Abstract
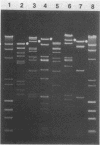
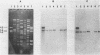
The Emr, Kmr, and Smr determinants of the Streptococcus faecalis R plasmid pJH1 were cloned in Streptococcus sanguis with a streptococcal plasmid vector, pVA380-1. Each cloned determinant was used as a probe in hybridization reactions with dot blots containing plasmid-enriched DNA from 91 group D streptococcal isolates resistant to erythromycin, kanamycin, and streptomycin; the isolates were obtained from animal and human sources in a variety of geographical locations. Nearly 70% of the strains contained DNA that hybridized to each of the three resistance determinants from pJH1. Five plasmids mediating resistance to erythromycin, kanamycin, and streptomycin were examined in more detail. These plasmids varied in size between 26 and 105 kilobase pairs (kbp) and exhibited very different EcoRI restriction patterns. However, each plasmid contained the resistance determinants on a single 13- to 20-kbp EcoRI fragment. Southern blot hybridizations and additional restriction endonuclease digests revealed extensive DNA sequence homology and virtually indistinguishable restriction endonuclease maps within a 9- to 11-kbp region of each plasmid which included the resistance determinants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., Gonda M. A., Ranhand J. M., LeBlanc D. J. Streptococcus faecalis R plasmid pJH1 contains a pAM alpha 1 delta 1-like replicon. J Bacteriol. 1985 Nov;164(2):626–632. doi: 10.1128/jb.164.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., LeBlanc D. J. Genetic, molecular, and functional analysis of Streptococcus faecalis R plasmid pJH1. J Bacteriol. 1983 Sep;155(3):1094–1104. doi: 10.1128/jb.155.3.1094-1104.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., LeBlanc D. J. Streptococcus faecalis R plasmid pJH1 contains an erythromycin resistance transposon (Tn3871) similar to transposon Tn917. J Bacteriol. 1984 Jun;158(3):1172–1174. doi: 10.1128/jb.158.3.1172-1174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basker M. J., Slocombe B., Sutherland R. Aminoglycoside-resistant enterococci. J Clin Pathol. 1977 Apr;30(4):375–380. doi: 10.1136/jcp.30.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Shaw W. V., Jacob A. E. Plasmid-mediated mechanisms of resistance to aminoglycoside-aminocyclitol antibiotics and to chloramphenicol in group D streptococci. Antimicrob Agents Chemother. 1978 May;13(5):716–725. doi: 10.1128/aac.13.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M. Emergence of antibiotic resistance in hospitals, 1935-1975. Rev Infect Dis. 1979 Jan-Feb;1(1):4–22. doi: 10.1093/clinids/1.1.4. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Huang R. T., Davies J. Aminocyclitol resistance in Staphylococcus aureus: presence of plasmids and aminocyclitol-modifying enzymes. Plasmid. 1983 Mar;9(2):147–158. doi: 10.1016/0147-619x(83)90017-3. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Delbos F., Bieth G. High-level aminoglycoside resistance in group A, B, G, D (Streptococcus bovis), and viridans streptococci. Antimicrob Agents Chemother. 1982 Jan;21(1):176–179. doi: 10.1128/aac.21.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N. Characterization of two tetracycline resistance determinants in Streptococcus faecalis JH1. J Bacteriol. 1982 May;150(2):835–843. doi: 10.1128/jb.150.2.835-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Virgili S. S., Scott C. L. Extrachromosomal gene systems in Streptococcus mutans. Adv Exp Med Biol. 1978;107:859–868. doi: 10.1007/978-1-4684-3369-2_96. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. Streptococcus plasmid pAM alpha 1 is a composite of two separable replicons, one of which is closely related to Bacillus plasmid pBC16. J Bacteriol. 1983 Aug;155(2):607–615. doi: 10.1128/jb.155.2.607-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J., Novick R. P. Closely related plasmids from Staphylococcus aureus and soil bacilli. Plasmid. 1982 Mar;7(2):152–162. doi: 10.1016/0147-619x(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Rollins L. D., Lee L. N., LeBlanc D. J. Evidence for a disseminated erythromycin resistance determinant mediated by Tn917-like sequences among group D streptococci isolated from pigs, chickens, and humans. Antimicrob Agents Chemother. 1985 Apr;27(4):439–444. doi: 10.1128/aac.27.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. A transposon (Tn917) in Streptococcus faecalis that exhibits enhanced transposition during induction of drug resistance. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1217–1221. doi: 10.1101/sqb.1979.043.01.138. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Damle S. P., Clewell D. B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979 Jun;15(6):828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Beaman A. J., Lee L. N. Tween 80 effect on glucosyltransferase synthesis by Streptococcus salivarius. J Bacteriol. 1978 Jan;133(1):231–239. doi: 10.1128/jb.133.1.231-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Moellering R. C., Jr, Weinberg A. N. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol. 1971 Mar;105(3):873–879. doi: 10.1128/jb.105.3.873-879.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., Engel H. W., van Klingeren B. Drug resistance in group D streptococci of clinical and nonclinical origin: prevalence, transferability, and plasmid properties. Antimicrob Agents Chemother. 1977 Jun;11(6):925–932. doi: 10.1128/aac.11.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]