Abstract
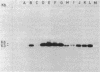
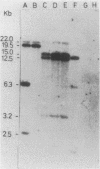
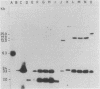
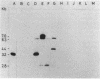
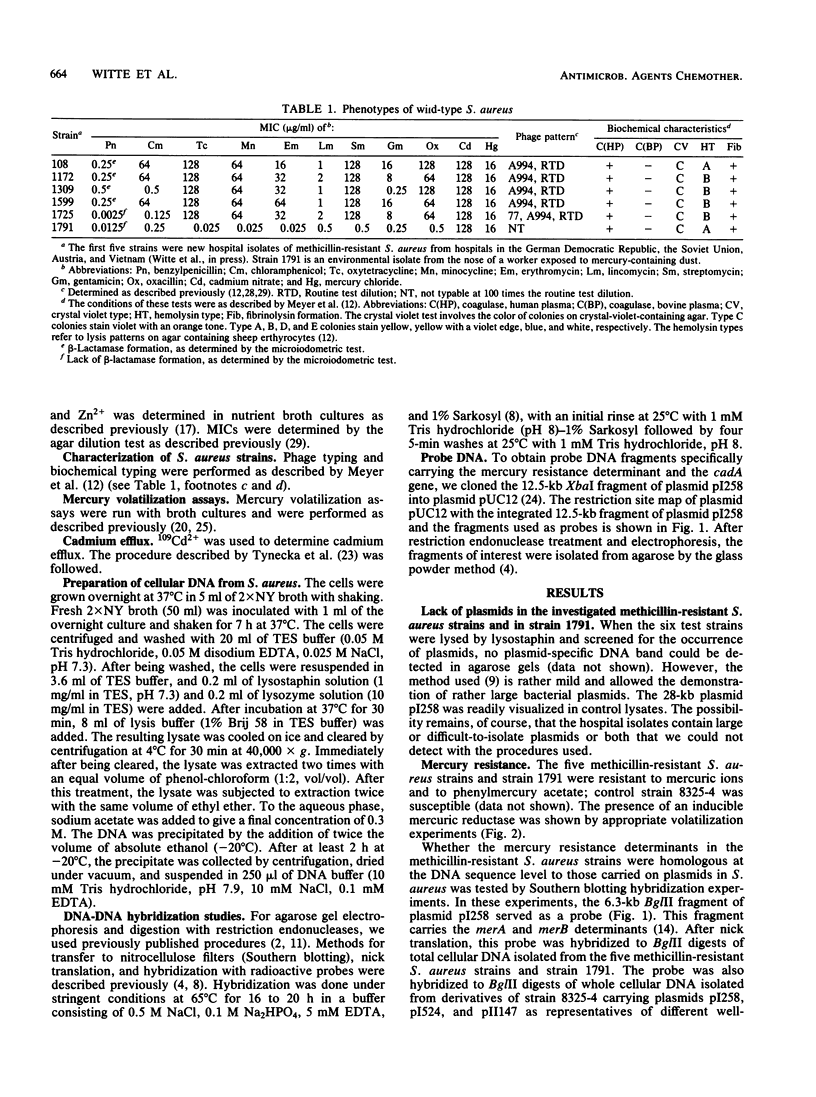
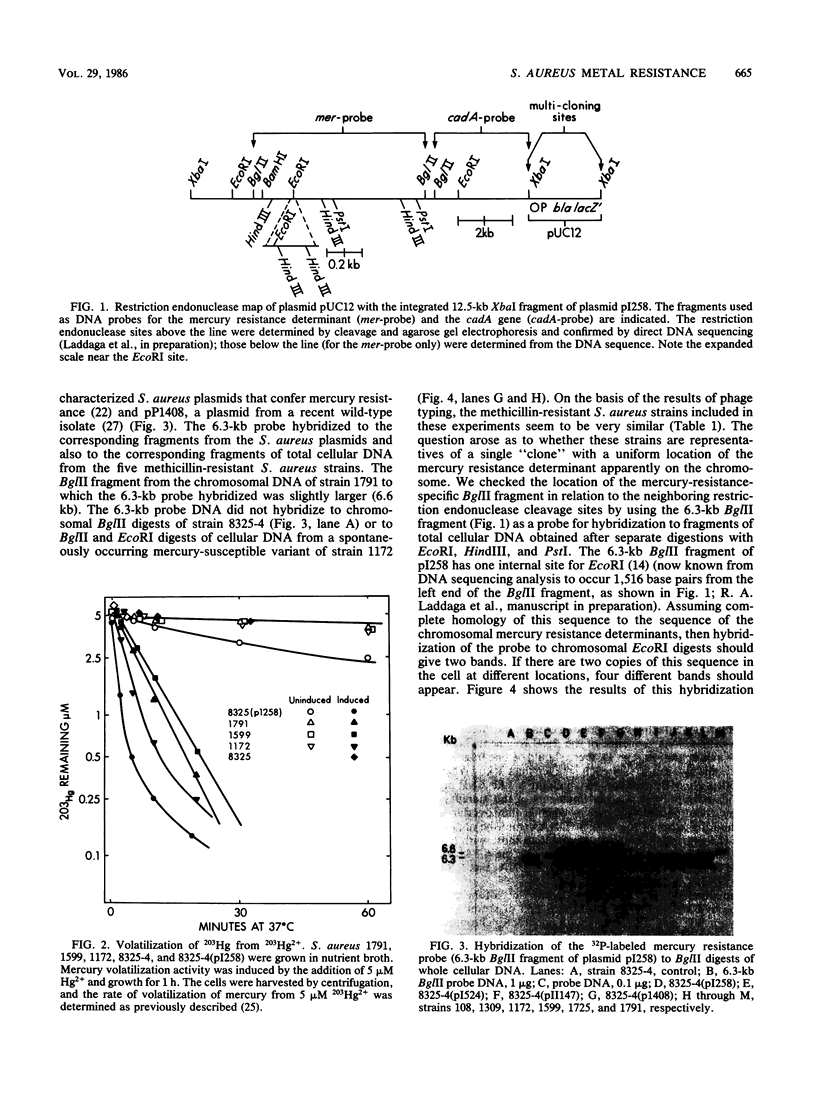
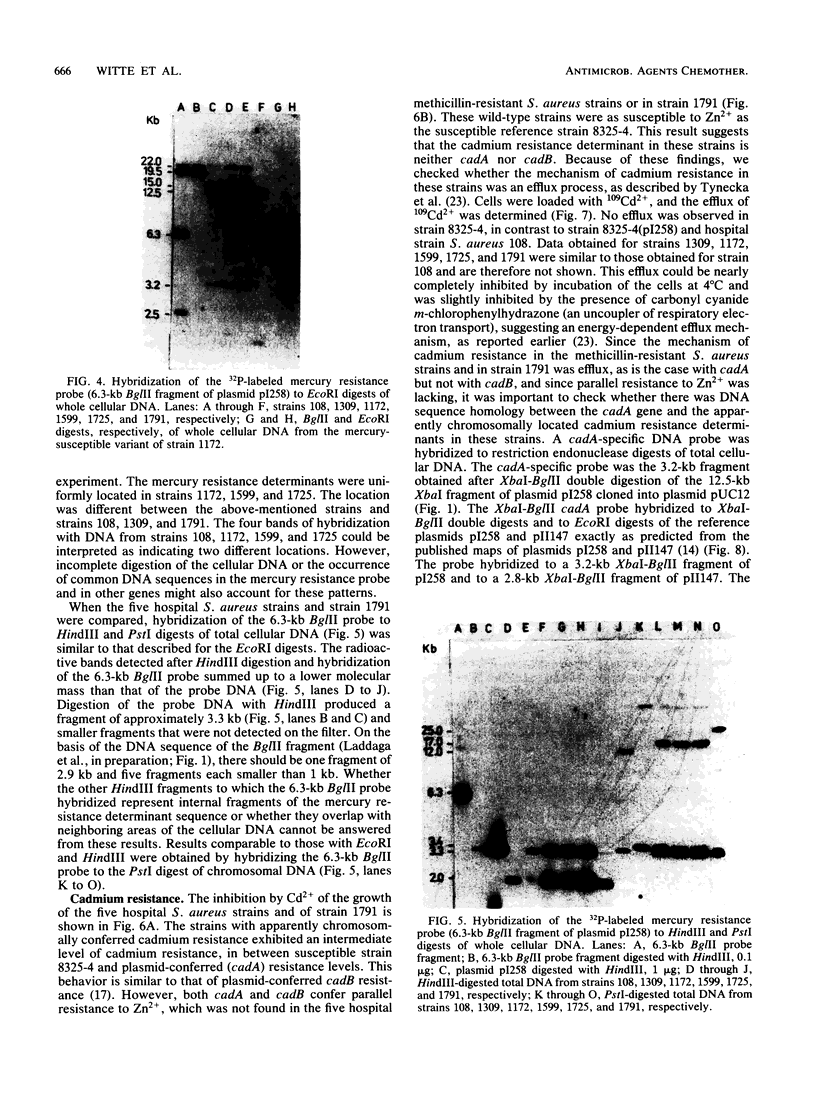
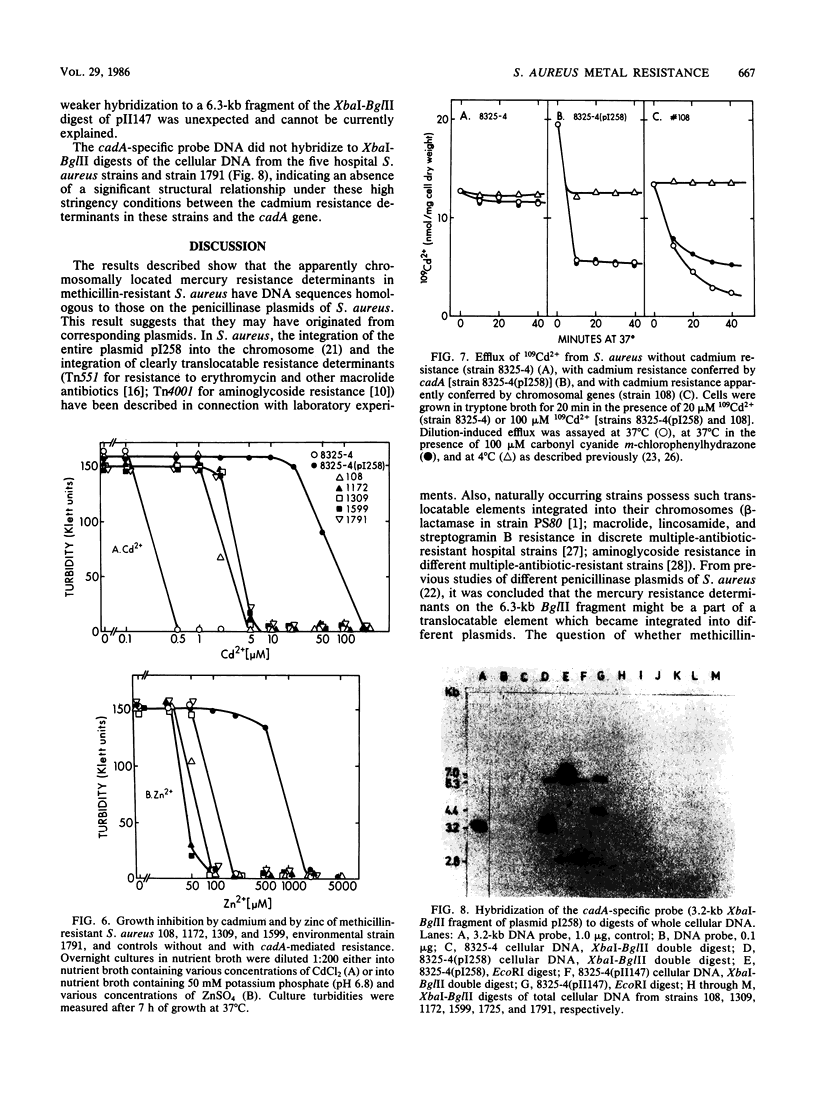
Apparently chromosomally located mercury resistance determinants in five methicillin-resistant Staphylococcus aureus strains of different geographical origin were structurally homologous to plasmid-located mercury resistance determinants in S. aureus. These were all located on a 6.3-kilobase (kb) Bg/II fragment, as evident from Southern hybridization experiments with the 6.3-kb Bg/II fragment of plasmid pI258 as the probe. These methicillin-resistant S. aureus strains exhibited similar phage susceptibility patterns and biochemical reactions. They differed, however, in the DNA location of the mercury resistance determinants, as evidenced by neighboring cleavage sites for restriction endonucleases EcoRI, HindIII, and PstI. In an environmental (nonhospital) strain in which mercury resistance was also apparently chromosomally conferred, these determinants were also homologous to pI258 DNA, but they were located on a 6.6-kb Bg/II fragment. Cadmium resistance determinants in the five methicillin-resistant S. aureus strains and the environmental S. aureus strain were not similar to the known plasmid-located determinants cadA and cadB. Cd2+ resistance was based on an efflux mechanism for Cd2+. However, no parallel resistance to zinc was conferred. The 3.2-kb XbaI-Bg/II fragment obtained from plasmid pI258 and used as a cadA-specific probe did not hybridize to total DNA digests of the strains with apparently chromosomally determined cadmium resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. The genetics of penicillinase production in Staphylococcus aureus strain PS80. J Gen Microbiol. 1969 Dec;59(3):289–301. doi: 10.1099/00221287-59-3-289. [DOI] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M., Son P. H. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. doi: 10.1016/0076-6879(83)01008-3. [DOI] [PubMed] [Google Scholar]
- Dubova V. G. Obrazovanie ustoichivykh k kloksatsillinu (5-metil-3-orto-khlorfenil-4-izoksazolil-penitsillin) shtammov stafilokokkov i kharakteristika ikh svoistv. Antibiotiki. 1965 Nov;10(11):969–973. [PubMed] [Google Scholar]
- Green L., Miller R. D., Dykhuizen D. E., Hartl D. L. Distribution of DNA insertion element IS5 in natural isolates of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4500–4504. doi: 10.1073/pnas.81.14.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hall B. M. Mercury resistance of Staphylococcus aureus. J Hyg (Lond) 1970 Mar;68(1):121–129. doi: 10.1017/s0022172400028576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman L., Riley M. Conservation and variation of nucleotide sequences in Escherichia coli strains isolated from nature. J Bacteriol. 1980 Nov;144(2):560–568. doi: 10.1128/jb.144.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E., Miller R. D., Green L., de Framond J. Transposable element IS50 improves growth rate of E. coli cells without transposition. Cell. 1983 Dec;35(2 Pt 1):503–510. doi: 10.1016/0092-8674(83)90184-8. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Distribution of resistances to metals and antibiotics of staphylococcal strains in Japan. Zentralbl Bakteriol Orig A. 1977 Apr;237(4):470–476. [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Thompson N. E., Haubrich D., Novick R. P. Chromosomal map locations of integrated plasmids and related elements in Staphylococcus aureus. Plasmid. 1977 Nov;1(1):38–51. doi: 10.1016/0147-619x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree P. M., Vickery A. M. Further observations on methicillin-resistant staphylococci. Med J Aust. 1973 May 26;1(21):1030–1034. doi: 10.5694/j.1326-5377.1973.tb110903.x. [DOI] [PubMed] [Google Scholar]
- Schaefler S., Jones D., Perry W., Ruvinskaya L., Baradet T., Mayr E., Wilson M. E. Emergence of gentamicin- and methicillin-resistant Staphylococcus aureus strains in New York City hospitals. J Clin Microbiol. 1981 Apr;13(4):754–759. doi: 10.1128/jcm.13.4.754-759.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Schwesinger M. D., Novick R. P. Prophage-dependent plasmid integration in Staphylococcus aureus. J Bacteriol. 1975 Aug;123(2):724–738. doi: 10.1128/jb.123.2.724-738.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalita Z., Murphy E., Novick R. P. Penicillinase plasmids of Staphylococcus aureus: structural and evolutionary relationships. Plasmid. 1980 May;3(3):291–311. doi: 10.1016/0147-619x(80)90042-6. [DOI] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Silver S., Kinscherf T. G. Cation transport alteration associated with plasmid-determined resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Dec;14(6):856–865. doi: 10.1128/aac.14.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Dünnhaupt K. Occurrence of a nonplasmid-located determinant for gentamicin resistance in strains of Staphylococcus aureus. J Hyg (Lond) 1984 Aug;93(1):1–8. doi: 10.1017/s0022172400060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Reissbrodt R. Standardisierte Bestimmung minimaler Hemmkonzentrationen (MHK) antibakteriell wirksamer Chemotherapeutika für schnell wachsende Keime im Plattenverdünnungstest. Z Gesamte Hyg. 1983 Mar;29(3):178–180. [PubMed] [Google Scholar]
- Witte W., Van Dip N., Hummel R. Resistenz gegen Quecksilber und Cadmium bei Staphylococcus aureus unterschiedlicher ökologischer Herkunft. Z Allg Mikrobiol. 1980;20(8):517–521. [PubMed] [Google Scholar]