Abstract
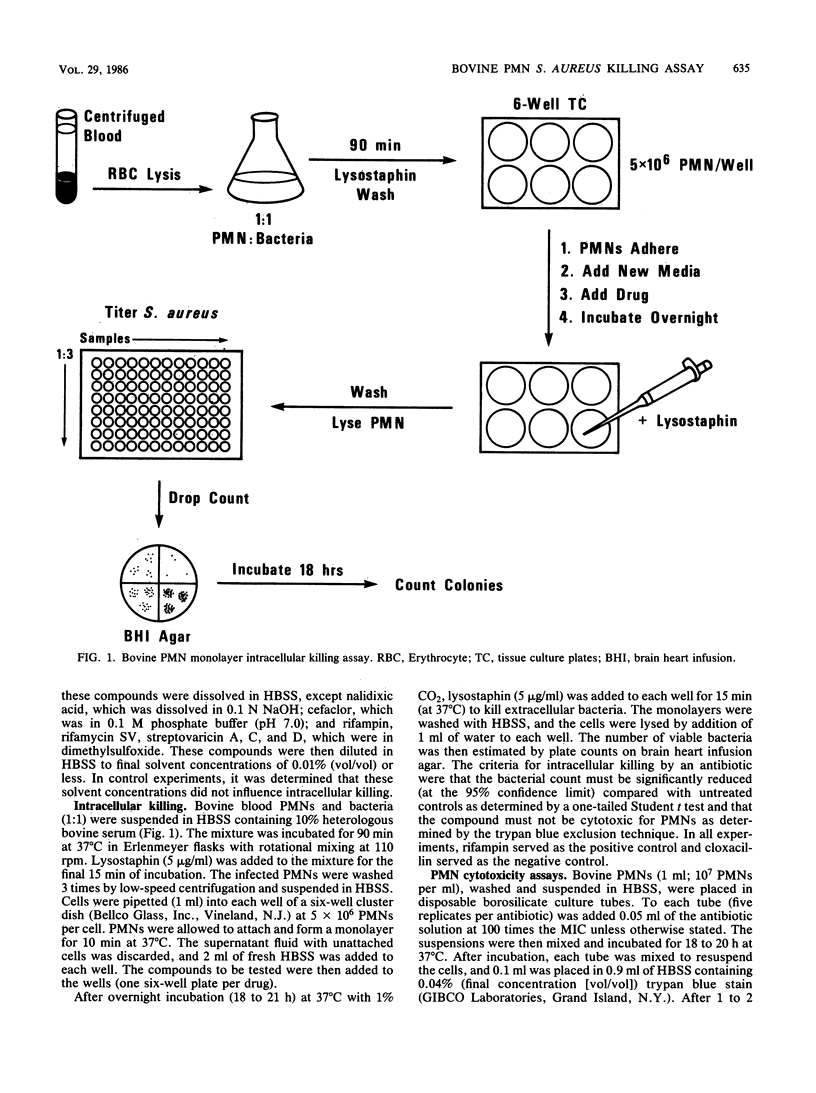
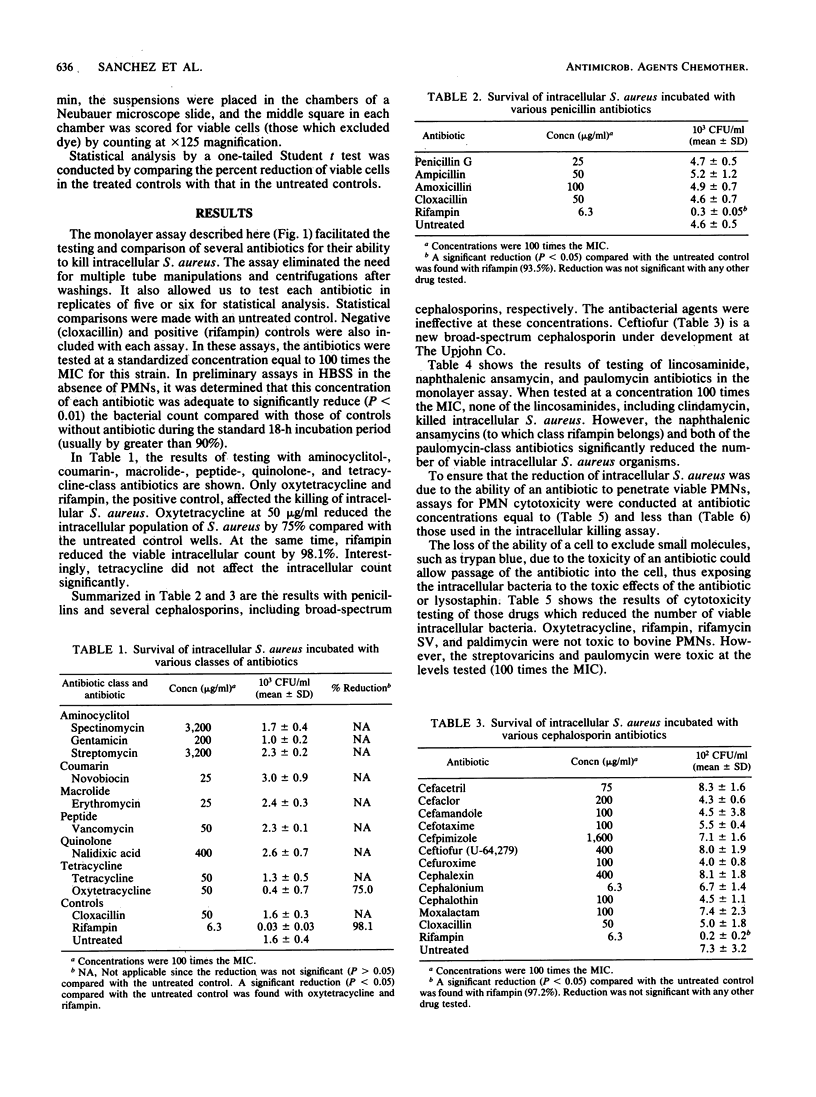
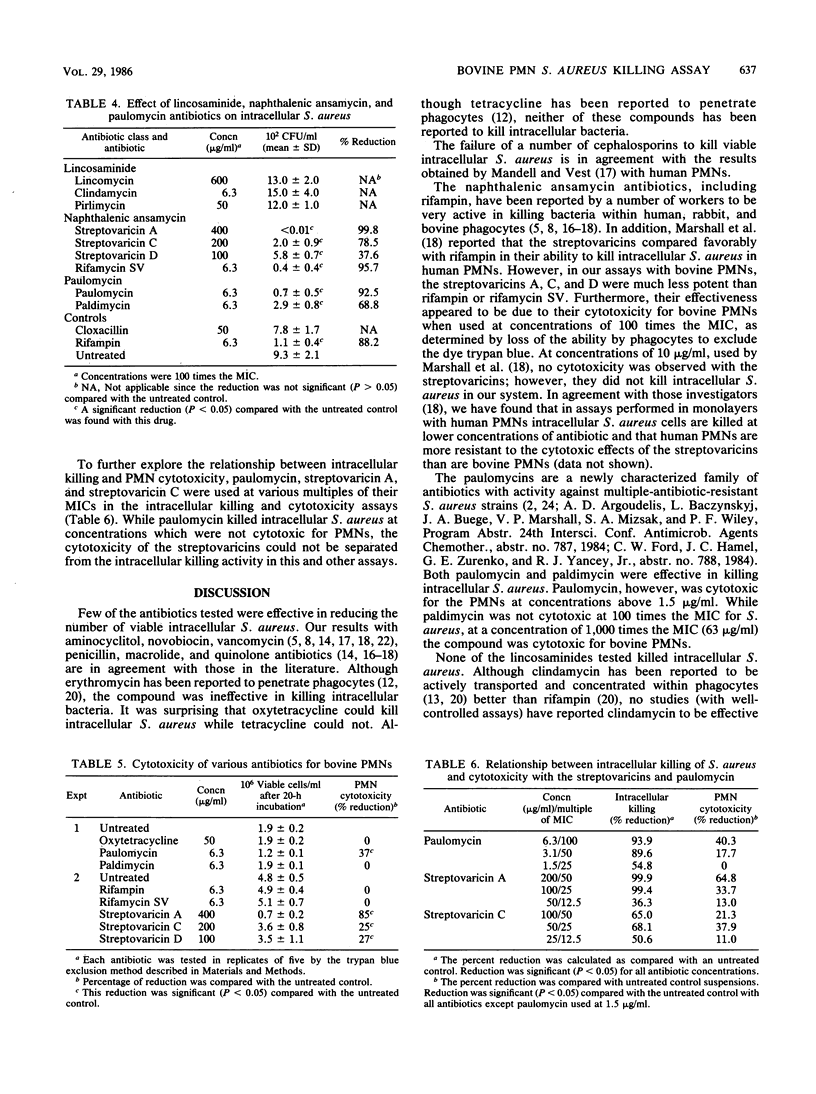
A bovine polymorphonuclear leukocyte (PMN) monolayer system was used to determine the ability of different antibiotics to kill surviving intracellular Staphylococcus aureus. The following classes of antimicrobial agents were tested in this high-volume assay procedure: aminocyclitol, beta-lactam, coumarin, lincosaminide, macrolide, naphthalenic ansamycin, paulomycin, peptide, quinolone, and tetracycline. The activities of these compounds were compared with those of positive (rifampin), negative (cloxacillin), and non-antibiotic-treated controls. Only oxytetracycline, the ansamycins (rifampin, rifamycin SV, streptovaricin A, C, and D), paulomycin, and paldimycin caused a significant reduction in the viable count of intracellular S. aureus. Of these, however, the intracellular killing by the streptovaricins was directly related to the cytotoxicity (as determined by trypan blue exclusion) of these compounds for the PMNs. Although the paulomycins were cytotoxic for the PMNs, the cytotoxic and the intracellular killing activity of these new compounds could be distinguished. The relevance of these results to the therapeutic effectiveness of these antibiotics in the treatment of bovine staphylococcal mastitis is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. C., Heneghan D. J. Extrapolation from experimental chronic staphylococcal mastitis in mice to experimental infections in cattle. Br Vet J. 1979 Nov-Dec;135(6):527–535. doi: 10.1016/s0007-1935(17)30005-2. [DOI] [PubMed] [Google Scholar]
- Argoudelis A. D., Brinkley T. A., Brodasky T. F., Buege J. A., Meyer H. F., Mizsak S. A. Paulomycins A and B. Isolation and characterization. J Antibiot (Tokyo) 1982 Mar;35(3):285–294. doi: 10.7164/antibiotics.35.285. [DOI] [PubMed] [Google Scholar]
- Carlson G. P., Kaneko J. J. Isolation of leukocytes from bovine peripheral blood. Proc Soc Exp Biol Med. 1973 Mar;142(3):853–856. doi: 10.3181/00379727-142-37131. [DOI] [PubMed] [Google Scholar]
- Craven N., Anderson J. C. Antibiotic activity against intraleukocytic Staphylococcus aureus in vitro and in experimental mastitis in mice. Am J Vet Res. 1983 Apr;44(4):709–712. [PubMed] [Google Scholar]
- Craven N., Anderson J. C. The effects of cloxacillin on staphylococci phagocytosed by bovine neutrophils. Res Vet Sci. 1980 Jul;29(1):57–62. [PubMed] [Google Scholar]
- Craven N., Anderson J. C. The location of Staphylococcus aureus in experimental chronic mastitis in the mouse and the effect on the action of sodium cloxacillin. Br J Exp Pathol. 1979 Oct;60(5):453–459. [PMC free article] [PubMed] [Google Scholar]
- Craven N., Anderson J. C. Therapy of experimental staphylococcal mastitis in the mouse with cloxacillin and rifampicin, alone and in combination. Res Vet Sci. 1981 Nov;31(3):295–300. [PubMed] [Google Scholar]
- Dodd F. H., Westgarth D. R., Griffin T. K. Strategy of mastitis control. J Am Vet Med Assoc. 1977 May 15;170(10 Pt 2):1124–1128. [PubMed] [Google Scholar]
- Easmon C. S., Crane J. P. Cellular uptake of clindamycin and lincomycin. Br J Exp Pathol. 1984 Dec;65(6):725–730. [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S. The effect of antibiotics on the intracellular survival of Staphylococcus aureus in vitro. Br J Exp Pathol. 1979 Feb;60(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1981 Nov;144(5):472–479. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- Lam C., Mathison G. E. Effect of low intraphagolysosomal pH on antimicrobial activity of antibiotics against ingested staphylococci. J Med Microbiol. 1983 Aug;16(3):309–316. doi: 10.1099/00222615-16-3-309. [DOI] [PubMed] [Google Scholar]
- Lam C., Mathison G. E. Intraphagocytic protection of staphylococci from extracellular penicillin. J Med Microbiol. 1982 Aug;15(3):373–385. doi: 10.1099/00222615-15-3-373. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973 Jul;52(7):1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Vest T. K. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis. 1972 May;125(5):486–490. doi: 10.1093/infdis/125.5.486. [DOI] [PubMed] [Google Scholar]
- Marshall V. P., Cialdella J. I., Ohlmann G. M., Gray G. D. MIC values do not predict the intraphagocytic killing of Staphylococcus aureus by naphthalenic ansamycins. J Antibiot (Tokyo) 1983 Nov;36(11):1549–1560. doi: 10.7164/antibiotics.36.1549. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyörälä S. Terapiresultat vid intracisternal behandling av subakuta och kroniska mastiter under laktationsperioden. Nord Vet Med. 1982 Dec;34(12):435–440. [PubMed] [Google Scholar]
- Solberg C. O. Protection of phagocytized bacteria against antibiotics. A new method for the evaluation of neutrophil granulocyte functions. Acta Med Scand. 1972 May;191(5):383–387. [PubMed] [Google Scholar]
- Wiley P. F., Mizsak S. A., Baczynskyj L., Argoudelis A. D. The structure of paulomycin. J Antibiot (Tokyo) 1984 Oct;37(10):1273–1275. doi: 10.7164/antibiotics.37.1273. [DOI] [PubMed] [Google Scholar]



