Abstract
Sophisticated imaging methods, such as computed tomography, magnetic resonance imaging and positron emission tomography, play an increasingly important role in the management of head and neck cancer. Pretreatment imaging findings have predictive value for patient outcome, independently from the currently used TNM classification, and may be used to tailor treatment to the individual patient. Based on per-treatment imaging, individualised replanning during radiotherapy may ameliorate tumour control rates and reduce toxic effects to normal tissues. Early posttreatment imaging studies contain important prognostic information, and allow selection of patients for further treatment or watchful waiting.
Keywords: Head and neck neoplasms, treatment outcome, patient selection, followup studies
Introduction
The TNM system, reflecting tumour extension, provides prognostic information in head and neck cancer patients. Computed tomography (CT) and magnetic resonance imaging (MRI) assist in pre-treatment planning by better defining the extent of the primary tumour and by detecting subclinical adenopathies. According to this staging system, patients are stratified towards surgery, radiotherapy, a combination of surgery and radiotherapy, or chemoradiotherapy.
Even in advanced head and neck cancers, modern concomitant chemoradiotherapy can achieve relatively high locoregional control and survival rates [1].
Concerns have been expressed about the weakness of the TNM classification for several head and neck cancer sites, as the cure rates reported in the literature vary and prognosis is not sufficiently related to the TNM values [2, 3]. A quantitative analysis of imaging findings, with tumour volume calculation from imaging studies, offers additional predictive information.
Intensity modulated radiotherapy (IMRT) was recently introduced into the clinic. In IMRT, multiple beams characterised by variable, non-uniform radiation intensity, are applied. This technique offers the opportunity of dose escalation to the tumour without increasing the dose to other organs at risk. These more sophisticated treatment techniques create higher demands on diagnostic imaging, requiring very accurate delineation of the gross tumour volume to avoid geographic miss. As intentional dose inhomogeneity within the target, or so-called dose painting is feasible, biological information on tumour subvolumes can allow selective dose escalation to more radioresistant tumour areas. Such an approach requires non-invasive identification of these tumour areas. Multimodality imaging can provide biological tumour information, such as on tumour hypoxia, cell proliferation, apoptosis, angiogenesis and receptor status of tumours [4]. This has led to the introduction of the biological tumour volume concept [5], integrating physical and biological conformality.
Predicting the outcome is therefore not only dependent on tumour stage determined according to the classic TNM criteria, but relies increasingly on imaging-derived objective tumour data. Also other factors, such as general patient status and comorbidity, should be taken into account.
Pretreatment imaging
Primary tumour extent
Correct delineation of the primary tumour and the involved neck lymph nodes is necessary to perform curative radiotherapy in head and neck cancer. The most peripheral borders of the neoplasm must be determined as accurately as possible. Improvement of target definition is one of the most critical steps to improve outcome after radiotherapy [6]. Conformal radiotherapy and IMRT use steep dose gradients; suboptimal delineation of the tumour volumes and normal tissues at risk (such as the spinal cord and parotid glands) may lead to underdosage of the tumour and/or overdosage of the normal tissues, potentially decreasing control probability and increasing the likelihood of treatment complications [7].
Laryngeal and pharyngeal neoplasms can be accurately imaged either by CT or MRI. Overall, there is no evidence for superiority of one modality over the other [8–11]. However, in nasopharyngeal cancer, the tumour extent is better defined by MRI [12–14]. It has been shown that the choice of the imaging modality influences the outcome of the patient with nasopharyngeal cancer: significantly better treatment results in terms of local control, disease-specific survival and overall survival are obtained with the use of MRI; the respective hazard ratios for CT versus MRI are 1.96, 1.48 and 1.85 [15]. The better treatment outcome is related to a better definition of tumour extent in this disease by using MRI.
During recent years, positron emission tomography (PET) imaging is increasingly used in the initial neck cancer staging process, complementary to CT and/or MRI. Recently, a study was published in which CT, MR and PET was obtained with the patient in the same position, and registration was performed between the different images and with the total laryngectomy specimen in nine patients. All three imaging modalities significantly overestimated the tumour volume. There was mismatch between each of the imaging modalities, as well as between the surgical specimen and the imaging modalities. All imaging techniques failed to show a fraction of the macroscopic tumour extent, and did not show superficial mucosal tumour extent. In this study, from all techniques, PET was the closest to the surgical reference volume [16]. However, as none of the imaging techniques displays superficial or microscopic tumour extent, a security margin has to be added to the gross tumour volume (GTV). Nevertheless, a significantly smaller GTV, clinical (CTV) and planning target volume (PTV) was determined based on pre-treatment fluorodeoxyglucose (FDG)-PET, using an automatic segmentation algorithm based on signal-to-background ratio, compared to CT [7].
It should be noted that the definition of GTV on PET images is not a trivial issue; using particular numerical values of FDG intensity levels is arbitrary, and may not be very well reproducible, as signal intensity varies from scanner to scanner and even from patient to patient [17, 18]. Using the combined information from a dedicated head and neck PET-CT study likely provides more accurate information on the true tumour extent [19].
Neck adenopathy
In head and neck cancer, the identification of lymphatic tumour spread is crucial, as lymphatic metastasis is the most important mechanism of tumour spread. The presence of neck adenopathies largely determines the likelihood of locoregional control and the risk for distant metastasis.
Clinical evaluation of neck lymph nodes is not very precise. None of the currently available imaging methods, including FDG-PET, can reliably depict small tumour deposits within non-enlarged lymph nodes, nor differentiate reactively enlarged lymph nodes from metastatic lymphadenopathy. Because of this shortcoming, in many patients the neck lymph nodes are treated electively. Currently, ultrasound-guided fine needle aspiration cytology is the most accurate method to evaluate the neck lymph nodes [20]. However, the results obtained with this method are operator-dependent; furthermore, apart from being an invasive technique, it does not offer precise spatial localisation of metastatic neck disease.
Improvement in the diagnostic capabilities of non-invasive cross-sectional imaging would allow elective treatment of the N0 neck to be avoided, and to confine radiation target volumes to the neck regions containing metastatic adenopathies. It would also offer more reliable surveillance imaging, with earlier detection of tumour recurrence. A few studies have addressed the value of a specific contrast agent (ultrasmall superparamagnetic iron oxide, USPIO) in MRI to detect metastatic neck adenopathies. From a theoretical point of view, this contrast agent should lead to more reliable results than with gadolinium-enhanced MRI; although promising, the reported results for neck adenopathies are somewhat variable [21–23]. Further improvement of MRI technology may lead to better results with this contrast agent [21].
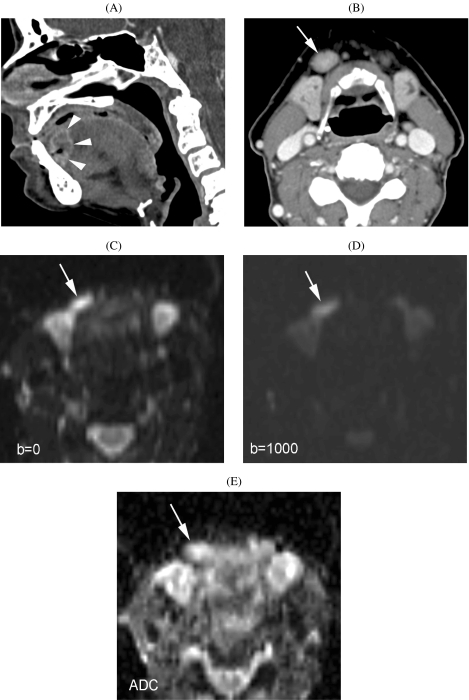
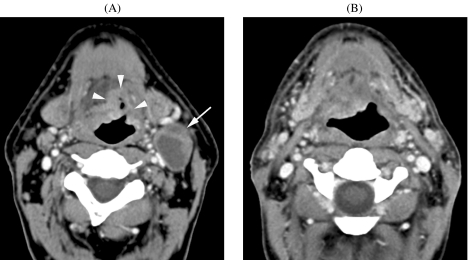
One study reports on the use of diffusion-weighted MRI in detecting metastatic adenopathies in the neck [24]. This is a new application of diffusion-weighted MRI that may have great potential (see also below) (Fig. 1).
Figure 1.
Patient suffering cancer of floor of the mouth. (A) CT image (sagittal reformatting) shows primary tumor at the junction of the floor of the mouth and oral tongue (arrowheads). (B) Axial CT image. Slightly enlarged lymph node (minimal axial diameter 12 mm) in the submandibular region (arrow): suspicious for metastatic adenopathy. (C)–(E) Axial diffusion-weighted MR-images. Compared to the 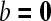 image (C), the signal clearly reduced in the adenopathy (arrow) on the
image (C), the signal clearly reduced in the adenopathy (arrow) on the 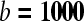 image (D), indicating easy diffusion of water protons. The ADC-map (E) shows a relative high signal (
image (D), indicating easy diffusion of water protons. The ADC-map (E) shows a relative high signal (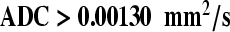 ). These findings are consistent with a benign adenopathy. Histological examination of the neck dissection specimen did not show tumour.
). These findings are consistent with a benign adenopathy. Histological examination of the neck dissection specimen did not show tumour.
In patients with positive neck nodes, imaging of extranodal tumour spread and/or involvement of critical structures, such as the carotid arteries, has prognostic and therapeutic relevance. In a multivariate analysis, extranodal spread, as visible on MRI, was shown to be the only independent predictor of distant metastasis [25].
Quantifying tumour volume
Success in controlling a tumour by radiotherapy depends on killing all clonogenic cells in the gross tumour volume. The probability of cure depends, among other factors, on the initial number of clonogenic cells. There are indications that the clonogen number increases linearly with tumour volume. Therefore, tumour volume can be an interesting predictor of local outcome. Volumetric assessment of soft tissue masses is possible with cross-sectional imaging techniques such as CT, using the summation-of-areas technique.
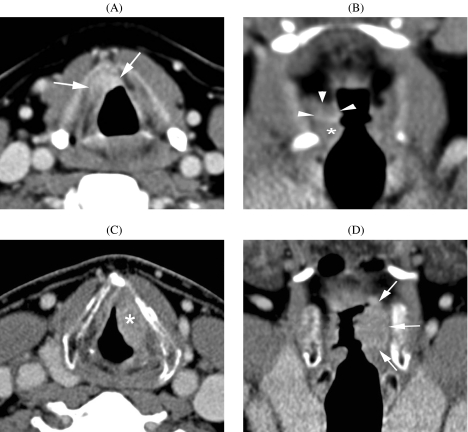
Several studies have confirmed the prognostic value of CT-determined tumour volume for outcome after definitive radiation therapy in head and neck cancer, including glottic, supraglottic, hypopharyngeal and nasopharyngeal cancer [26–31]. Such quantitative information is helpful in the treatment decision process; high risk patients can be identified and followed more closely (Fig. 2).
Figure 2.
(A), (B) Squamous cell carcinoma of the left false vocal cord. A small infiltrating lesion is seen in the right paraglottic space (arrows). Normal true vocal cord (asterisk). Because of paraglottic space infiltration, this lesion was classified as a T3 tumour. Measured tumour volume on CT images was 0.3 ml. No evidence of disease 2 years after end of radiotherapy. (C), (D) Another patient suffering supraglottic squamous cell cancer, also centered on the false vocal cord (asterisk), but more extensively infiltrating the paraglottic space (arrows). This lesion was also classified as a T3 tumour. Tumour volume was 6.1 ml. Local failure occurred 6 months after the end of radiotherapy.
However, recent studies on oropharyngeal cancer did not reveal an important impact of primary tumour volume on the likelihood of local control after radiotherapy [32, 33]. It is not clear why the relation between oropharyngeal tumour volume and local outcome is less pronounced than in other head and neck sites, but this finding reflects the existence of other factors influencing the tumour response to irradiation.
Biological tumour volume
Besides tumour volume, there are other factors determining the resistance against radiation and chemotherapy, for example a genetically determined inherent resistance, and physiological factors. The presence of inadequate and heterogeneous vascular networks, resulting in tumour hypoxia, is critical. In some human tumours, especially in head and neck cancer [34], treatment may fail due to the presence of hypoxia. Therefore, tumour hypoxia needs to be identified and quantified, not only as a predictor of outcome, but also to select patients for concomitant therapy to overcome the hypoxia effect. Currently, the evaluation of tumour oxygenation requires application of invasive methods, such as biopsy-based immunohistochemistry, or the use of oxygen-sensitive electrodes.
A clear need exists for non-invasive methods to investigate the tumour micro-environment, including oxygenation. With both CT and MRI, additional, biological information can be acquired [35, 36]. One approach is to apply dynamic contrast-enhanced (DCE) imaging techniques; such methodology allows a combined estimation of tissue perfusion, blood volume and permeability of vessels. A relationship between DCE-MRI parameters and tumour oxygenation has been shown, e.g. in uterine cervical carcinoma, using polarographic needle electrodes as reference. In a series of 105 patients with advanced head and neck cancer, tumour perfusion, as measured by DCE-CT, was the strongest independent predictor of local outcome after radiotherapy [35].
Several other approaches to measuring tumour oxygenation are available with MRI. One possibility is to use blood as an intrinsic contrast agent. The blood oxygenation level-dependent (BOLD) contrast depends on the endogenous switch from paramagnetic deoxyhaemoglobin to diamagnetic oxyhaemoglobin, a conversion translated in changes of MR signals [37]. A disadvantage of this method is its high susceptibility to artefacts, making it difficult to apply in the investigation of head and neck cancer.
Diffusion-weighted MRI (DW-MRI) allows non-invasive evaluation of the Brownian movement of water molecules; this movement can be quantified by calculating the apparent diffusion coefficient (ADC). DW-MRI provides information about microscopic structures such as cell density, cell integrity and vascularity [38], and is applicable in the head and neck region. This technique is very sensitive to structural changes in pathologic tissue, even during their very early stages of development. It has been shown that viable tumour can be differentiated from necrotic tumour with DW-MRI in the larynx [39]. As it is a completely non-invasive technique, not requiring an external contrast agent, DW-MRI potentially has important advantages for evaluating biological properties of tumoural masses.
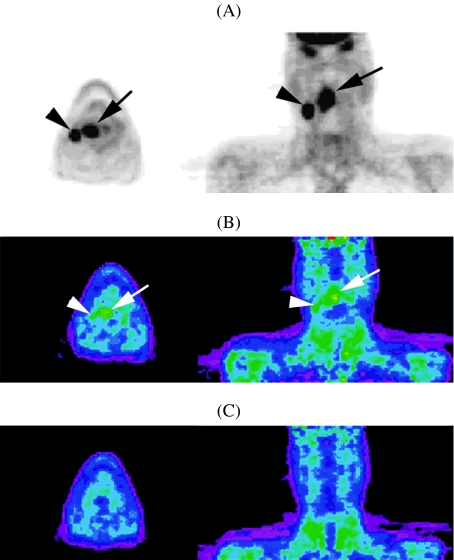
In PET-imaging, FDG is the most frequently used tracer; its uptake reflects the metabolic active tumour volume. Apart from FDG, other tracers can be employed, providing more specific information about other elements of tumoral metabolism. For example, [18F]misonidazole (F-MISO) is reduced in hypoxic conditions and its metabolite binds to intracellular molecules (Fig. 3). Registration of F-MISO-PET with FDG-PET images reflects the hypoxic tumour fraction. Another PET tracer employed to detect hypoxic tumour regions in head and neck cancer is Cu-diacetyl-bis(N4-methylthiosemicarbazone (Cu-ATSM) [40]. Registration of such PET images, showing the hypoxic regions but lacking spatial resolution, with CT images, allows hypoxia imaging-guided IMRT. Selective dose escalation to the hypoxic, more radioresistant tumour subvolumes, while sparing the normal tissues, is feasible with IMRT. Theoretically, use of such imaging-derived biological information should improve the treatment outcome after IMRT. Recently, a randomised trial was reported, in which patients showing tumour hypoxia on F-MISO-PET had a worse locoregional control rate after chemoradiation than those without tumour hypoxia; also, a better locoregional outcome was obtained in patients showing tumour hypoxia on F-MISO-PET, in whom chemoradiotherapy was associated with tirapazamine, a cytotoxin selectively targeting hypoxic cells [41].
Figure 3.
Patient suffering squamous cell carcinoma of the oropharynx, stage T3N2b. A base-line FDG-PET (A) shows uptake in the primary tumour (black arrow) and in regional adenopathy (black arrowhead). F-MISO-PET (B) acquired 1 day later, shows uptake in the primary tumour (white arrow) and the adenopathy (white arrowhead), indicating the presence of hypoxia. Repeat F-MISO-PET after 4 weeks of radiotherapy (C) shows loss of F-MISO uptake, suggesting tumour reoxygenation (images courtesy of Sandra Nuyts, MD, PhD).
Imaging during (chemo)radiotherapy
Measurable anatomic changes occur during the course of radiotherapy for head and neck cancers, mainly during the second half of the treatment. Such volumetric and geometric changes can have a potential dosimetric impact when conformal treatment techniques are applied. Replanning of treatment by deforming the intensity distributions of each beam based on deformations of anatomy, as observed in daily CT studies during therapy, has been reported; this led to significant differences compared to the intended dose distributions [42].
Compared to pre-treatment CT, the per-treatment tumour volume appeared significantly smaller on MRI than on CT. Per-treatment automatic segmentation of tumour volume on FDG-FET is not possible due to tremendous increase in background signal by radiation-induced inflammation [7].
Non-invasive tumour response evaluation during (chemo)irradiation opens news possibilities for treatment tailoring, not only by adapting the target volume, but also by dose escalation to non-responding tumour subvolumes, or by adding concomitant treatment such as carbogen breathing, bioreductive drugs or radiosensitising drugs.
Posttreatment imaging
Early tumour recurrence may be difficult to distinguish from tissue changes induced by therapy. Therefore, it is recommended to obtain a follow-up CT or MR study after surgical, radiation or combined treatment for a head and neck neoplasm with high-risk profile [43, 44]. Probably the best time to obtain such a baseline study is about 2–4 months after the end of treatment. Such a baseline study allows treatment-caused changes in the head and neck tissues to be documented. By comparing subsequent studies with the baseline study, it becomes possible to predict and detect tumour recurrences with more confidence and this at an earlier stage than is possible with clinical follow-up alone (Fig. 4). In most patients, CT is an adequate imaging modality for pre- and posttreatment imaging; MRI is preferred in patients with nasopharyngeal, sinonasal and skull base tumours.
Figure 4.
Axial CT images in a patient with supraglottic cancer. (A) Pretreatment image. Enhanced soft tissue mass, arising from the right lateral border of the epiglottis, infiltrating the paraglottic fat (arrow); on lower sections, also infiltration of the preepiglottic space was seen. Small metastatic lymph node (arrowhead). The tumour was staged as T3N1. (B) Four months after the end of radiotherapy, a baseline follow-up CT study was performed. Clinically, the patient was doing well. The asymmetrical and slightly enhancing tissue infiltration in the tumour bed (arrows) is doubtful. Follow-up CT was recommended. (C) Eight months after the end of radiotherapy. The patient was now experiencing some pain and swallowing difficulties, but clinical examination did not reveal abnormal findings. On CT, the soft tissue infiltration in the supraglottis (arrows) is progressive compared to the baseline study, very suspicious for tumour recurrence. At direct laryngoscopy, an intact mucosa was found, and biopsy was negative. Because of the very worrisome findings on CT, direct laryngoscopy was repeated and deep (submucosal) tissue was sampled; histopathologic examination of this tissue confirmed the presence of cancer. Subsequently, total laryngectomy was performed.
The baseline study itself carries important predictive information regarding the eventual local outcome: CT achieves a sensitivity of 83%, and a specificity of 95% in the early differentiation of treatment responders from non-responders in irradiated laryngeal and hypopharyngeal cancer [44]. In advanced head and neck cancer, treated by chemoradiation, MRI performed 6–8 weeks after the end of treatment was able to predict residual/recurrent disease at the primary site with a sensitivity of 48% and a specificity of 85% [45]. In this same study, it was shown that a clinical examination of the head and neck under general anaesthesia, at the same time points, had limited value, as most local recurrences remained undetected. These authors recommended performing such an examination only in patients with suspicious findings on early follow-up MRI [45].
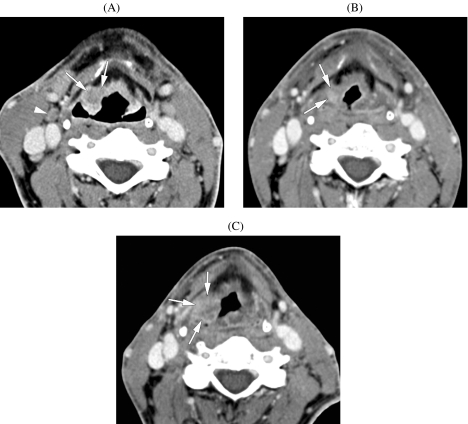
In patients with nodal disease, the role of planned neck dissection after (chemo)radiotherapy is not well defined [46]. Such a planned neck dissection may reduce the regional failure rate and improve survival. However, many of these neck resection specimens do not contain tumor, meaning that the patient was exposed to the inherent risks of surgery, with no benefit. CT of the neck, obtained early after the end of (chemo)radiotherapy, was shown to be a reliable method to predict the absence of residual nodal disease (Fig. 5). Absence of lymph nodes larger than 1.5 cm, and lack of any focal nodal abnormality had a negative predictive value of 94%, while the clinical evaluation had a negative predictive value of 77% [47]; in this particular study, the CT study was performed at a median of 1 month after the end of (chemo)radiotherapy.
Figure 5.
Axial CT images in a patient suffering from a T3N2c tongue base squamous cell cancer. (A) Before chemoradiotherapy. Infiltrating tongue base tumour (arrowheads). Necrotic neck adenopathy (arrow); several other, bilateral adenopathies were visible on this study. (B) Follow-up study obtained 6 months after completion of therapy. Residual deformity of tongue base, but no enhancing mass is seen; the neck adenopathies disappeared. Patient is now 4 years after therapy, without evidence of disease.
Some authors recommend FDG-PET for detecting residual nodal disease. However, FDG-PET obtained early after the end of therapy appears to be unreliable. In a prospective study on 12 patients, treated by radiotherapy alone, a negative predictive value of 14% was obtained when PET was done 1 month after treatment [48]. This high level of false-negative findings may be related to temporary changes in tumour glucose metabolism, induced by therapy [49]. More reliable results are obtained when FDG-PET is performed 3–4 months after the end of treatment, resulting in a negative predictive value of 97–100% [50, 51]. Although there is discussion about the optimal timing of planned neck dissection after (chemo)radiation, usually this is recommended to take place 4–8 weeks after the end of treatment.
Conclusion
Sophisticated imaging methods play an increasingly important role in the management of head and neck cancer. Pretreatment imaging findings have predictive value for patient outcome, independently from the currently used TNM classification, and may be used to tailor treatment plans. Based on per-treatment imaging, individualised replanning during radiotherapy may ameliorate tumour control rates and reduce toxic effects to normal tissues. Early posttreatment imaging also carries important prognostic information, allowing selection of patients for further treatment or watchful waiting.
References
- 1.Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99-14. J Clin Oncol. 2005;23:3008–15. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 2.Piccirillo JF. Purposes, problems, and proposals for progress in cancer staging. Arch Otolaryngol Head Neck Surg. 1995;121:145–9. doi: 10.1001/archotol.1995.01890020009003. [DOI] [PubMed] [Google Scholar]
- 3.Kowalski LP, Carvalho AL. General tumor factors. In: Baatenburg de Jong RJ, editor. Prognosis in Head and Neck Cancer. London: Taylor & Francis; 2006. pp. 127–33. [Google Scholar]
- 4.Chapman JD, Bradley JD, Eary JF, et al. Molecular (functional) imaging for radiotherapy applications: an RTOG symposium. Int J Radiat Oncol Biol Phys. 2003;55:294–301. doi: 10.1016/s0360-3016(02)04215-3. [DOI] [PubMed] [Google Scholar]
- 5.Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–60. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 6.Nuyts S. Use of imaging data in radiotherapy planning of head and neck cancer: improved tumour characterisation, delineation and treatment verification. In: Hermans R, editor. Head and Neck Cancer Imaging. Berlin: Springer; 2006. pp. 345–59. [Google Scholar]
- 7.Geets X, Daisne JF, Tomsej M, Duprez T, Lonneux M, Gregoire V. Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol. 2006;78:291–7. doi: 10.1016/j.radonc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Hermans R. Staging of laryngeal and hypopharyngeal cancer: value of imaging studies. Eur Radiol. 2006 doi: 10.1007/s00330-006-0301-7. May 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Dammann F, Horger M, Mueller-Berg M, et al. Rational diagnosis of squamous cell carcinoma of the head and neck region: comparative evaluation of CT, MRI, and 18FDG PET. AJR Am J Roentgenol. 2005;184:1326–31. doi: 10.2214/ajr.184.4.01841326. [DOI] [PubMed] [Google Scholar]
- 10.Bolzoni A, Cappiello J, Piazza C, et al. Diagnostic accuracy of magnetic resonance imaging in the assessment of mandibular involvement in oral-oropharyngeal squamous cell carcinoma: a prospective study. Arch Otolaryngol Head Neck Surg. 2004;130:837–43. doi: 10.1001/archotol.130.7.837. [DOI] [PubMed] [Google Scholar]
- 11.Hsu WC, Loevner LA, Karpati R, et al. Accuracy of magnetic resonance imaging in predicting absence of fixation of head and neck cancer to the prevertebral space. Head Neck. 2005;27:95–100. doi: 10.1002/hed.20128. [DOI] [PubMed] [Google Scholar]
- 12.Poon PY, Tsang VH, Munk PL. Tumour extent and T stage of nasopharyngeal carcinoma: a comparison of magnetic resonance imaging and computed tomographic findings. Can Assoc Radiol J. 2000;51:287–95. [PubMed] [Google Scholar]
- 13.King AD, Teo P, Lam WW, Leung SF, Metreweli C. Paranasopharyngeal space involvement in nasopharyngeal cancer: detection by CT and MRI. Clin Oncol (R Coll Radiol) 2000;12:397–402. doi: 10.1053/clon.2000.9199. [DOI] [PubMed] [Google Scholar]
- 14.Chung N-N, Ting L-L, Hsu W-C, Lui LT, Wang P-M. Impact of magnetic resonance imaging versus CT on nasopharyngeal carcinoma: Primary tumor target delineation for radiotherapy. Head and Neck. 2004;26:241–6. doi: 10.1002/hed.10378. [DOI] [PubMed] [Google Scholar]
- 15.Chang JT, Lin CY, Chen TM, et al. Nasopharyngeal carcinoma with cranial nerve palsy: the importance of MRI for radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1354–60. doi: 10.1016/j.ijrobp.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93–100. doi: 10.1148/radiol.2331030660. [DOI] [PubMed] [Google Scholar]
- 17.Paulino AC, Johnstone PA. FDG-PET in radiotherapy treatment planning: Pandora’s box? Int J Radiat Oncol Biol Phys. 2004;59:4–5. doi: 10.1016/j.ijrobp.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 18.MacManus M, Hicks R, Bayne M, Leong T, Peters L, Ball D. In regard to Paulino and Johnstone: Use of PET and CT imaging data in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2004;59:4–5. doi: 10.1016/j.ijrobp.2004.07.667. Int J Radiat Oncol Biol Phys 2004; 60: 1005–6. [DOI] [PubMed] [Google Scholar]
- 19.Beyer T, Antoch G, Muller S, et al. Acquisition protocol considerations for combined PET/CT imaging. J Nucl Med. 2004;45 (Suppl 1):S25–35. [PubMed] [Google Scholar]
- 20.van den Brekel MW, Castelijns JA. What the clinician wants to know: surgical perspective and ultrasound for lymph node imaging of the neck. Cancer Imaging. 2005;5(A):S41–9. doi: 10.1102/1470-7330.2005.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigal R, Vogl T, Casselman J, et al. Lymph node metastases from head and neck squamous cell carcinoma: MR imaging with ultrasmall superparamagnetic iron oxide particles (Sinerem MR)—results of a phase-III multicenter clinical trial. Eur Radiol. 2002;12:1104–13. doi: 10.1007/s003300101130. [DOI] [PubMed] [Google Scholar]
- 22.Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ. Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology. 2002;222:239–44. doi: 10.1148/radiol.2221010225. [DOI] [PubMed] [Google Scholar]
- 23.Baghi M, Mack MG, Hambek M, et al. The efficacy of MRI with ultrasmall superparamagnetic iron oxide particles (USPIO) in head and neck cancers. Anticancer Res. 2005;25:3665–70. [PubMed] [Google Scholar]
- 24.Sumi M, Sakihama N, Sumi T, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. Am J Neuroradiol. 2003;24:1627–34. [PMC free article] [PubMed] [Google Scholar]
- 25.van den Brekel MW, Ljumanovic R, Castelijns JA. Imaging characteristics of regional metastasis. In: Baatenburg de Jong RJ, editor. Prognosis in Head and Neck Cancer. London: Taylor & Francis; 2006. pp. 197–213. [Google Scholar]
- 26.Hermans R, Van den Bogaert W, Rijnders A, Baert AL. Value of computed tomography as outcome predictor of supraglottic squamous cell carcinoma treated by definitive radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:755–65. doi: 10.1016/s0360-3016(99)00039-5. [DOI] [PubMed] [Google Scholar]
- 27.Hermans R, Van den Bogaert W, Rijnders A, Doornaert P, Baert AL. Predicting the local outcome of glottic squamous cell carcinoma after definitive radiation therapy: value of computed tomography-determined tumour parameters. Radiother Oncol. 1999;50:39–46. doi: 10.1016/s0167-8140(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso AA, Mukherji SK, Schmalfuss I, et al. Preradiotherapy computed tomography as a predictor of local control in supraglottic carcinoma. J Clin Oncol. 1999;17:631–7. doi: 10.1200/JCO.1999.17.2.631. [DOI] [PubMed] [Google Scholar]
- 29.Chong VF, Zhou JY, Khoo JB, Huang J, Lim TK. Nasopharyngeal carcinoma tumor volume measurement. Radiology. 2004;231:914–21. doi: 10.1148/radiol.2313030358. [DOI] [PubMed] [Google Scholar]
- 30.Pameijer FA, Mancuso AA, Mendenhall WM, et al. Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck. 1998;20:159–68. doi: 10.1002/(sici)1097-0347(199803)20:2<159::aid-hed10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Chua DT, Sham JS, Kwong DL, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997;39:711–9. doi: 10.1016/s0360-3016(97)00374-x. [DOI] [PubMed] [Google Scholar]
- 32.Nathu RM, Mancuso AA, Zhu TC, Mendenhall WM. The impact of primary tumor volume on local control for oropharyngeal squamous cell carcinoma treated with radiotherapy. Head Neck. 2000;22:1–5. doi: 10.1002/(sici)1097-0347(200001)22:1<1::aid-hed1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Hermans R, Op de Beeck K, Van den Bogaert W, et al. The relation of CT-determined tumor parameters and local and regional outcome of tonsillar cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2001;50:37–45. doi: 10.1016/s0360-3016(00)01559-5. [DOI] [PubMed] [Google Scholar]
- 34.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 35.Hermans R, Meijerink M, Van den Bogaert W, Rijnders A, Weltens C, Lambin P. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1351–6. doi: 10.1016/s0360-3016(03)00764-8. [DOI] [PubMed] [Google Scholar]
- 36.Rijpkema M, Kaanders JH, Joosten FB, van der Kogel AJ, Heerschap A. Method for quantitative mapping of dynamic MRI contrast agent uptake in human tumors. J Magn Reson Imaging. 2001;14:457–63. doi: 10.1002/jmri.1207. [DOI] [PubMed] [Google Scholar]
- 37.Rijpkema M, Kaanders JH, Joosten FB, van der Kogel AJ, Heerschap A. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head-and-neck tumors as measured by magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002;53:1185–91. doi: 10.1016/s0360-3016(02)02825-0. [DOI] [PubMed] [Google Scholar]
- 38.Thoeny HC, De KF, Chen F, et al. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234:756–64. doi: 10.1148/radiol.2343031721. [DOI] [PubMed] [Google Scholar]
- 39.Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Evaluation of the larynx for tumour recurrence by diffusion-weighted MRI after radiotherapy: initial experience in four cases. Br J Radiol. 2006;79:681–7. doi: 10.1259/bjr/89661809. [DOI] [PubMed] [Google Scholar]
- 40.Chao KS, Bosch WR, Mutic S, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–82. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 41.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 42.Mohan R, Zhang X, Wang H, et al. Use of deformed intensity distributions for on-line modification of image-guided IMRT to account for interfractional anatomic changes. Int J Radiat Oncol Biol Phys. 2005;61:1258–66. doi: 10.1016/j.ijrobp.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Pameijer FA, Hermans R, Mancuso AA, et al. Pre- and post-radiotherapy computed tomography in laryngeal cancer: imaging-based prediction of local failure. Int J Radiat Oncol Biol Phys. 1999;45:359–66. doi: 10.1016/s0360-3016(99)00149-2. [DOI] [PubMed] [Google Scholar]
- 44.Hermans R, Pameijer FA, Mancuso AA, Parsons JT, Mendenhall WM. Laryngeal or hypopharyngeal squamous cell carcinoma: can follow-up CT after definitive radiation therapy be used to detect local failure earlier than clinical examination alone? Radiology. 2000;214:683–7. doi: 10.1148/radiology.214.3.r00fe13683. [DOI] [PubMed] [Google Scholar]
- 45.van den Broek GB, Rasch CR, Pameijer FA, Peter E, van den Brekel MW, Balm AJ. Response measurement after intraarterial chemoradiation in advanced head and neck carcinoma: magnetic resonance imaging and evaluation under general anesthesia? Cancer. 2006;106:1722–9. doi: 10.1002/cncr.21786. [DOI] [PubMed] [Google Scholar]
- 46.Pellitteri PK, Ferlito A, Rinaldo A, et al. Planned neck dissection following chemoradiotherapy for advanced head and neck cancer: is it necessary for all? Head Neck. 2006;28:166–75. doi: 10.1002/hed.20302. [DOI] [PubMed] [Google Scholar]
- 47.Liauw SL, Mancuso AA, Amdur RJ, et al. Postradiotherapy neck dissection for lymph node-positive head and neck cancer: the use of computed tomography to manage the neck. J Clin Oncol. 2006;24:1421–7. doi: 10.1200/JCO.2005.04.6052. [DOI] [PubMed] [Google Scholar]
- 48.Rogers JW, Greven KM, McGuirt WF, et al. Can post-RT neck dissection be omitted for patients with head-and-neck cancer who have a negative PET scan after definitive radiation therapy? Int J Radiat Oncol Biol Phys. 2004;58:694–7. doi: 10.1016/S0360-3016(03)01625-0. [DOI] [PubMed] [Google Scholar]
- 49.Castaigne C, Muylle K, Flamen P. Positron emission tomography in head and neck cancer. In: Hermans R, editor. Head and Neck Cancer Imaging. Berlin: Springer; 2006. pp. 329–43. [Google Scholar]
- 50.Yao M, Graham MM, Hoffman HT, et al. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001–10. doi: 10.1016/j.ijrobp.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 51.Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27:175–81. doi: 10.1002/hed.20130. [DOI] [PubMed] [Google Scholar]