Abstract
Platelet-derived growth factor (PDGF) isoforms lead to mitogenic, survival, and chemotactic responses in a variety of mesenchymal cell types during development and in the adult. We have studied the importance of phosphatidylinositol-3′ kinase (PI3K) signaling in these responses by mutating the PI3K-binding sites in the PDGF-β receptor by gene targeting in embryonic stem cells. Homozygous mutant mice developed normally; however, cells derived from the mutants were less chemotactic and had largely lost their ability to contract collagen gels in response to PDGF. Injection of a mast cell degranulating agent in mice led to a decrease in interstitial fluid pressure resulting in edema formation. In contrast to wild-type mice, mutant mice were unable to normalize the pressure after treatment with PDGF. Taken together, these observations suggest a function for PDGF signaling through PI3K in interstitial fluid homeostasis by modulating the tension between cells and extracellular matrix structures.
Platelet-derived growth factor (PDGF) isoforms are potent mitogens, survival factors, and chemoattractants for many mesenchymal cell types. PDGF consists of homo- or heterodimers of A- and B-polypeptide chains, which exert their biological effects by binding to two structurally related tyrosine kinase receptors, PDGF-α receptor and β receptor (1).The PDGF signal transduction machinery has been analyzed extensively for the PDGF-β receptor (for reviews, see refs. 2 and 3). Upon ligand binding, PDGF receptors homo- or heterodimerize and phosphorylate each other in trans on specific tyrosine residues, initiating signaling cascades that lead to growth, actin cytoskeleton rearrangements, and chemotaxis (1). A large body of evidence indicates that the latter two effects are dependent on activation of phosphatidylinositol-3′ kinase (PI3K) through binding to two phosphorylated tyrosines (tyrosines 739 and 750 in the mouse sequence; refs. 4 and 5). These two tyrosines are located in Tyr-Xaa-Xaa-Met motifs and thus are recognized by the Src homology 2 (SH2) domains of p85, the regulatory subunit of the PI3K. p85, in turn, associates with the catalytic subunit p110, which phosphorylates the membrane lipids phosphatidylinositol (4)-phosphate and phosphatidylinositol (4,5)-bisphosphate in the D-3 position of their inositol rings to the corresponding (3,4)-diphosphate and (3,4,5)-triphosphate derivatives (reviewed in ref. 6). The effect of PI3K on the actin cytoskeleton, resulting in formation of lamellipodia, probably is mediated by activation of the small GTP-binding protein Rac (7–9).
Targeted disruptions of the genes encoding PDGF ligands and receptors have indicated a role for these proteins in various aspects of muscle and vascular development (10–13). Very similar phenotypes are observed with PDGF-B or PDGF-β receptor mutants and include perinatal death, generalized bleedings and edema, hematological abnormalities, and defects in kidney glomeruli. Some of these defects appear to be associated with the loss of specialized smooth muscle cells such as pericytes (14) and kidney mesangial cells (15), but the cellular basis for these defects, which may involve cell proliferation, survival, or migration, remain unknown. In particular, it is possible that one or another specific signal transduction pathway may play an essential role in mediating various aspects of the phenotypes observed in knock-out experiments. We therefore specifically mutated the tyrosines at positions 739 and 750 to phenylalanine residues in the PDGF-β receptor by gene targeting to investigate the importance of PI3K signaling in vivo.
MATERIALS AND METHODS
Generation of Mutant Mice.
The PDGF-β receptor locus was isolated from a 129Sv genomic library (11). A 4-kb genomic EcoRV fragment containing the exon coding for the PI3K-binding sites was cloned into the pAlter-1 vector (Promega) for site-directed mutagenesis by using the following oligonucleotides: 5′-GACGGTGGCTTCATGGATATGAGC-3′ (Y739F) and 5′-TCTATAGATTTCGTGCCCATGTTGG-3′ (Y750F), respectively (base exchange indicated in bold and underlined). Selected clones were verified by sequencing. The targeting vector consists of a 1.7-kb EcoRV-SpeI genomic 5′ fragment, followed by PGKneobpA expression cassette flanked by loxP sites, a 5-kb SpeI-XhoI genomic 3′ fragment, and a herpes simplex virus thymidine kinase (HSV-TK; negative selection) expression cassette in pBluescript II. The construct was linearized for electroporation into 129Sv-derived embryonic stem (ES) cells (AK7), and colonies were selected with G418 and FIAU. Homologous recombination events were screened by PCR as described (16), using primers from the neo gene (5′-TGGCTACCCGTGATATTGCT-3′) and genomic sequence outside the targeting construct (5′-CCGAAATGTGTACCAGTCTGAAA-3′), resulting in a 3-kb amplification product for correct integration. The cycling parameters were as follows: initial denaturation at 93°C for 2 min, followed by 40 cycles of 93°C for 30 sec, 54°C for 30 sec, and 65°C for 4 min. The PCR product was sequenced to verify the presence of the mutations (primer used: 5′-CCCAGCCACTTGAACCTG-3′). Southern blots were done by using a 177-bp PCR amplification product corresponding to nucleotides 1957–2133 of the mouse PDGF-β receptor cDNA, hybridizing to genomic DNA 5′ outside of the targeting construct [5.2-kb wild-type (wt) allele; 6.9-kb mutant allele]. The blots were rehybridized with neo to show that there was only a single insertion of the vector and with plasmid to verify the absence of a concatemer. Tissue culture and blastocyst injections were as described previously (16).
Embryonic Fibroblasts.
3T3-like embryonic fibroblasts were derived from E13 embryos according to standard procedures (17). Cells were grown in EF medium [DMEM (GIBCO) supplemented with 10% FBS (Sigma)/0.1 mM 2-mercaptoethanol/2 mM l-glutamine]. All experiments involving mouse embryonic fibroblasts were done repeatedly with two or more different cell isolates.
In Vitro PI3K Assay.
Subconfluent embryonic fibroblasts in 10-cm tissue culture dishes were starved in DMEM containing 0.5% FCS overnight. For ligand stimulation, medium was removed except for 1 ml, followed by the addition of 10 μl of starvation medium with or without 50 ng PDGF-BB. After 5 min at 37°C, the stimulation was stopped by rinsing the dishes twice with 10 ml of ice-cold PBS/0.1 mM Na3VO4. Preparation of cell lysates and the in vitro PI3K assay was done according to ref. 5.
Immunoprecipitation Studies.
Cells were grown, stimulated, and lysed as above. Protein concentrations of clarified lysates were determined by using BCA Protein Assay (Pierce), and equal amounts of protein were used for the immunoprecipitations. Immunoprecipitations and analyses thereof was done as described in ref. 18.
Assay for Phosphorylation of Protein Kinase B (PKB)/Akt.
Subconfluent to confluent cell cultures in six-well plates were starved in EF medium containing 0.02% FBS (Sigma) overnight, washed twice with PBS, and incubated with serum-free EF medium for 1 hr. Cells were preincubated with 100 ng PDGF-AA per ml of medium at 37°C for 2 hr, followed by a stimulation at 37°C for 5 min with 10 μl of serum-free EF medium containing either no ligand or PDGF-AA or PDGF-BB to give a final concentration of 25 ng ligand per ml of medium as indicated. Lysate preparation and analysis were done according to the manufacturer’s instruction (New England Biolabs).
Antisera.
The PDGF-β receptor antiserum has been described (19). The antisera for the precipitation of p85/PI3K (U13) was a kind gift of Ivan Gout (Ludwig Institute for Cancer Research, London), and phospholipase C-γ (PLC-γ) (raised against C terminus) was kindly provided by Lars Rönnstrand (Ludwig Institute for Cancer Research, Uppsala, Sweden). The monoclonal phosphotyrosine antibody PY20 was from Affiniti Research Products (Exeter, U.K.). Peroxidase-conjugated swine anti-rabbit Ig (1:30,000 in PBS/0.05% Tween-20) and peroxidase-conjugated sheep anti-mouse Ig (1:2,500 in PBS/0.05% Tween-20) were from Amersham Pharmacia and Dakopatts (Glostrup, Denmark), respectively. The polyclonal rabbit IgG antibody recognizing Ser-473-phosphorylated PKB/Akt and the secondary anti-rabbit horseradish peroxidase-conjugated antibody was from New England Biolabs.
Immunoprecipitation Studies.
Cells were grown, stimulated, and lysed as above. Protein concentrations of clarified lysates were determined by using BCA Protein Assay (Pierce), and equal amounts of protein were used for the immunoprecipitations. Immunoprecipitates were captured by using protein A-Sepharose (Immunosorb; MEDICAGO AB, Uppsala, Sweden) and washed three times with lysis buffer (see above) and once with 20 mM Tris⋅HCl, pH 7.5, before separation on a reducing, 7% SDS-polyacrylamide gel. The gel then was blotted onto Immobilon-P (Millipore) membranes by using the Mini-Protean II System (Bio-Rad) according to the manufacturer’s instructions. The membrane was blocked in PBS/0.2% Tween-20 at 4°C overnight before incubation with first and secondary antibodies in PBS/0.05% Tween-20.
Chemotaxis Assays.
Cell migration was studied by using the modified Boyden Chamber method with either the Transwell System (Costar) or the 48-well chamber (Neuroprobe). We followed the procedure as described in ref. 4 with the following exceptions: the membranes were coated with collagen dissolved in PBS; the α-receptor was down-regulated by preincubation of the cells with 100 ng PDGF-AA at 37°C for 1 hr before stimulation with 10 ng PDGF-BB/ml or mock stimulation with medium only. Thirty thousand cells (Transwell chamber, duplicate) or 16,000 cells (Neuroprobe chamber, triplicate) were applied per well. Cells were counted under the microscope by using either a ×160 magnification and seven small fields of vision per well (Transwell, duplicate) or a ×200 magnification and one large field of vision per well (Neuroprobe, triplicate).
Collagen Gel-Contraction Assay.
We followed the procedure for determination of fibroblast-mediated contraction of collagen lattices as described earlier (20) with the following exception: MCDB 104 medium (National Veterinary Institute, Uppsala, Sweden) was used instead of MEM or DMEM. Measurements were made in triplicates for each experiment.
Animals and Interstitial Fluid Pressure (Pif) Measurements.
Pif measurements were performed by using wt (6 animals) or mutant (11 animals) 6 months old 129Sv × C57BL/6 mice. The animals were fed ad libitum before experimentation. Mice were kept under a heating lamp throughout the experimental procedure. The mice were anesthetized by an s.c. injection of a 1:1 mixture (0.05 ml/10 g body weight) of Midazolam (Dormicum; Roche) and Fentanyl/Fluanison (Hypnorm; Janssen). Mice were injected i.v. (0.05 ml) with 100 μg C48/80 (Sigma) to anaphylaxis and edema formation. Circulatory arrest was induced within 5 min after injection of C48/80 and during anesthesia, as part of the experimental protocol to limit transcapillary fluid flux, by i.v. injection of saturated KCl (0.05 ml). This procedure allows for the distribution of C48/80 to the peripheral tissues, whereas edema formation is still at a minimum. Without circulatory arrest, the edema formation would be accompanied by a rise in the interstitial fluid volume, because of capillary influx, and lead to misinterpretation or underestimation of the decreased Pif (21). Pif was measured with sharpened glass capillaries (tip diameter, 3–7 μm) filled with 0.5 M NaCl colored with Evans Blue and connected to a servocontrolled counterpressure system (22). Measurements were performed on the dorsal side of the hind paw, puncturing through intact skin at a depth of 0.2–0.5 mm below the skin surface (dermal layers). The animal was placed in a supine position during the experiment. To eliminate movement of the hind paw, the distal part was fixed carefully to the puncture table with surgical tape.
Pressure recordings were performed in the following sequence: (i) with intact circulation before administration of C48/80; (ii) during the first 30 min after administration of C48/80 and circulatory arrest; and (iii) the subsequent 60 min after subdermal injection of PDGF-BB. The measurements were averaged for the following time periods: 0–10, 11–20, 21–30, 41–50, 51–60, and 61–90 min after the onset of C48/80 infusion. PDGF-BB (2 μg) was injected subdermally in a volume of 1 μl by using a 5-μl chromatography syringe with a 34-gauge needle. The needle was inserted through intact skin, and the substance was deposited carefully subdermally. Measurements were made at the edge of the injected volume and controlled visually.
All data are given as means ± SD. Statistical analysis was performed by one-way ANOVA with repeated measures and subsequent Bonferroni and Student’s t test. A value of P < 0.05 was considered statistically significant. The procedures were carried out with the approval of and in accordance with the recommendations laid down by the Norwegian State Commission for Laboratory Animals.
RESULTS
Generation of Mice with Mutant PDGF-β Receptor.
We subcloned the exon corresponding to tyrosines 739 and 750 in the PDGF-β receptor gene and mutated these codons into phenylalanines (Fig. 1A). The mutated exon then was introduced into a targeting vector (Fig. 1B) and electroporated into 129Sv ES cells. G418-resistant clones that had undergone homologous recombination were identified by PCR and subsequently verified by Southern blot analysis (data not shown). The presence of the two point mutations was confirmed by sequencing PCR-amplified DNA fragments from targeted ES cells. Two independent ES cell clones were used for the generation of germ-line chimeras. Heterozygous mice did not display an overt phenotype and were intercrossed to generate homozygous mutant offspring, which were recovered in normal Mendelian proportions (data not shown). No hemorrhages or edemas could be detected in newborn homozygotes. Histological examination of the kidneys showed no difference between wild-type and mutant mice (data not shown).
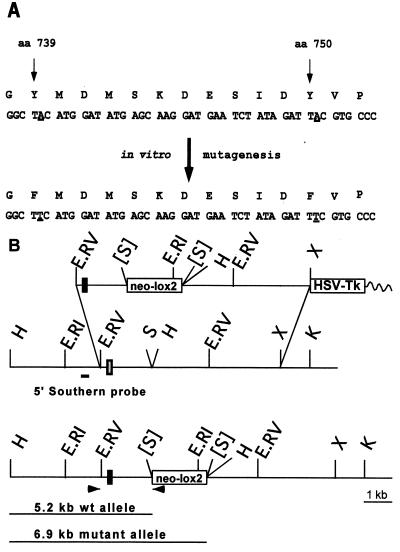
Figure 1.
Introduction of point mutations at the PDGF-β receptor locus. (A) Changes introduced by in vitro mutagenesis in the amino acid sequence encompassing the PI3K-binding sites in the PDGF-β receptor are marked in bold letters. (B) Targeting of the locus. (Top) Targeting vector. (Middle) Wild-type locus. (Bottom) Targeted locus. Shaded bar, wild-type exon; solid bar, mutated exon. Oligonucleotide primers (arrowheads) used for PCR detection and probes used for Southern analysis are indicated. The position and expected fragment sizes of wild-type and targeted HindIII (H) fragments detected with the 5′ Southern are shown. E.RV, EcoRV; S, SalI; E.RI, EcoRI; H, HindIII; X, XhoI; K, KpnI; [S], site destroyed; HSV-Tk, herpes virus simplex thymidine kinase; neo-lox2, neomycin phosphotransferase expression cassette flanked by lox sites.
Biochemical Analysis of Cells Carrying Wild-Type or Mutant PDGF-β Receptors.
To characterize the mutation introduced into the PDGF-β receptor, 3T3/fibroblast-like cell lines were derived from E12 embryos. At first we verified that the mutated PDGF-β receptor was still an active kinase by immunoprecipitating it from lysates of nonstimulated or PDGF-BB-stimulated cells and blotting with the antiphosphotyrosine antibody PY-20 (Fig. 2A). As expected, the regulatory subunit of PI3K could only be coimmunoprecipitated with activated wild-type receptor, but not with activated mutant receptor, confirming the successful mutation of the PI3K-binding site and the absence of additional binding sites for PI3K on the mutant β receptor (Fig. 2B). As a control, both wild-type and mutant receptors were coimmunoprecipitated with PLC-γ, a PDGF-β receptor substrate binding to phosphorylated Tyr-1021 (ref. 23; Fig. 2C). This indicates that the mutant β receptor could interact with other important signal transduction molecules known to associate with the wild-type β receptor.
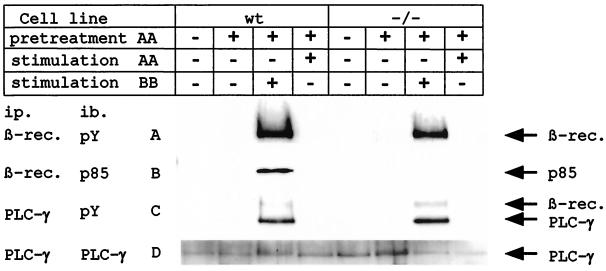
Figure 2.
The mutant PDGF-β receptor is unable to associate with PI3K. Fibroblasts derived from homozygous mutant or wild-type embryos were either completely unstimulated or preincubated with PDGF-AA followed by no further stimulation or stimulation with PDGF-BB or PDGF-AA. Cell lysates derived thereof, containing equal amounts of proteins, were split in two. (A) One-half of the cell lysates was immunoprecipitated with anti-PDGF-β receptor antibodies and blotted with PY20 for the detection of kinase active β receptor. The difference in signal intensity reflects different receptor numbers of the two cell lines (data not shown). (B) The same filter was stripped and reblotted with an anti-PI3K (p85) antibody. (C) The second half of the cell lysates was immunoprecipitated with anti-PLC-γ antibody and blotted with PY20 showing tyrosine-phosphorylated PLC-γ and coimmunoprecipitated, tyrosine-phosphorylated PDGF-β receptor. (D) The same filter was stripped and reblotted with anti-PLC-γ antibody showing similar amounts of PLC-γ. ip, immunoprecipitation; ib, immunoblotting.
To analyze the abilities of wild-type and mutant β receptors to activate PI3K, we performed an in vitro PI3K assay on receptor-coimmunoprecipitated material. Compared with activated, wild-type PDGF-β receptor, only residual PI3K activity of about 2% was associated with activated, mutated PDGF-β receptor (Fig. 3A). Thus, within the technical limitations of the assay, there was no significant, direct activation of PI3K by the mutated PDGF-β receptor. Because it had been demonstrated that PI3K also can be activated by molecules of other PDGF-induced signaling pathways, such as Ras and Src (17, 18), we used the phosphorylation of PKB/Akt, which depends on PI3K products (reviewed in ref. 24), to investigate possible indirect activation of PI3K upon β receptor stimulation. The PDGF-α receptor, which also is stimulated by PDGF-BB, was first specifically down-regulated by preincubation of the cells with PDGF-AA because the α receptor is very potent in the activation of PKB/Akt phosphorylation (Fig. 3B, lanes 2, 4, and 5 and 8, 10, and 11). After stimulation with PDGF-BB, a significantly reduced PKB/Akt phosphorylation was observed in mutant cells compared with wild-type cells, indicating that only very low amounts of PI3K products are formed after activation of mutant PDGF-β receptors (Fig. 3B, lanes 6 and 12).
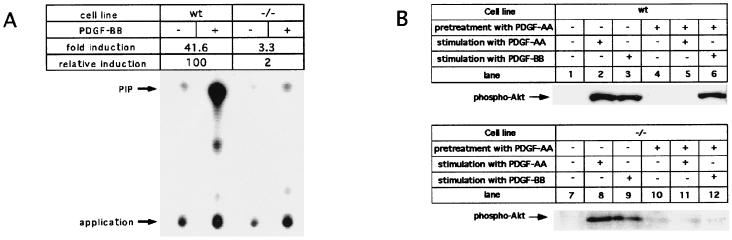
Figure 3.
PI3K activity is not associated with mutant PDGF-β receptor. (A) In vitro PI3K kinase assay. TLC of PI3K reaction products from unstimulated and PDGF-BB-stimulated wild-type and homozygous mutant cells. Cells were incubated without (−) or with (+) 100 ng PDGF-BB/ml at 37°C for 5 min; cell lysates containing identical amounts of total protein (see also Fig. 2) were immunoprecipitated with β receptor-specific antiserum PDGFR3. Immunoprecipitates were subjected to PI3-kinase assay, and PI3K reaction products were analyzed by TLC and autoradiography. The positions of the reaction product, phosphatidylinositol phosphate (PIP), and the application origin are indicated. (B) Phosphorylation of PKB/Akt induced by wt or mutant PDGF receptor. Serum-starved cells were incubated in serum-free starvation medium without ligand (lanes 1 and 7) or in serum-free starvation medium with 25 ng/ml PDGF-AA (lanes 2 and 8) or 25 ng/ml PDGF-BB (lanes 3 and 9) for 5 min; to down-regulate the α receptor, cells were preincubated in serum-free starvation medium for 2 hr with 100 ng/ml PDGF-AA (lanes 4–6 and 10–12), followed by stimulation with 25 ng/ml PDGF-AA (lanes 5 and 11) or 25 ng/ml PDGF-BB (lanes 6 and 12).
Effect of Y739/750F Mutation on PDGF-Induced Chemotactic Response.
It had been shown previously that the chemotactic response of the human PDGF-β receptor depends on the activation of PI3K via phosphotyrosines 740/751 of the kinase insert (4, 5). We tested PDGF-BB-induced migration of embryonic fibroblasts through a collagen-coated, 8-μm-thick membrane by using two different types of modified Boyden chambers, the 12-well Transwell system and the 48-well Neuroprobe device. As expected, fibroblasts from homozygous mutant embryos exhibited a 3- to 5-fold reduced chemotactic response when compared with wt fibroblasts (Fig. 4). In addition, we observed a total absence of circular actin ruffles when mutants cells were stimulated with PDGF-BB, whereas 1–5% of wt fibroblasts were positive for these structures (data not shown), consistent with previous findings of an important role for PI3K in actin reorganization (5). The regulated turnover of the actin cytoskeleton generally is seen as a prerequisite for chemotactic movements of cells toward the source of a growth factor (25).
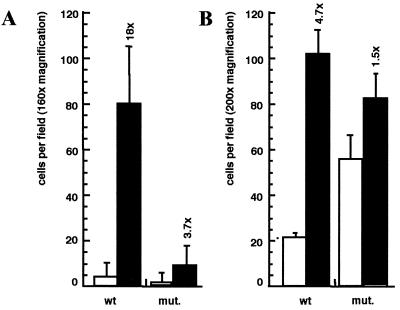
Figure 4.
PDGF-induced chemotaxis is reduced in mutant fibroblasts. Chemotactic response of wt or mutant cells toward no ligand (□) or 10 ng PDGF-BB (■) was tested by using either the “Transwell”- (A) or the “Neuroprobe”-modified Boyden Chamber assay (B; see Materials and Methods). Fold induction values are added above the bars for SD. Results from one representative experiment are shown.
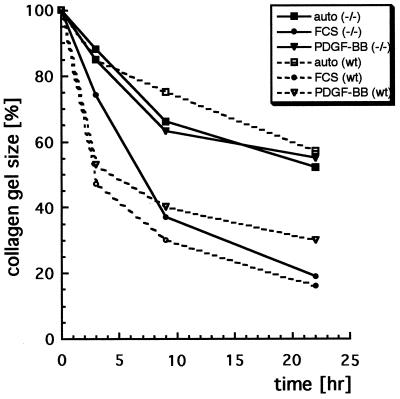
Cells from Mice with Mutated β Receptors Are Impaired in Their Ability to Contract Collagen Gels.
Fibroblasts have the ability to contract three-dimensional collagen gels in a process previously shown to be mediated by β1 integrins (20) and stimulated by PDGF-BB or serum. In addition, collagen gel contraction depends on actin turnover, because it is inhibited by treatment of cells with cytochalasin D, which blocks actin polymerization (26). A mixture of cells carrying wt or mutant receptors and a solution of native collagen type I were seeded into wells of a 96-well plate, where the collagen was allowed to polymerize. Wild-type cells effectively contracted collagen gels after stimulation with PDGF-BB or FCS (Fig. 5). In contrast, cells with mutant β receptors contracted the collagen gel above the inherent autocontraction ability only after stimulation with FCS, but not after stimulation with PDGF-BB. The lack of PDGF-BB-mediated contraction by β receptor mutant cells did not result from apoptosis as a result of missing PI3K signaling, because a later addition of serum to PDGF-BB-stimulated cells led to a rapid contraction of collagen gel by cells with mutant β receptors (data not shown). In addition, there was no difference between cells with wild-type or mutant β receptors in spreading on fibronectin or collagen type 1 or in mitogenic response to PDGF-BB (data not shown).
Figure 5.
PDGF-induced collagen gel contraction depends on PI3K signaling. Embryonic fibroblast-populated collagen gels were formed in serum-free MCDB 104 medium in microtiter plates. Time curves for spontaneous contraction (squares), FCS-stimulated contraction (circles), and PDGF-BB-stimulated contraction (triangles) of wt cells (open symbols) or homozygous mutant cells (filled symbols) are shown. Factors were added in triplicate in each experiment. Results from one representative experiment are shown.
The PDGF-β Receptor/PI3K Pathway Plays a Role in Fluid Pressure Homeostasis of Loose Connective Tissue.
In vivo, extracellular matrices serve to anchor and support cells within a tissue. Within the vascular system, this array has a major role in transcapillary fluid homeostasis. One important parameter in transcapillary fluid homeostasis is the Pif (for review, see ref. 27). Pif is a function of the interstitial volume, which, in turn, is governed by pressure differences that act across the capillary vascular wall and the lymphatic drainage of fluid from the tissue. Interstitial pressure is conventionally considered a passive controller of interstitial volume, that is, increasing capillary filtration will raise interstitial volume and pressure, and the latter will act, in turn, across the capillary wall to limit further filtration. Acute inflammatory reactions, as seen in burn injuries of the skin or generalized anaphylactic reactions, are accompanied by edema formation in the dermis. The dermal and subepidermal loose connective tissue recently has been found to play an active role in this process. Normal Pif in rat dermis is slightly subatmospheric around −0.5 mmHg (1 mmHg = 133 Pa), but in an anaphylactic model induced by i.v. injection of dextran, Pif drops to around −4 mmHg within only a few minutes concomitant with edema formation. Subsequent subdermal injection of PDGF-BB but not PDGF-AA counteracts this fall in interstitial pressure (21). Interestingly, a similar fall in interstitial pressure as in dextran anaphylaxis can be obtained by s.c. injection of wortmannin, a PI3K inhibitor (28).
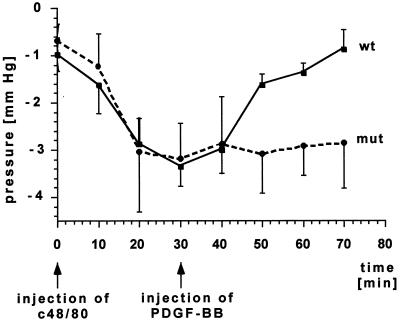
We therefore investigated the possibility that Pif might be regulated by PI3K in a PDGF-β receptor-dependent manner. Because our mice, in contrast to rats, did not demonstrate an allergic response toward dextran, we used the mast cell degranulating agent C48/80 (29) to induce a fall in interstitial fluid pressure. The change of the interstitial fluid pressure over time was measured in the paws of mice with sharpened glass capillaries connected to a servocontrolled counterpressure system. In wild-type mice the injection of C48/80 resulted in a drop of Pif from around −1 mmHg to an average of −3.4 mmHg after about 30 min. After injection of PDGF-BB, Pif recovered to about −0.9 mmHg within 40 min (Fig. 6). These results are similar to the observations made with the rat dextran anaphylaxis model (21). In mice with mutated β receptor, there was a similar initial drop in Pif after injection of C48/80. In sharp contrast to wild-type mice, however, the mutant mice were unable to restore interstitial fluid pressure in response to PDGF-BB injection (Fig. 6).
Figure 6.
Pif in the hind paw of wild-type or mutant mice after anaphylactic shock. PDGF-BB does not restore Pif after injection of PDGF-BB into mutant mice (●). SDs are given for 6 wild-type (■) animals and 11 mutant animals (●). A value of P < 0.05 was considered statistically significant.
DISCUSSION
We have engineered small mutations in the PDGF-β receptor gene that disrupt the ability of the receptor to bind to PI3K and generated the corresponding mouse mutants. In contrast to the β receptor null mutant phenotype, which shows hemorrhages, edema, lack of glomerular mesangial cells, and neonatal lethality (11), mice with a PDGF-β receptor mutant for PI3K binding develop normally and do not exhibit an overt phenotype. This might indicate that β receptor-mediated signaling through activated PI3K is only of minor importance during embryonic development or that other signaling molecules activated by the mutant β receptor are able to compensate for the loss of PI3K signaling. The latter possibility is underscored by the recent finding that a chimeric mutant PDGF-β receptor, unable to associate with PI3K, RasGAP, SHP2, and PLC-γ, induces an almost identical set of immediate early genes as a chimeric wt PDGF-β receptor (30). The α receptor also may be able to compensate, to a certain extent, for the loss of PI3K activation by the mutant β receptor. However, phosphorylation of Akt/PKB, a downstream target of PI3K, was reduced substantially but was still detectable after exposure of mutant cells to PDGF-BB. This observation suggests that although PI3K is unable to bind to the mutant receptor, activation of the PI3K signaling pathway may not be entirely eliminated. Nonetheless, our results as well as similar approaches reported for the c-Met or FGFR-1 receptors (31, 32) provide insights into the importance of specific signaling pathways in mice that are not possible to obtain by conventional knock-out approaches.
The inability of fibroblast-like cells with mutant β receptors to contract collagen gels suggests a mechanism by which interstitial fluid pressure and volume might be regulated under conditions such as acute inflammatory reactions. In subdermal loose connective tissue, hyaluronans and glycosaminoglycans exert swelling pressure (33), but this is also restrained by the cellular tension exerted on a collagenous fiber network (27, 34). A perturbation of the interactions between the cells and the extracellular matrix may allow the hyaluronan gel to expand, enabling a fall in interstitial pressure until a new balance is reached between the expanding hyaluronan/glycosaminoglycan gel and cell-mediated tension on the fiber networks. The PDGF-mediated reversion of this process, i.e., the contraction of the collagen matrix by fibroblast-like cells, depends on PI3K signaling via the PDGF-β receptor, as is the chemotactic response toward PDGF-BB, which was reduced in mutant fibroblasts. In light of these results it is tempting to speculate that the process of collagen contraction might involve a migratory element. The actual source of PDGF-BB in dermis is unknown. However, macrophages are a likely source of PDGF-BB in wound healing and chronic inflammations of the skin (35), and in normal human skin, PDGF-AB/BB is expressed by subepidermal, HLA-DR-positive cells as well as in peripheral nerves (36).
PDGF-BB modulates collagen-binding β1 integrin activity, as observed in fibroblast-mediated collagen gel contraction, where PDGF-BB stimulation rendered fibroblasts less sensitive for inhibition by anti-β1 integrin IgG or monovalent Fab fragments (ref. 20 and unpublished findings). Furthermore, infusion of PDGF-BB overcomes the lowering of interstitial pressure induced by monoclonal anti-α2β1 IgG in rat dermis (21). The latter finding is compatible with the notion that PDGF-BB stimulation increases the apparent activity of the collagen-binding integrin α2β1 in vivo. The present results extend on these findings, demonstrating that PI3K activation is necessary for PDGF-BB to counteract the lowering of Pif induced by anaphylaxis. Based on these observations, we suggest that an in vivo role of PDGF-BB is to modulate the mechanical activity of fibroblasts in collagenous matrices through activation of PI3K and, indirectly, the collagen-binding integrin α2β1. Our findings also might have potential medical implications. Stimulators of PI3K activity may serve as therapeutics counteracting edema formation in various inflammatory conditions. In addition, PI3K also may serve as a drug target to reduce the increased intratumoral interstitial fluid pressure, which has been postulated to complicate the uptake of antitumor agents (37).
The results presented in this work point to the complexity of signaling events downstream of the PDGF-β receptor in vivo. Our observations underscore the importance of a cross-talk between integrin and growth factor-signaling pathways in vivo, implicate PI3K signaling in this process, and identify a previously unrecognized role for PDGF-β receptor in adult mice.
Acknowledgments
We thank Chris Auger, Karin Weismann, and Aive Åhgren for excellent technical assistance and our colleagues for critical reading of the manuscript. R.H., A.B., and M.T. were supported by postdoctoral fellowships from the Deutsche Forschungsgemeinschaft, the Norwegian Research Council and the Nordic Academy for Advanced Study, and the American Cancer Society, respectively. This work was supported, in part, by grants from Axel och Margaret Ax:son Johnsons Stiftelse (C.-H.H.), the Swedish Cancer Society (K.R.), and the National Institutes of Health (P.S.).
ABBREVIATIONS
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol-3′ kinase
- Pif
interstitial fluid pressure
- PLC-γ
phospholipase C-γ
- ES
embryonic stem
- PKB
protein kinase B
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Heldin C-H, Westermark B. In: The Molecular and Cellular Biology of Wound Repair. Clark R A F, editor. New York: Plenum; 1996. pp. 249–273. [Google Scholar]
- 2.Heldin C H, Östman A, Ronnstrand L. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosenkranz S, Kazlauskas A. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 4.Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Nature (London) 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 5.Wennström S, Siegbahn A, Yokote K, Arvidsson A-K, Heldin C-H, Mori S, Claesson-Welsh L. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- 6.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins P T, Eguinoa A, Qiu R-G, Stokoe D, Cooke F T, Walters R, Wennström S, Claesson-Welsh L, Evans T, Symons M, et al. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 8.Nobes C D, Hall A. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 10.Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 11.Soriano P. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 12.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, et al. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 13.Soriano P. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl P, Johansson B R, Levéen P, Betsholtz C. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 15.Lindahl P, Hellström M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 16.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 17.Todaro G J. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wennström S, Landgren E, Blume-Jensen P, Claesson-Welsh L. J Biol Chem. 1992;267:13749–13756. [PubMed] [Google Scholar]
- 19.Claesson-Welsh L, Eriksson A, Morén A, Severinsson L, Ek B, Östman A, Betsholtz C, Heldin C-H. Mol Cell Biol. 1988;8:3476–3486. doi: 10.1128/mcb.8.8.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gullberg D, Tingström A, Thuresson A-C, Olsson L, Terracio L, Borg T K, Rubin K. Exp Cell Res. 1990;186:264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- 21.Rodt S Å, Åhlén K, Berg A, Rubin K, Reed R K. J Physiol (London) 1996;495:193–200. doi: 10.1113/jphysiol.1996.sp021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiig H, Reed R K. Acta Physiol Scand. 1981;113:307–315. doi: 10.1111/j.1748-1716.1981.tb06901.x. [DOI] [PubMed] [Google Scholar]
- 23.Rönnstrand L, Mori S, Arvidsson A-K, Eriksson A, Wernstedt C, Hellman U, Claesson-Welsh L, Heldin C-H. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downward J. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 25.Stossel T P. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 26.Guidry C, Grinnell F. J Cell Sci. 1985;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Reed K R, Woie K, Rubin K. Int Union Physiol Sci Am Physiol Soc. 1997;12:42–48. [Google Scholar]
- 28.Åhlén K, Berg A, Stiger F, Tengholm A, Siegbahn A, Gylfe E, Reed R K, Rubin K. Cell Adhes Commun. 1998;5:461–473. doi: 10.3109/15419069809005604. [DOI] [PubMed] [Google Scholar]
- 29.Koller M E, Woie K, Reed R K. J Appl Physiol. 1993;74:2135–2139. doi: 10.1152/jappl.1993.74.5.2135. [DOI] [PubMed] [Google Scholar]
- 30.Fambrough D, McClure K, Kazlauskas A, Lander E S. Cell. 1999;97:727–741. doi: 10.1016/s0092-8674(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 31.Maina F, Casagranda F, Audero E, Simeone A, Comoglio P M, Klein R, Ponzetto C. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 32.Partanen J, Schwartz L, Rossant J. Genes Dev. 1998;12:2332–2344. doi: 10.1101/gad.12.15.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer F A. Biochim Biophys Acta. 1983;755:388–399. doi: 10.1016/0304-4165(83)90242-8. [DOI] [PubMed] [Google Scholar]
- 34.Rubin K, Sundberg C, Åhlen K, Reed R K. In: Interstitium, Connective Tissue and Lymphatics. Reed R K, Bert J L, Winlove P, Laine G A, editors. London: Portland Press; 1995. [Google Scholar]
- 35.Ross R, Masuda J, Raines E W, Gown A M, Katsuda S, Sasahara M, Malden L T, Masuko H, Sato H. Science. 1990;248:1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- 36.Reuterdahl C, Sundberg C, Rubin K, Funa K, Gerdin B. J Clin Invest. 1993;91:2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain R K. Science. 1996;271:1079–1080. doi: 10.1126/science.271.5252.1079. [DOI] [PubMed] [Google Scholar]