Abstract
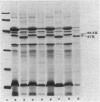
Mutants with multiple low-level antibiotic resistance were isolated from virulent wild-type Aeromonas salmonicida strains exposed to a low concentration of any one of several low-molecular-mass (approximately 635 daltons or less) antibiotics. Multiple resistance was toward beta-lactam compounds (penicillin G, ampicillin, cloxacillin), quinolones (flumequine, oxolinic acid, nalidixic acid), tetracyclines, chloramphenicol, and novobiocin. Susceptibilities of the mutants toward several higher-molecular-mass (greater than 700 daltons) hydrophobic or polycationic antibiotics such as rifampin, erythromycin, polymyxin B, and streptomycin sulfate were not affected. The mutants were obtained at frequencies suggesting point mutations. Outer membrane protein profiles, examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, revealed that all multiple low-level resistant mutants were deficient in a major protein of approximately 38.5 kilodaltons and contained a major protein of approximately 37 kilodaltons which was not present in significant amounts in the wild-type strains. In addition, these mutants lacked exoprotease activity. Furthermore, mutants isolated as deficient in exoprotease were found, with the exception of one avirulent strain, to exhibit multiple low-level antibiotic resistance and the outer membrane protein changes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan B. J., Stevenson R. M. Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can J Microbiol. 1981 Oct;27(10):1114–1122. doi: 10.1139/m81-174. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Aoki T., Egusa S., Kimura T., Watanabe T. Detection of R factors in naturally occurring Aeromonas salmonicida strains. Appl Microbiol. 1971 Oct;22(4):716–717. doi: 10.1128/am.22.4.716-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., MacIntyre S., Buckley J. T., Hancock R. E. Purification and reconstitution in lipid bilayer membranes of an outer membrane, pore-forming protein of Aeromonas salmonicida. J Bacteriol. 1983 Dec;156(3):1006–1011. doi: 10.1128/jb.156.3.1006-1011.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecycz I. T., Campbell J. N. Mechanisms of activation and secretion of a cell-associated precursor of an exocellular protease of Pseudomonas aeruginosa 34362A. Eur J Biochem. 1985 Jan 2;146(1):35–42. doi: 10.1111/j.1432-1033.1985.tb08616.x. [DOI] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Intracellular accumulation of extracellular proteins by pleiotropic export mutants of Aeromonas hydrophila. J Bacteriol. 1983 Apr;154(1):413–418. doi: 10.1128/jb.154.1.413-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Phillippe L., Teng Tseng J., Stemke G. W., Campbell J. N. Purification and characterization of exocellular proteases produced by a clinical isolate and a laboratory strain of Pseudomonas aeruginosa. Can J Microbiol. 1980 Jan;26(1):77–86. doi: 10.1139/m80-012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maier T. W., Zubrzycki L., Coyle M. B., Chila M., Warner P. Genetic analysis of drug resistance in Neisseria gonorrhoeae: production of increased resistance by the combination of two antibiotic resistance loci. J Bacteriol. 1975 Nov;124(2):834–842. doi: 10.1128/jb.124.2.834-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., Mooi F. R., de Graaf F. K. Excretion of proteins by gram-negative bacteria: export of bacteriocins and fimbrial proteins by Escherichia coli. Antonie Van Leeuwenhoek. 1984;50(5-6):569–584. doi: 10.1007/BF02386227. [DOI] [PubMed] [Google Scholar]
- Rossouw F. T., Rowbury R. J. Effects of the resistance plasmid R124 on the level of the OmpF outer membrane protein and on the response of Escherichia coli to environmental agents. J Appl Bacteriol. 1984 Feb;56(1):63–79. doi: 10.1111/j.1365-2672.1984.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F., Blackman E., Lewis E. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol. 1975 Nov;124(2):750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudamore R. A., Beveridge T. J., Goldner M. Penetrability of the outer membrane of Neisseria gonorrhoeae in relation to acquired resistance to penicillin and other antibiotics. Antimicrob Agents Chemother. 1979 Jun;15(6):820–827. doi: 10.1128/aac.15.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]