3 hypermethylation in the vicinity of the ENaCα promoter. This latter effect, which is reported in this issue of the JCI (10), is predicted to enhance MR access to chromatin, binding to MREs, and stimulation of transcription. IRS1, insulin receptor substrate 1; Meth, methylation; α, β, γ, ENaCα, -β, and -γ subunits, respectively. Modified from ref. 10 and with permission from American Journal of Physiology. Renal Physiology (9).
Future directions
One of the most interesting questions regarding the role of SGK1 in controlling ENaCα expression is the teleological one: Why? Both ENaCα and SGK1 have in their regulatory regions canonical MREs that are functional and mediate MR activation of the gene. What would be the selection pressure that would favor the introduction of SGK1 itself as an intermediary in the control of ENaC expression? To us, the answer to this question lies in the economy, flexibility, and capacity for integration of inputs that this system provides. As noted above, SGK1 is an immediate early gene that responds rapidly to changes in aldosterone levels. In contrast, ENaCα is a late-response gene whose expression increases only after several hours of sustained hormone elevation. Because aldosterone rises and falls rapidly over a limited range during the course of a normal day in response to minor changes in dietary salt intake, SGK1 is similarly rising and falling and can rapidly influence ENaC trafficking and potentially single-channel activities. ENaCα transcription under these conditions would not be expected to change; rather, the expression level would represent an integrated response to the combination of activated MRs (largely reflecting only the effect of aldosterone) and activated SGK1. Not only is the expression level of the latter regulated by inputs other than aldosterone (e.g., osmotic shock), its activity is controlled by the PI3K pathway and its vast array of regulatory inputs. Hence, the commitment of a cell to increase ENaC expression would presumably reflect a sustained commitment to enhance Na+ reabsorption, such as one might see on a chronic low-salt diet. It will be of mechanistic and potentially pathophysiologic interest to examine the dependence of MR-stimulated ENaCα expression on activated SGK1. The work of Zhang, Kone, and colleagues (10) predicts that activated SGK1 will enhance MR occupancy at the ENaCα promoter, synergize with MR in stimulating ENaCα expression, and alter the time course of aldosterone action. SGK1 inhibitors would be predicted to have the opposite effect and could be clinically useful as a potassium-sparing diuretic or for treatment of hypertension associated with the metabolic syndrome (also referred to as syndrome X). However, SGK1 has a variety of effects on glucose metabolism, growth, and proliferation in many cell types, including renal tubule and mammary epithelial cells and hepatocytes, and the potential clinical use of chemicals that modulate this important mediator must be approached with caution.
Acknowledgments
This work was supported by NIH grants DK056695 and DK054354.
Footnotes
Nonstandard abbreviations used: AF9, ALL1-fused gene from chromosome 9; Dot1a, disruptor of telomeric silencing alternative splice variant a; ENaC, epithelial Na+ channel; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; MRE, mineralocorticoid response element; NR, nuclear receptor; SGK1, serum- and glucocorticoid–induced kinase 1.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:592–595 (2007). doi:10.1172/JCI31538.
See the related article beginning on page 773.
References
- 1.Hughey R.P., et al. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J. Biol. Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 2.Tong Q., Gamper N., Medina J.L., Shapiro M.S., Stockand J.D. Direct activation of the epithelial Na(+) channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J. Biol. Chem. 2004;279:22654–22663. doi: 10.1074/jbc.M401004200. [DOI] [PubMed] [Google Scholar]
- 3.Diakov A., Korbmacher C. A novel pathway of epithelial sodium channel activation involves a serum- and glucocorticoid-inducible kinase consensus motif in the C terminus of the channel’s alpha-subunit. J. Biol. Chem. 2004;279:38134–38142. doi: 10.1074/jbc.M403260200. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth M.B., Edinger R.S., Johnson J.P., Frizzell R.A. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J. Gen. Physiol. 2005;125:81–101. doi: 10.1085/jgp.200409124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S.-Y., et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd C., Naray-Fejes-Toth A. Gene regulation of ENaC subunits by serum- and glucocorticoid-inducible kinase-1. Am. J. Physiol. Renal Physiol. 2005;288:F505–F512. doi: 10.1152/ajprenal.00242.2004. [DOI] [PubMed] [Google Scholar]
- 7.Webster M.K., Goya L., Ge Y., Maiyar A.C., Firestone G.L. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wulff P., et al. Impaired renal Na+ retention in the sgk1-knockout mouse. . J. Clin. Invest. 2002;110:1263–1268. doi: 10.1172/JCI200215696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhalla V., Soundararajan R., Pao A.C., Li H., Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am. J. Physiol. Renal Physiol. 2006;291:F714–F721. doi: 10.1152/ajprenal.00061.2006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W., et al. Aldosterone-induced Sgk1 relieves Dot1a-AF9–mediated transcriptional repression of epithelial Na+ channel α. . J. Clin. Invest. 2007;117:773–783. doi: 10.1172/JCI29850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mick V.E., et al. The alpha-subunit of the epithelial sodium channel is an aldosterone-induced transcript in mammalian collecting ducts, and this transcriptional response is mediated via distinct cis-elements in the 5′-flanking region of the gene. Mol. Endocrinol. 2001;15:575–588. doi: 10.1210/mend.15.4.0620. [DOI] [PubMed] [Google Scholar]
- 12.Lee D.Y., Teyssier C., Strahl B.D., Stallcup M.R. Role of protein methylation in regulation of transcription. Endocr. Rev. 2005;26:147–170. doi: 10.1210/er.2004-0008. [DOI] [PubMed] [Google Scholar]
- 13.Feng Q., et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr. Biol. 2002;12:1052–1058. doi: 10.1016/s0960-9822(02)00901-6. [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen F., Gafken P.R., Gottschling D.E. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109:745–756. doi: 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 15.Ng H.H., et al. Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. . Genes Dev. 2002;16:1518–1527. doi: 10.1101/gad.1001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., et al. Aldosterone-sensitive repression of ENaCalpha transcription by a histone H3 lysine-79 methyltransferase. Am. J. Physiol., Cell Physiol. 2006;290:C936–C946. doi: 10.1152/ajpcell.00431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., Xia X., Reisenauer M.R., Hemenway C.S., Kone B.C. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J. Biol. Chem. 2006;281:18059–18068. doi: 10.1074/jbc.M601903200. [DOI] [PMC free article] [PubMed] [Google Scholar]