Abstract
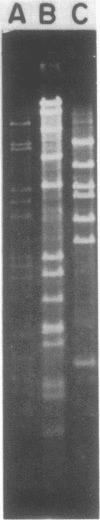
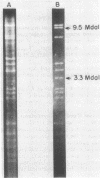
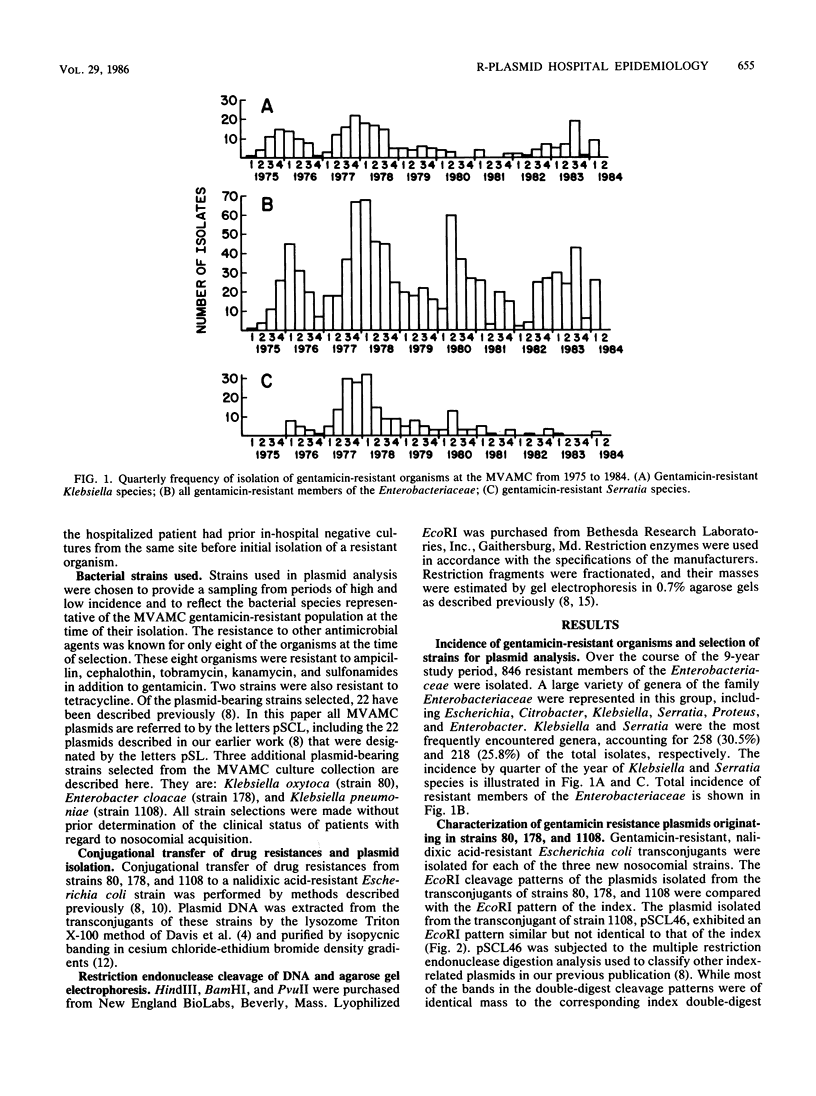
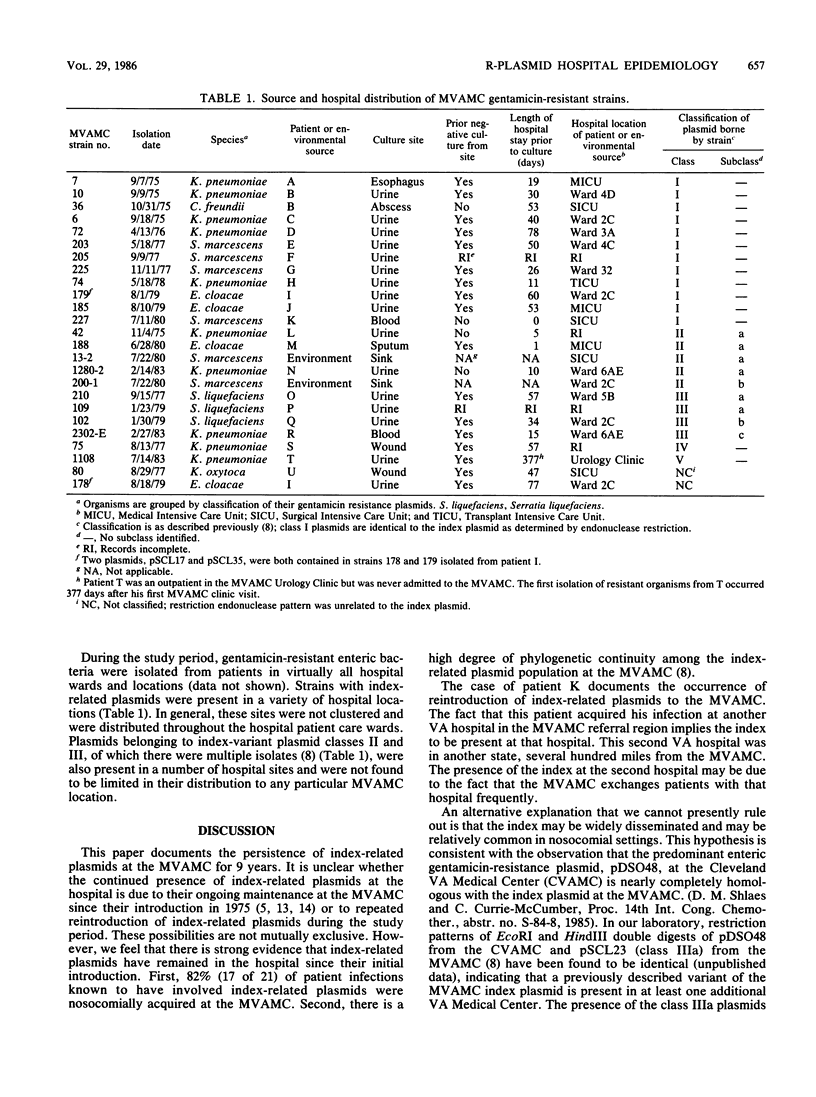
A single plasmid clone was predominantly responsible for gentamicin resistance at the Minneapolis Veterans Administration Medical Center (MVAMC) for 9 years, although two unrelated R plasmids were found. Epidemiological data and restriction endonuclease analysis of 25 plasmid isolates suggested that the clonally derived plasmid population had persisted at the hospital. However, one case of reintroduction of the original epidemic plasmid from a hospital in another state was documented 4 years after the introduction of the original plasmid to the MVAMC. Resistance by the clonally derived plasmid population was not localized geographically within the MVAMC but rather was a hospital-wide problem. Furthermore, previously described classes of DNA rearrangement of the original plasmid were also widely disseminated in the hospital, implying that spread of strains bearing index-related plasmids was relatively unimpeded within the MVAMC despite extensive barrier isolation and cohorting measures. Potential environmental reservoirs of the plasmids were identified in hospital sinks and drains, but their relation to continued patient infection is not known.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtney M. A., Miller J. R., Summersgill J., Melo J., Raff M. J., Streips U. N. R-factor responsible for an outbreak of multiply antibiotic-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 1980 Dec;18(6):926–929. doi: 10.1128/aac.18.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hughes V. M., Nugent M. E., Richards H. Plasmids and transposons and their stability and mutability in bacteria isolated during an outbreak of hospital infection. Plasmid. 1979 Apr;2(2):182–196. doi: 10.1016/0147-619x(79)90037-4. [DOI] [PubMed] [Google Scholar]
- Davey R. B., Pittard J. Endonuclease fingerprinting of plasmids mediating gentamicin resistance in an outbreak of hospital infections. Aust J Exp Biol Med Sci. 1980 Aug;58(4):331–338. doi: 10.1038/icb.1980.32. [DOI] [PubMed] [Google Scholar]
- Gerding D. N., Buxton A. E., Hughes R. A., Cleary P. P., Arbaczawski J., Stamm W. E. Nosocomial multiply resistant Klebsiella pneumoniae: epidemiology of an outbreak of apparent index case origin. Antimicrob Agents Chemother. 1979 Apr;15(4):608–615. doi: 10.1128/aac.15.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Larson T. A. Aminoglycoside resistance in gram-negative bacilli during increased amikacin use. Comparison of experience in 14 United States hospitals with experience in the Minneapolis Veterans Administration Medical Center. Am J Med. 1985 Jul 15;79(1A):1–7. doi: 10.1016/0002-9343(85)90184-6. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F. Characteristics of Serratia marcescens containing a plasmid coding for gentamicin resistance in nosocomial infections. J Infect Dis. 1981 Jun;143(6):810–817. doi: 10.1093/infdis/143.6.810. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Gerding D. N., Cleary P. P. Plasmid macroevolution in a nosocomial environment: demonstration of a persistent molecular polymorphism and construction of a cladistic phylogeny on the basis of restriction data. Mol Gen Genet. 1984;194(1-2):173–178. doi: 10.1007/BF00383513. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HARADA K., HASHIMOTO H. Multiple resistance of enteric bacteria and transmission of drug-resistance to other strain by mixed cultivation. Jpn J Exp Med. 1960 Jun;30:179–184. [PubMed] [Google Scholar]
- Markowitz S. M., Veazey J. M., Jr, Macrina F. L., Mayhall C. G., Lamb V. A. Sequential outbreaks of infection due to Klebsiella pneumoniae in a neonatal intensive care unit: implication of a conjugative R plasmid. J Infect Dis. 1980 Jul;142(1):106–112. doi: 10.1093/infdis/142.1.106. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Ross D. G., Guzman M. A., Medeiros A. A., Hedges R. W., Botstein D. Dissemination of an antibiotic resistance plasmid in hospital patient flora. Antimicrob Agents Chemother. 1980 Apr;17(4):537–543. doi: 10.1128/aac.17.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. L., Peterson B. C., Gerding D. N., Cleary P. P. Physical characterization of ten R plasmids obtained from an outbreak of nosocomial Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 1979 Apr;15(4):616–624. doi: 10.1128/aac.15.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tantulavanich S., Olexy V. M., Prasad T. R., Bird T. J., Talanda-Fath C., Grieble H. G., Farrand S. K. An R plasmid of broad host-range, coding for resistance to nine antimicrobial agents endemic in Gram-negative nosocomial isolates. J Med Microbiol. 1981 Nov;14(4):371–380. doi: 10.1099/00222615-14-4-371. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Plorde J. J., Falkow S. Molecular analysis of R-factors from multiresistant nosocomial isolates. J Infect Dis. 1980 May;141(5):625–636. doi: 10.1093/infdis/141.5.625. [DOI] [PubMed] [Google Scholar]