Abstract
Mutations that increase the longevity of the soil nematode Caenorhabditis elegans could define genes involved in a process specific for aging. Alternatively, these mutations could reduce animal metabolic rate and increase longevity as a consequence. In ectotherms, longevity is often negatively correlated with metabolic rate. Consistent with these observations, environmental conditions that reduce the metabolic rate of C. elegans also extend longevity. We found that the metabolic rate of long-lived C. elegans mutants is reduced compared with that of wild-type worms and that a genetic suppressor that restored normal longevity to long-lived mutants restored normal metabolic rate. Thus, the increased longevity of some long-lived C. elegans mutants may be a consequence of a reduction in their metabolic rate, rather than an alteration of a genetic pathway that leads to enhanced longevity while maintaining normal physiology. The actual mechanism responsible for the inverse correlation between metabolic rate and longevity remains unknown.
Despite the universality of aging and death, we lack a widely accepted explanation from either evolutionary or mechanistic perspectives for why organisms senesce and die (1, 2). The free-living soil nematode Caenorhabditis elegans is a popular model organism in aging studies. Use of C. elegans for aging studies has increased rapidly because of the identification of mutations that can increase worm life span (3–8). These mutations potentially identify genes specifically involved in aging and may provide insight into the molecular basis of senescence. The existence of these long-lived mutants has been interpreted as evidence for a genetic program that controls aging (9). Here, we propose an alternative view: that some of these mutations may increase longevity as a secondary consequence of lower metabolic rates. As such, the mutational effects are comparable to the effect observed with such simple environmental manipulations as an alteration in ambient temperature.
MATERIALS AND METHODS
Strains Used.
The following C. elegans strains were used: (i) wild type, standard wild-type strain, N2; (ii) age-1, the first long-lived C. elegans strain identified (4), which is a double mutant containing fer-15(b26) and age-1(hx546); (iii) daf-2-(e1370), a long-lived mutant originally identified as a dauer-constitutive mutant (10, 11); and (iv) clk-1 daf-2, a double mutant containing clk-1(e2519) and daf-2(e1370), which was reported to have the greatest increase in longevity of any C. elegans strain (6). We found that the single age-1 mutant [age-1(hx546)] strain consistently had wild-type longevity at 20°C. Because we did observe an extended longevity in the original double-mutant strain, but not the single age-1 strain, we used the original age-1 fer-15 strain in this study.
Previous studies have found that, relative to wild-type worms, these mutations increase longevity by 50–65% for the age-1 fer-15 mutants (4, 6), 60–110% for the daf-2 mutants (5, 7), and 130–475% for the clk-1 daf-2 mutants (6). Growth temperature is an important factor affecting the relative increase in longevity of these long-lived worms.
Survivorship Analysis.
Worms were maintained on nematode growth medium on 35-mm Petri plates and fed live Escherichia coli (strain OP50). Growth temperatures were controlled by placing worms in different incubators in which temperature was monitored at 2-h intervals with Hobo temperature-recording data loggers (Onset Computer Corp., Bourne, MA). All long-lived mutants and a wild-type control were maintained and assayed at 20°C. Initial worm density was identical between all groups (50 worms per plate), and worms were transferred regularly to new plates to ensure that the worms were in optimal environmental conditions. A worm was scored as dead when it ceased moving and did not respond to a mechanical stimulus. Many (15–45%) daf-2 and clk-1 daf-2 worms died containing larvae inside. These worms were excluded from the survivorship analysis.
Metabolic Measurements.
Metabolic rate was measured on groups of 50 worms in a 2.2-ml glass vial. Worms were placed on a small agarose spot and sealed in the vial, flushed with CO2-free air, and placed in an incubator for 5–6 h. A 1-ml gas sample was then removed from the vial with a Hamilton SampleLock syringe and injected into a Sable System TR-2 carbon dioxide gas respirometry system. The vial was then reflushed with CO2-free air and a second sample was taken 5 h later. Each group of worms was run in duplicate, and each set of experiments included at least five samples without worms as a control for background CO2 production. Control experiments showed that the agarose spot neither increased nor decreased CO2 production significantly and that groups of worms have a constant metabolic rate over the time-course of these experiments. The amount of CO2 produced by each group was calculated by using datacan software (Sable Systems International, Henderson, NV). This value was converted to SI units by using a respiratory quotient of 0.70 to convert CO2 production to oxygen consumption. An energy equivalent of 20.1 J/ml oxygen was used for all energy calculations. The respiratory quotient was calculated by simultaneously measuring oxygen depletion and carbon dioxide generation of several hundred wild-type worms by using a Sable System TR-2 respirometry system coupled to a Sable System PA-1 oxygen analyzer. This value is consistent with that predicted for free-living nematodes (12). To ensure that the worms were well-fed during the measurement, worms were supplied with an abundance of heat-killed E. coli. In a series of control experiments, we determined that worms both grow and have normal longevity when grown on heat-killed bacteria. Metabolic rate of all long-lived C. elegans strains and a wild-type control was measured for worms maintained at 20°C, with metabolic rate measured when the worms were 3, 6, 9, and 12 days old. Lifetime metabolic output of wild-type worms grown at different temperatures was calculated by using a series of 4–5 metabolic measurements starting from when worms became egg-laying adults and continuing until 25% of the population had died. This time period includes the vast majority of the lifetime metabolic output of a worm. To avoid potential sampling bias, we did not measure metabolic rates in older populations because of the significant mortality that had started to occur in the populations. This measurement interval varied from 2–9 days for worms grown at 25°C to 9–40 days for worms at 10°C.
RESULTS AND DISCUSSION
Two environmental factors strongly influence C. elegans longevity: food availability and growth temperature. When starved, young C. elegans larvae can enter into a dormant state called the dauer larva (11, 13). Although dauer larvae are capable of moving, they are characterized by a unique morphology and enter a period of dormancy with reduced activity and metabolic rate (13, 14). Dauer worms can survive for months in this state and the time spent as dauer larvae is additive to their total longevity (13). Also, as is the case in most ectotherms, C. elegans longevity is negatively correlated with growth temperature. Wild-type worms grown at low temperature live about four times longer than their high temperature counterparts (Fig. 1a), and the worm life span at low temperature is approximately as long as the longest-lived mutants. The large effect of temperature on longevity is thought to be caused by the influence of temperature on metabolic rate (15–18).
Figure 1.
Survivorship, metabolic rate, and metabolic output of wild-type worms grown at 10–25°C. (a) Survivorship of wild-type worms at 10–25°C. Each bar is the mean (± SEM) of ≈100 worms. (b) Metabolic rate of wild-type worms at different temperatures. Metabolic rates were compared between worms of comparable developmental age (young, egg-laying adults). (c) Lifetime metabolic output of wild-type worms at different growth temperatures. Bars represent the means (± SEM) of four measurement values per temperature.
To test the hypothesis that C. elegans longevity is inversely correlated with metabolic rate, we compared the longevity and metabolic rate among two groups of worms: wild-type worms grown at different temperatures and long-lived mutants. For these experiments, we conducted both metabolic and longevity assays under environmental conditions that maximized the reproductive output of the worms. The worms were well fed with bacteria and maintained on solid agar medium within their thermal optimum. As pointed out by Shock (as reviewed in ref. 19), a basic premise of aging research is that the study organism must be maintained under optimum environmental conditions to allow the animal to develop a normal aging phenotype. Because of technical limitations, previous measurements of the metabolic rate of C. elegans involved placing unfed worms in liquid and monitoring changes in the dissolved oxygen levels (14, 20). Although C. elegans can be grown in liquid culture, it is a terrestrial, not an aquatic, nematode. In liquid culture, worms grow much more slowly, have an altered morphology, cannot mate, and have reduced fertility. Maintaining worms in such conditions makes it difficult to determine whether the animal undergoes normal aging. Additionally, we found that unfed worms quickly reduced their metabolic rate and that the metabolic rate of C. elegans grown under optimal environmental conditions is several times higher than previously reported (14, 20).
Recent advances in the sensitivity of carbon dioxide gas analyzers and in analysis software make it possible to measure metabolic rates of a small number of worms under optimum environmental conditions. Using such a system, we compared the metabolic rate of wild-type C. elegans grown at various temperatures. As expected, worms reared at lower temperatures had extended longevity and a reduced metabolic rate (Fig. 1 a and b). These results show that metabolic rate is inversely correlated with C. elegans longevity. Although the metabolic rate differed, at 15 or 20°C, the worms had a similar metabolic output (Fig. 1c). The reduced metabolic output of worms grown at 10 and 25°C may be caused by growing the worms at temperatures close to their thermal limits. Evidence that C. elegans is physiologically stressed at 10 and 25°C is seen in fecundity comparisons between worms grown at these temperatures. C. elegans hermaphrodites grown at 10 and 25°C laid an average of 109 (SEM = 7.0; n = 80) progeny and 228 (SEM = 5.4; n = 68) progeny, respectively. In contrast, hermaphrodites grown at 15 and 20°C laid an average of 281 (SEM = 7.4; n = 85) and 287 (SEM = 5.1; n = 87) progeny, respectively.
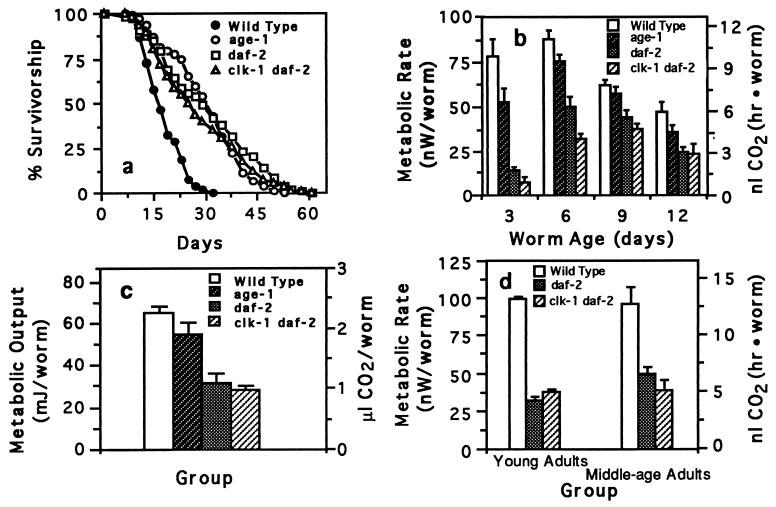
We then compared metabolic rates of wild-type C. elegans to long-lived C. elegans mutants. If mutations that increase C. elegans longevity act by reducing the animals’ metabolic rate, such mutants may have a chronologically extended life span, but their physiological life span (21), the relative number of physiological events carried out, would be equivalent to that of wild-type animals. We compared the metabolic rate and longevity of several long-lived C. elegans mutant strains, age-1 fer-15, daf-2, and clk-1 daf-2. These strains all lived longer than wild type (Fig. 2a), and all had reduced metabolic rates (Fig. 2b).
Figure 2.
Survivorship, metabolic rate, and metabolic output of wild-type C. elegans and long-lived C. elegans mutants. (a) Survivorship of wild-type (n = 168), age-1 (n = 202), daf-2 (n = 167), and clk-1 daf-2 worms (n = 106) maintained at 20°C. (b) Metabolic rate of wild-type and long-lived worms. Metabolic rate was measured over a 9-day period with worms maintained at 20°C. Worms from each strain were measured when 3, 6, 9, and 12 days old. The double age-1 fer 15 mutant was used in all of these experiments. Each bar represents the mean (± SEM) of six separate measurements with 50 worms used per sample measurement. The metabolic rate of daf-2 and clk-1 daf-2 worms is significantly less than wild-type worms at every time point (P < 0.02, Mann–Whitney U test). (c) Metabolic output of wild-type and long-lived worms over a 12-day period. Metabolic output was calculated by integrating the area under a plot of metabolic rate for 9 days. Each bar represents the mean (± SEM) of four integration values. Metabolic output of daf-2 and clk-1 daf-2 is significantly less than wild type (P < 0.01; unpaired Student’s t test). Although reduced, the metabolic output of age-1 was not significantly less than wild type (P = 0.09). Further experiments showed that, despite its longer life span, the lifetime metabolic output of age-1 is not significantly different from that of wild type (data not shown). (d) Metabolic rate comparison between wild-type, daf-2, and clk-1 daf-2 worms when worms are at equivalent body size and development. Each bar plots the mean (± SEM) of two measurements.
A potentially confounding variable in comparing the metabolic rates of wild type with daf-2 and clk-1 daf-2 worms is that daf-2 and clk-1 daf-2 mutants have a reduced growth rate compared with wild-type worms (see Fig. 4). As a control for this effect, we compared metabolic rates of worms between these groups at two points when they were of equivalent size and developmental stage. The first point was when the different groups of worms were young egg-laying adults (the wild-type worms were 3 days old and the daf-2 and clk-1 daf-2 worms were 6 days old). The second point occurred when the groups were near the end of their reproductive period. When compared in this manner, wild-type worms had, on average, metabolic rates 2.5 times higher than daf-2 or clk-1 daf-2 mutants (Fig. 2d).
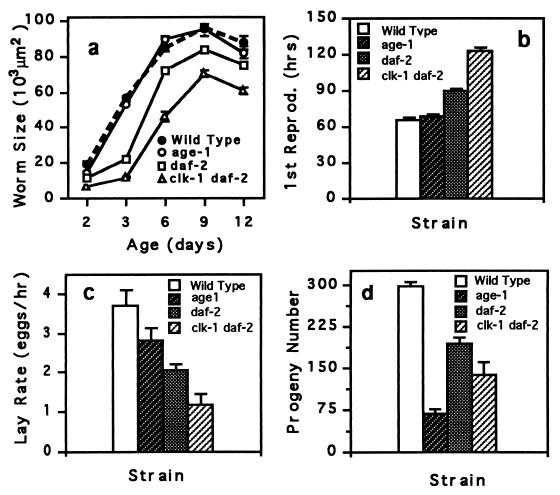
Figure 4.
Life-history traits of long-lived mutant and wild-type hermaphrodites. (a) Worm size is the area of worms measured from an video image of a worm. (b) First reproduction is the time taken for an egg to hatch and develop into an egg-laying adult. (c) Lay rate is the rate of egg laying for young adult worms. (d) Progeny number is the total number of offspring. The large reduction in age-1 fertility is probably caused by the fer-15 mutation, which is also present in this strain. All traits were assayed at 20°C. Means (± SEM) for 15 individuals per strain are plotted.
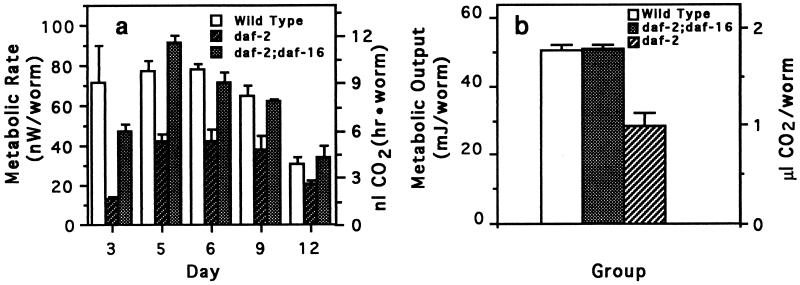
Mutations in the daf-16 gene, which seems to act downstream of the daf-2 gene (5, 10), suppress the extended longevity of daf-2 mutants. As well as restoring normal longevity to daf-2 mutants, mutations in the daf-16 gene restored normal metabolic rate (Fig. 3 a and b). These double mutants also had normal growth rates (data not shown).
Figure 3.
Metabolic rate and metabolic output of wild-type, long-lived daf-2 worms and of daf-2 worms genetically suppressed by a mutation in the daf-16 gene. daf-2;daf-16 worms are identical to daf-2 worms, except that they have an additional mutation in the daf-16 gene. This gene is required for worms to form dauer larvae. (a) Metabolic rate of wild-type, daf-2;daf-16, and daf-2 worms. Bars represent the means (± SEM) of four measurement values per temperature, with 50 worms used per measurement. (b) Metabolic output of wild-type, daf-2;daf-16, and daf-2 worms. Metabolic output was calculated as described in Fig. 2c. Each bar represents the mean (± SEM) of four integration values with each integration value calculated from metabolic rates measured at five time points.
If mutations that extend the longevity of C. elegans affected only aging, then mutant animals should have wild-type growth and development. Although it is known that clk and daf-2 mutations affect development and fecundity, it has been proposed that these factors are not responsible for the extended longevity of these strains (5, 6). We compared several life-history characteristics of wild type and long-lived mutants (Fig. 4). Consistent with these animals being metabolically compromised, long-lived mutants of C. elegans generally developed more slowly and had reduced growth rates and fecundity, characteristics common to worms grown at low temperatures.
The reduced metabolic rates of the long-lived mutants are consistent with the molecular characterization of these mutations. These genes seem to fall into two classes that would be expected to affect metabolic function: clk genes, which are involved in developmental timing, with mutations slowing down development and extending life span (6), and daf genes, which are involved in the pathways that lead to formation of the dauer larvae. The clock gene, clk-1, resembles a yeast gene involved in central metabolic regulation and derepression of an enzyme in glucose catabolism (22), and clk-1 mutations seem to affect longevity in a manner similar to caloric restriction (23). The daf-2 gene is similar to a human insulin receptor gene (24). Based on this observation, it had been hypothesized that these mutations would also affect worm metabolism (ref. 25, but see also ref. 9). The other long-lived mutant examined, age-1, encodes a phosphatidylinositol kinase family member that acts in the dauer signaling pathway (8). The age-1 strain used in this study contained mutations in both the age-1 and the fer-15 genes. Because fer-15 mutants alone have normal longevity (4, 8), we presume that the increased longevity of these mutants is caused by mutations in the age-1 gene, but we do not know why the single age-1 strain we tested displayed normal longevity.
It has been proposed that increased longevity of long-lived C. elegans mutants is due to these mutations either increasing antiaging mechanisms that allow the animal to reduce or repair metabolic damage or due to disrupting programmed aging (9, 26, 27). Alternatively, our experiments indicate that C. elegans has a relatively constant metabolic output and that worm longevity is inversely correlated with metabolic rate. The increased longevity of the long-lived C. elegans mutants tested seems to be a consequence of a reduction in their metabolic output rather than an alteration of a metabolically independent genetic mechanism specific for aging. Rather than aging in a fundamentally different way, these long-lived mutants live as though they are at a low temperature, even when at higher temperatures. Other experiments with Drosophila and houseflies (Musca domestica) have found a similar negative correlation between metabolic rate and longevity (refs. 15–17; but see also ref. 28). It is thought that metabolic rate is coupled to aging by the cumulative damage caused to biomolecules by free radicals and other reactive oxygen intermediates that are the inevitable by-products of aerobic metabolism (18, 26, 29, 30). Our results with C. elegans are consistent with the oxidative-damage hypothesis of aging and with observations showing that, in C. elegans, a mutation that increases oxidative stress reduces worm life span (31). However, the mechanism responsible for the inverse correlation between metabolic output and longevity in C. elegans remains unknown.
Acknowledgments
We thank L. Abbott, J. Bonner, B. Davis, E. Little, J. Lighton, S. Melov, P. Muhlrad, J. Nance, C. Martinez del Rio, R. Parker, D. Vleck, B. Wolf, and two anonymous reviewers for helpful review or discussion of this research and the manuscript. Worm strains were supplied by the Caenorhabditis Genetics Center. This research was supported by a National Institutes of Aging Grant AG11659.
References
- 1.Finch C E. Longevity, Senescence, and the Genome. Chicago: Univ. Chicago Press; 1990. [Google Scholar]
- 2.Rose M. The Evolutionary Biology of Aging. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 3.Van Voorhies W A. Nature (London) 1992;360:457–459. doi: 10.1038/360456a0. [DOI] [PubMed] [Google Scholar]
- 4.Friedman D B, Johnson T E. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenyon C, Chang J, Genach E, Rudner A, Tabtlang R. Nature (London) 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 6.Lakowski B, Hekimi S. Science. 1996;272:1010–1013. doi: 10.1126/science.272.5264.1010. [DOI] [PubMed] [Google Scholar]
- 7.Larsen P L, Albert P S, Riddle D L. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris J Z, Tissenbaum H A, Ruvkun G. Nature (London) 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 9.Kenyon C. Cell. 1996;84:501–504. doi: 10.1016/s0092-8674(00)81024-7. [DOI] [PubMed] [Google Scholar]
- 10.Riddle D L, Swanson M M, Albert P S. Nature (London) 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 11.Riddle D L, Blumenthal T, Meyer B J, Priess J R. C. elegans II. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [PubMed] [Google Scholar]
- 12.O’Riordan V B, Burnell A M. Comp Biochem Physiol B Biochem Mol Biol. 1990;95:125–130. [Google Scholar]
- 13.Klass M, Hirsh D. Nature (London) 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- 14.Anderson G L. Can J Zool. 1978;56:1786–1791. [Google Scholar]
- 15.Miquel J, Lundgren P R, Bensch K G, Atlan H. Mech Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 16.Ragland S S, Sohal R S. Exp Gerontol. 1975;10:279–289. doi: 10.1016/0531-5565(75)90005-4. [DOI] [PubMed] [Google Scholar]
- 17.McArthur M C, Sohal R S. J Gerontol. 1982;37:268–274. doi: 10.1093/geronj/37.3.268. [DOI] [PubMed] [Google Scholar]
- 18.Sohal R S, Orr W C. In: Molecular Aspects of Aging. Esser K, Martin G, editors. New York: Wiley; 1995. pp. 109–127. [Google Scholar]
- 19.Baker G T, III, Achenbaum W A. Exp Gerontol. 1992;27:261–273. doi: 10.1016/0531-5565(92)90054-4. [DOI] [PubMed] [Google Scholar]
- 20.Vanfleteren J R, De Vreese A. J Exp Zool. 1996;274:93–100. doi: 10.1002/(SICI)1097-010X(19960201)274:2<93::AID-JEZ2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Lindstedt S L, Calder W A. Q Rev Biol. 1981;56:1–16. [Google Scholar]
- 22.Ewbank J J, Barnes T M, Lakowski B, Lussier M, Bussey H, Hekimi S. Science. 1997;275:980–983. doi: 10.1126/science.275.5302.980. [DOI] [PubMed] [Google Scholar]
- 23.Lakowski B, Hekimi S. Proc Natl Acad Sci USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura K, Tissenbaum H A, Liu Y, Ruvkun G. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 25.Tissenbaum H A, Ruvkun G. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin G M, Austad S N, Johnson T E. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 27.Lithgow G J, Kirkwood T B L. Science. 1996;273:80. doi: 10.1126/science.273.5271.80. [DOI] [PubMed] [Google Scholar]
- 28.Lints F A. Gerontology. 1989;35:36–57. doi: 10.1159/000212998. [DOI] [PubMed] [Google Scholar]
- 29.Sohal R S, Allen R G. Exp Gerontol. 1990;25:499–522. doi: 10.1016/0531-5565(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 30.Sohal R S, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii N, Fujii M, Hartman P S, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa S, Suzuki K. Nature (London) 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]