Abstract
Accurate staging of cancer is of fundamental importance to treatment selection and planning. Current staging paradigms focus, first, on a detailed delineation of the primary tumour in order to determine its suitability for resection, and, thereafter, on assessment of the presence of metastatic spread that would alter the surgical approach, or mandate non-surgical therapies. This approach has, at its core, the assumption that the best, and sometimes the only, way to cure a patient of cancer is by surgical resection. Unfortunately, all non-invasive techniques in current use have imperfect ability to identify those primary tumours that are able to be completely excised, and even worse ability to define the extent of metastatic spread. Nevertheless, because of relatively low cost and widespread availability, computed tomography (CT) scanning is the preferred methodology for tumour, nodal and systemic metastasis (TNM) staging. This is often supplemented by other tests that have improved performance in particular staging domains. For example, magnetic resonance imaging (MRI), mammography, or endoscopic ultrasound may be used as complementary tests for T-staging; surgical nodal sampling for N-staging; and bone scanning, MRI or ultrasound for M-staging. Accordingly, many patients undergo a battery of investigations but, even then, are found to have been incorrectly staged based on subsequent outcomes. Even for those staged surgically, pathology can only identify metastases within the resection specimens and has no capability for detecting remote disease. As a result of this, many patients undergo futile operations for disease that could never have been cured by surgery. In the case of restaging, the situation is even worse. The sequelae of prior treatment can be difficult to differentiate from residual cancer and the likelihood of successful salvage therapy is even less than at presentation. More deleteriously, patients may be subjected to additional morbid treatments when cure has already been achieved. Thus, in post-treatment follow-up, the presence and extent of disease is equally critical to treatment selection and patient outcome as it is in primary staging. One of the major strengths of positron emission tomography (PET)/CT as a cancer staging modality is its ability to identify systemic metastases. At any phase of cancer evaluation, demonstration of systemic metastasis has profound therapeutic and prognostic implications. Only in the absence of systemic metastasis does nodal status become important, and only when unresectable nodal metastasis has been excluded does T-stage become important. There are now accumulating data that PET/CT could be used as the first, rather than the last test to assess M- and N-stage for evaluating cancers with an intermediate to high pre-test likelihood of metastatic disease based on poor long-term survival. In this scenario, there is great opportunity for subsequently selecting and tailoring the performance of anatomically based imaging modalities to define the structural relations of abnormalities identified by PET, when this information would be of relevance to management planning. Primary staging of oesophageal cancer and restaging of colorectal cancer are illustrative examples of a new paradigm for cancer imaging.
Keywords: Correlative imaging, hybrid imaging, staging, fluorodeoxyglucose (FDG), restaging
Introduction
The innovator makes enemies of all those who prospered under the old order, and only lukewarm support is forthcoming from those who would prosper under the new. Their support is lukewarm, partly from fear of their adversaries … and partly because men are generally incredulous, never really trusting new things unless they have tested them by experience. In consequence, whenever those who oppose the changes can do so, they attack vigorously, and the defence made by the others is lukewarm. So both the innovator and his friends come to grief.
Machiavelli, Il Principe, c1200
Cancer is a major cause of death in the developed world, and is becoming a significant issue for developing countries as they adopt a more westernised lifestyle, including greater consumption of alcohol and tobacco [1]. Unfortunately, despite huge expenditure on cancer research [2], survival of many cancers remains poor, especially for non-haematological malignancies. For common cancers like non-small cell lung, oesophageal, and colorectal cancer, survival of those presenting with all but the earliest stages of disease remains depressingly poor and has barely changed over the past 20 years [3]. These data suggest that there needs to be a concerted effort to improve cancer survival. Almost certainly, any improvement in cancer outcomes is going to require multifactorial advances in cancer management. Based on the premise that accurate diagnosis of the presence and extent of cancer is an essential first step in selecting and planning the most appropriate treatment for an individual patient, it is logical to conclude that improvement in staging accuracy has the potential to improve cancer outcomes. There is now a very large volume of literature [4] demonstrating that fluorodeoxyglucose (FDG) PET is more accurate than conventional staging paradigms, and, therefore, it is reasonable to postulate that more routine use of PET should improve patient outcomes either by optimising delivery of potentially curable treatments, or by avoiding unwarranted interventions.
Despite its superior diagnostic performance compared to other non-invasive staging techniques, the high cost of PET and relatively restricted availability has fostered a widespread belief that it is best reserved for cases with equivocal conventional imaging results. Such thinking has led to FDG PET being performed after anatomical imaging in most clinical settings. Indeed, this sequence of imaging has been the dominant situation in series reported in the literature. Moreover, entrenched familiarity with existing staging paradigms has also led many oncologists to consider it inappropriate to omit diagnostic CT from the cancer evaluation process in the absence of proof that this can be performed without compromising patient care. Nevertheless, the recent development of hybrid PET/CT devices that can combine high-quality anatomical imaging with the superior diagnostic accuracy of FDG PET in cancer evaluation [5] warrants a reappraisal of how and when this technology should be used in the cancer imaging paradigm. Already within a few years of commercial release of these scanners, several analyses of PET/CT demonstrated that hybrid imaging is significantly more accurate than either diagnostic CT or stand-alone PET for cancer staging in a range of tumours [6–10]. This is also true of the use of PET/CT in the restaging setting [11]. Given that PET/CT also provides moderate fidelity anatomical information by virtue of the low-dose, non-contrast CT used to provide attenuation-correction, could PET/CT obviate the need for diagnostic CT in some clinical scenarios? Such a paradigm shift could reduce radiation exposure and patient inconvenience without necessarily compromising the accuracy of the staging process. Recent evaluations have, for example, questioned whether diagnostic CT adds incremental diagnostic information to PET/CT in lymphoma [12]. This is, however, a disease in which surgery offers little as a therapeutic approach. Could the same be true of non-haematological malignancies?
There are considerable cost and resource implications of using PET/CT as the initial imaging investigation of choice in the evaluation of cancer patients. Accordingly, any change in the current paradigm, which utilises diagnostic CT to select patients requiring a PET or PET/CT, would require careful consideration of the reasons for which we perform cancer staging, the limitations of the current approach, and the potential benefits of change.
The importance of cancer staging to patient management
Definition of the extent of malignant involvement is the foundation on which current oncology practice is based. This information defines applicable therapeutic strategies and provides a guide to the patient’s prognosis. Diagnostic imaging modalities, particularly CT, are the primary techniques that are used to detect and stage cancer. As such, these modalities play a fundamental role in cancer management. Traditionally, stage groupings from I to IV have been used to divide patients into groups of decreasing suitability for treatment with curative intent and, consequently, worsening prognostic outlook. Recognising the limitations of previous disease-specific staging systems, there has been a recent move towards standardising stage based on characteristics of the primary tumour (T), draining lymph nodes (N), and distant metastases (M) that have been determined to be of management or prognostic importance. For each tumour site category, an alphanumeric value is assigned reflecting the burden, or prognostic significance of the disease identified, with a higher value representing more advanced disease, or a worse prognosis. The ‘T-stage’ is of great relevance to the surgical oncologist, because it defines the local growth and invasion pattern of the primary lesion. Generally, the size of the lesion and its relationship to key anatomical landmarks determine the T-stage assignment. A higher T-stage is associated with larger tumours, or for those that have crossed tissue planes that would normally restrict the radial growth of tumour. For most cancers, T-stage reflects the likelihood that the primary lesion can be successfully, and safely, resected en bloc. At some stage in their evolution many malignancies develop the ability to metastasize. This can occur to regional lymph nodes via lymphatic vessels draining the primary tumour, and generally proceeds in a hierarchical manner from proximal to distal nodal sites. The extent of nodal spread is designated by the ‘N-stage’. Higher nodal stage is reflective of spread to more distant nodal echelons, by larger nodal size, or both. The presence and extent of nodal involvement is critical to decisions regarding suitability for treatment with loco-regional therapies such as surgery and radiotherapy. Metastasis can also occur via the blood, either secondarily from lymphatics such as the thoracic duct, or directly through invasion of vessels by the primary tumour. These metastases to distant tissues generally develop in a haphazard manner, although many tumours display characteristic patterns of spread. For example, prostate cancer spreads primarily to bone, whereas colorectal cancer most often spreads to the liver. The presence of remote metastases and, sometimes, the extent of this spread, are designated by the ‘M-stage’. Although most tumours are simply rated as M0 or M1, some tumours have sub-classification of the M1 grouping to reflect disease burden, thereby differentiating between patients with limited and, perhaps, locally treatable metastases from those with widespread dissemination requiring systemic therapy or palliation alone. In a few tumours, such as oesophageal cancer, remote lymph node disease is also designated as M1, reflecting the inability to cure this disease by local therapies alone, and the poor associated prognosis.
Is TNM stage based on CT a valid basis on which to select patient management?
The utility of TNM status at presentation as a guide to management planning has been primarily validated by its ability to stratify survival of subgroups of patients with a given type of cancer. However, it needs to be recognised that prognostic implications of a given TNM stage are related to the accuracy of staging methods used, the actual therapies employed at the differing stages of disease, and the effectiveness of those therapies. Of these factors, the accuracy of the staging method has a compounding effect, since stage generally determines the selection and delivery of therapies of significantly differing efficacy. TNM stage is usually determined from a composite of clinical examination findings, the results of various investigations including imaging, and pathology, when it is available.
Each of the various methodologies used for determining cancer stage has inherent inaccuracies. Because of relatively low cost, widespread availability, and ability to define primary tumour relations, the status of draining lymph nodes and to screen for metastatic deposits in disparate tissues, contrast-enhanced CT scanning is the preferred methodology for initial TNM staging of most cancers. Unfortunately, diagnostic CT is something of a Jack-of-all-trades but master of none. Like other anatomical imaging techniques, diagnostic CT is generally quite good at demonstrating anatomical relations of the primary tumour and, variably, whether it has crossed important tissue planes. However, distortion of normal anatomical by local scarring, secondary mechanical effects of the primary tumour, such as obstructive atelectasis, or reactive changes in adjacent tissues, such as oedema, may significantly compromise T-stage assessment. Furthermore, the lack of sufficient contrast between tumour and many normal tissues renders CT incapable of detecting cases where the primary tumour may have crossed a critical boundary that renders it unable to be resected. Accordingly, supplementary anatomical imaging studies such as MRI, mammography and endoscopic ultrasound may be used in combination with CT for T-staging. Even after such combinations of tests, some patients cannot avoid operative evaluation to ascertain whether resection is feasible.
The ability of diagnostic CT to detect regional nodal involvement is also limited by both imperfect sensitivity and specificity inherent to size-based criteria of nodal involvement since normal-sized nodes can potentially harbour metastases and enlarged nodes can represent reactive changes [13]. This necessitates nodal sampling in many situations to either confirm or rule-out metastatic involvement. Although pathology is often deemed to be the ‘gold-standard’ for staging, because of its near perfect positive predictive value and its unrivalled ability to detect microscopic nodal spread in the sampled tissues, the negative predictive value of pathology is partially dependent on the intensity of the tissue processing, and is fundamentally compromised where tissues have not been sampled. Were this not true, no patient with a pathologically ‘proven’ complete resection (R0) of a primary lung cancer should ever die of loco-regional recurrence. Unfortunately, this is not the case. Furthermore, pathological staging is a morbid and costly procedure that can only logically improve treatment selection in the absence of systemic metastasis.
In the hope that the patient will be curable by surgery, the prescription of diagnostic CT is generally optimised to display regional anatomy of the primary tumour and draining lymph nodes basins. However, this focus potentially compromises optimal assessment of the many possible sites of metastatic disease. As a result of this, further diagnostic tests are often performed to evaluate specific sites of potential metastasis in specific high-risk individuals. These tests might include ultrasound of the liver, MRI of the brain and bone scanning. Thus, as a result of the real and perceived limitations of diagnostic CT for TNM staging, many patients undergo a battery of diagnostic tests, each of which adds inconvenience, delay, cost and potential morbidity to the staging process. Even then, many patients are found to have been inadequately staged based on subsequent outcomes.
The case for PET/CT being the first, rather than the last staging investigation
The most critical impediment to the cure of cancer is the presence of distant metastases. Exclusion of definite systemic metastasis is therefore of fundamental importance to selecting patients for aggressive local therapies. There is now a very large volume of literature attesting to the superior accuracy of FDG PET compared to diagnostic CT for detecting distant metastases. The powerful impact of FDG PET on modifying patient management has been demonstrated in a randomised-controlled trial in non-small cell lung cancer wherein detection of remote metastatic disease prevented futile thoracotomy in 20% of patients [14]. In our own experience, the major incremental impact of FDG PET in patients with non-haematological malignancies has been prevention of futile procedures in patients in whom PET detected metastatic disease that had been unrecognised by conventional staging techniques, thereby avoiding substantial associated cost and morbidity. For example, in non-small cell lung cancer, we found that over 25% patients with stage III disease had occult metastases that rendered local radiotherapy with curative intent an inappropriate treatment [15]. Furthermore, even among stage I patients by conventional staging almost 10% of had occult metastatic disease (M1) disease, and, therefore, could also never have been cured by either surgery or radiotherapy. Thus, as conventional stage increased, so too did the likelihood of M1 disease, consistent with Bayesian principles. The rate of occult metastatic disease in patients with even relatively early stage disease based on conventional imaging that was documented in this series was sufficient to convince our clinicians to routinely perform PET in all patients with potentially curable non-small cell lung cancer. We have demonstrated that this approach has resulted in an improvement in survival of patients treated with radical radiotherapy compared to a comparable cohort of patients receiving this therapy based on conventional staging [16].
Once definite systemic metastases have been excluded, the next most important question is whether there is macroscopic nodal metastasis that would compromise the ability to deliver local therapies as definitive treatment with curative intent. Several meta-analyses have documented the superior diagnostic performance of PET compared to diagnostic CT for N-staging in various cancers [17–24]. Again, in our own evaluations of the management impact of FDG PET, we found significant change in therapeutic choice, as well modification of delivery of a chosen therapy based on better definition of nodal involvement by PET (Fig. 1). By better defining macroscopic nodal involvement, expensive and morbid loco-regional therapies can be more effectively directly delivered. Clearly, it is only possible to cure patients with loco-regional disease if all disease sites are adequately treated. In patients in whom radical radiotherapy would be the only potential curative treatment due to performance status or other co-morbidity, the inability to perform pathological nodal sampling particularly supports the case for use of the most accurate non-invasive technique [25]. Similar results, regarding the clinical impact of FDG PET through improved N- and M-staging, have been reported by many other investigators and have generally used equipment and techniques of lower quality than modern PET/CT devices. More precise characterisation of the nature and location of focal FDG accumulations identified by PET by comparison with a contemporaneous CT is likely to further improve diagnostic performance and thereby, treatment selection and planning.
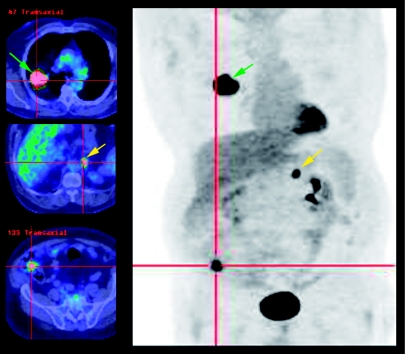
Figure 1.
The limitations of the current staging paradigm are demonstrated in this example of metastatic caecal carcinoma initially misdiagnosed as a T2N0M0 primary lung adenocarcinoma on conventional staging, including biopsy and diagnostic CT. The patient was deemed suitable for surgery or radical radiotherapy but FDG PET/CT indicated a probable caecal primary and an additional adrenal metastasis. Colonoscopy confirmed the presence of a caecal adenocarcinoma and histopathological features indicated that the lung lesion was likely a metastasis. Instead of planned mediastinal nodal biopsy followed by neoadjuvant chemoradiation and surgery, systemic chemotherapy for metastatic colon cancer was chosen.
Although stand-alone FDG PET clearly has limited capacity to define local primary tumour relations, a non-contrast CT, performed as part of PET/CT for the dual purposes of allowing attenuation correction of emission data and anatomical correlation, probably provides sufficient delineation of macroscopic primary tumour extent in the setting of remote nodal or systemic metastases to obviate a contrast CT. In the absence of remote metastatic disease, a more detailed analysis of loco-regional tumour extent using the best anatomical imaging technique for the particular location could be employed for tumours in specific locations which present particular surgical problems. For some patients this might involve performing a localised repeat CT with intravenous contrast, while in others, it may be preferable to utilise MRI or ultrasound to characterise local tumour stage. Delaying diagnostic CT until after PET/CT may also allow more specific functional information to be obtained. For example tumour perfusion could be defined by way of dynamic contrast CT of target lesions identified on PET/CT [26].
The difficulty with the concept of replacing diagnostic CT with PET/CT is, thus, not related to quality-of-care issues, which would clearly be benefited, but rather by the resource and cost issues. PET/CT scans generally take 20–40 min on equipment costing 2–3 times that of CT scanners, which can perform scans on a patient every 5–10 min. After amortisation of equipment and staffing expenditure, the cost of CT is, consequently, substantially less than for PET/CT. The cost of FDG also needs to be added to the cost of PET. Although the unit dose price of FDG is falling due to more efficient production and distribution facilities, it is likely to remain significantly more expensive than CT contrast. From this discussion it might appear impossible to make a case for substitution. However, if rather than considering the unit cost of individual investigations, one looks at the cumulative cost of staging and therapy, it is possible to argue that a PET/CT might be cost-effective based on comparisons of strategies that have found that PET plus CT can be more cost-effective than using CT alone [27–29]. First, if a single diagnostic test can provide a sufficiently high post-test likelihood of disease to allow confident assignment of therapy, it can potentially replace a number of less expensive but also less accurate tests that need to be used in combination to achieve a similar level of clinical confidence (Fig. 2). Second, by more accurately defining disease extent, it may obviate the need for expensive or morbid confirmatory tests that may otherwise be required when using a less accurate staging technique (Fig. 3). Third, since the cost of most cancer therapies vastly exceed the cost of diagnostic tests performed for staging, by preventing futile therapies, or by improving planning to the extent that cure of patients is more likely, more accurate staging can reduce the overall costs of cancer care. These considerations are dependent on the degree to which incremental diagnostic information can be supplied by PET/CT compared with diagnostic CT when either is performed as a stand-alone investigation. This, in turn, is a function of the clinical scenario in which staging is performed.
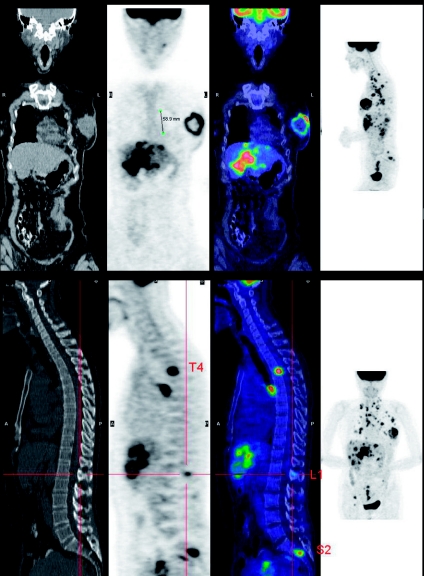
Figure 2.
In the setting of locally advanced breast cancer the ability to demonstrate the primary lesion, draining lymph nodes, and systemic metastases to bone, liver and lungs renders PET a potential replacement for a battery of tests including, mammography, sentinel lymph node biopsy, diagnostic CT, bone scan, and liver ultrasound. In this setting, PET/CT also provides a baseline for therapeutic monitoring.
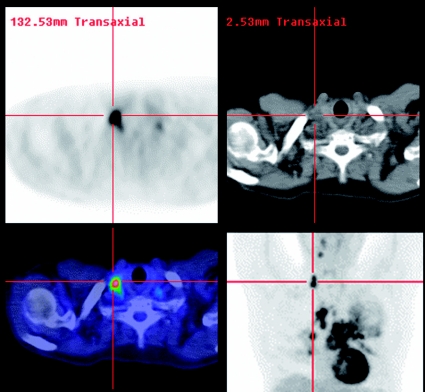
Figure 3.
Equivocal enlargement of mediastinal lymph nodes on diagnostic CT usually requires mediastinal lymph node sampling by various approaches including mediastinoscopy. These add to the cost and potential morbidity of the staging process. By identifying nodes that are more likely to be involved based on increased FDG uptake, and particularly more easily accessed, PET may improve the selection of the best nodal sites to sample in order to confirm, or exclude, metastatic involvement. In this case extrathoracic non-small cell lung cancer was confirmed by ultrasound-guided biopsy on a neck node identified on PET/CT and thus the patient was spared a surgical procedure.
The importance of disease prevalence to the application of staging paradigms
According to Bayesian principles, the post-test likelihood of disease is a function of both the pre-test likelihood of disease, and the diagnostic performance of the test. The pre-test likelihood of disease is related to the prevalence of disease in the population being assessed. In diseases like cancer where the true burden of disease can be impossible to characterise due to lack of a gold standard, true estimates of diagnostic performance and disease prevalence are problematic [30]. However, in general terms, where the likelihood of disease is either very high or very low, little incremental diagnostic information is provided even by very accurate tests compared to less accurate tests. Conversely, where there is an intermediate likelihood of disease, there is substantial incremental diagnostic value associated with using more accurate diagnostic techniques. Although our studies addressing the impact of FDG PET were generally preselected by the absence of unequivocal metastatic disease on conventional staging techniques, and a clinical intention to treat the patient in the hope of cure, we relied on PET-findings when they were discrepant with those from other techniques. Using this approach we found that PET significantly altered management in a somewhat surprisingly high percentage of patients. For example, in a prospective evaluation of FDG PET in diagnosis, staging and restaging of lung cancer, we found PET findings altered planned management in around two-thirds of cases [31]. This high rate of modification of treatment plans was validated as being appropriate in the vast majority of cases based on follow-up. These results almost certainly reflect selection of patients with an intermediate to high likelihood of metastatic disease based on clinical presentation. In such patients, the superior sensitivity of PET for M- and N-stage would be predicted to be best able to provide incremental diagnostic information compared to less accurate staging techniques. Also, in line with superior diagnostic staging accuracy, our data have demonstrated that FDG PET stage treatment status better stratifies for survival than does conventional stage in a number of different cancers [32–34]. We have also demonstrated that FDG PET consistently outperforms diagnostic CT in the restaging of solid malignancies treated with surgery or radiotherapy and in whom structural abnormality suggests the presence of residual or recurrent disease [35–39]. This relates to the fact that the accuracy of anatomical imaging, such as CT, is compromised when normal anatomy is distorted by prior treatment. In head and neck cancer patients, for example, we were able to show that FDG PET was able to dichotomise a group of otherwise similar patients into groups with and without evidence of recurrence [36]. The group with negative scans generally avoided active salvage therapies that had been planned and yet had the best survival. Even with active treatment, the group with positive PET scans had relatively poor survival. We have also reported similar results in the restaging of non-small cell lung cancer [35], ovarian cancer [39] and breast cancer [37], wherein diagnostic CT demonstrated poor ability to predict survival related to its inability to reliably differentiate between post-treatment changes and residual disease. In these studies the prevalence of disease was approximately 50% and therefore strongly favoured demonstration of significant incremental benefit with use of a more accurate test.
Accordingly, it would be appropriate to reason that if FDG PET/CT could cost-effectively replace diagnostic CT as the first imaging test in oncological stage evaluation, it would be in patients meeting the criteria of an intermediate a priori likelihood of regional or systemic metastasis. Based on the known survival of various cancers, there are many that are likely to have metastatic disease at diagnosis, simply because of their poor survival with surgery alone. However, let us just consider a couple of potential scenarios that would fulfil these requirements, one in the primary staging setting and another in the restaging setting, as illustrative examples.
PET/CT as the initial staging investigation of oesophageal cancer
The incidence of oesophageal adenocarcinoma is rising rapidly in the Western world [40]. The prognosis of patients with oesophageal cancer remains relatively poor, despite improvements in surgical technique, reduced peri-operative mortality, and the introduction of multimodality therapies [41]. This is because oesophageal cancer often presents relatively late in patients who ignore symptoms of dysphagia. Diagnosis is now frequently made by direct visualisation of the tumour at endoscopy and biopsy confirmation at that time. Accordingly, definitive staging is usually not performed until after the histopathological diagnosis is already made. Thus, there is potentially a choice of initial staging investigation. Primary therapies currently include loco-regional therapies (such as surgery, neo-adjuvant chemo-radiotherapy followed by surgery, and chemo-radiation alone), or systemic therapy with chemotherapy. Because of the relatively poor outcome of patients with this disease there has been a trend towards multimodality therapy and increasing intensity of therapy. These approaches add significantly to the overall cost and morbidity of therapy but have yielded only a modest improvement in survival [42, 43]. Exclusion of macroscopic metastatic disease is pivotal to the selection of therapy, as this makes curative loco-regional procedures feasible. Only in the absence of systemic or remote nodal metastases does T-stage become relevant to treatment planning.
Contrast-enhanced CT of chest and abdomen is currently the most favoured initial staging test of choice on the grounds that it provides some information regarding loco-regional disease, but can also detect systemic metastases to liver, lung and other tissues in the imaging field of view. If there is no obvious distant metastasis on CT, EUS is increasingly being employed for loco-regional staging because of its superiority for defining local tumour relations and peri-tumoral nodal involved compared to CT. Unfortunately, even in patients deemed to be suitable for aggressive loco-regional therapy by this conventional sequence of imaging, we recently found that incremental PET findings still altered the treatment of 27 (40%) of 68 patients resulting in change in treatment intent or modality in 32% of cases, including a change from curative to palliative therapy in 12 cases with metastases detected only by PET [44]. This high management impact raises the possibility that PET/CT could be used upfront in patients with locally advanced oesophageal cancer, as the a priori likelihood of remote nodal and systemic disease is at least intermediate. Preliminary evaluation of PET/CT has suggested that this technology further improves on the already superior performance of stand-alone PET compared to CT [45].
Demonstration of remote metastatic disease on PET/CT would allow image-guided biopsy for confirmation, if required, and also provide a baseline for therapeutic monitoring of systemic chemotherapy. In this setting, diagnostic CT may be completely superfluous. If no evidence of systemic metastases were found, documentation of remote nodal disease would allow planning of radiotherapy to incorporate all macroscopic nodal deposits. As we have also demonstrated in a recent prospective trial, PET/CT altered treatment plans in over 2/3 of patients with radiotherapy planned provisionally on the basis of CT [46]. If radiation oncologists can be convinced that radiotherapy can be adequately, and safely, planned on the basis of the extent of metabolically active disease in combination with a non-contrast CT, diagnostic CT may again not be required.
Conversely, the performance of PET/CT as the initial staging test could, however, lead to prescription of a diagnostic CT tailored to resolving ongoing uncertainty with respect to stage. For example, if PET/CT results in any given site were considered equivocal and that site of abnormality would alter treatment selection or delivery, an appropriately prescribed contrast CT may be helpful to assess differential diagnoses and to guide further investigation. Alternatively, if no nodal or systemic metastases are found on PET/CT, endoscopic ultrasound is the most accurate technique to define T-stage and to detect peri-tumoral nodes that may not be resolved as separate from the primary lesion on PET. Accordingly, this may be the preferred technique after PET/CT unless access is limited by obstruction, in which case, newer multi-detector techniques may improve diagnostic CT performance for T-staging [47].
Accordingly, use of this staging paradigm would likely lead to a reduction in the number of diagnostic CT scans performed for staging oesophageal, a potentially shorter and more definitive process of selection of the most appropriate treatment and selective use of the optimal anatomical imaging technique to more accurately define regional anatomy in the subgroup of patient who remain surgical candidates after PET/CT. Most importantly, it could prevent a cascade of cost and morbidity associated with performance of inappropriate management based on inaccurate staging, particularly insensitive detection of remote metastases.
PET/CT as the initial restaging investigation of colorectal cancer
As detailed above, residual structural abnormalities are common following definitive surgery or radiotherapy of cancer. Differentiating residual or recurrent cancer from scar tissue is problematic, especially in patients with ongoing symptoms. Biopsy of residual masses has the potential for sampling errors and may also lead to complications related to impaired healing in previous radiotherapy fields. For cancers associated with tumour markers, presence of a residual mass may erroneously suggest local recurrence when, in fact, systemic metastasis may be the real source of the biochemical abnormality.
Colorectal cancer is one of the most common cancers in western societies. Unless detected at an early stage, it still has a relatively poor prognosis. Around 20% of patients treated surgically for pathological stage II disease will be dead within 5 years and this increases to over 40% of patients with stage III disease at diagnosis. For patients presenting with stage IV disease, the outlook is bleak with less than 10% surviving at 5 years [48]. This significant risk of recurrent disease and the demonstration that resection of limited hepatic metastases can improve survival [49] has led to some oncologists performing ongoing surveillance of patients who have undergone curative resection of locally advanced colorectal cancers.
The value of surveillance and the best method to use for this purpose remain controversial [50]. Clinical review and measurement of carcino-embryonic antigen (CEA) levels are relatively common and inexpensive options but early intervention based on elevated CEA levels has not been shown to improve patient outcomes. In order to more definitively detect local anastomotic recurrences and liver metastases, the two most common sites of recurrent disease, colonoscopy and CT scanning, respectively, are sometimes added to the surveillance scheme. Assuming that the most likely cause of elevated CEA levels in a patients previously treated for colorectal cancer is the development of hepatic metastases and that, if these are of limited extent, that these may be amenable to surgical resection, the prescription of diagnostic CT in patients with elevated CEA levels is usually optimised for evaluation of the liver.
However, particularly in rectal cancer, residual structural abnormalities at the site of resection or previous radiotherapy are common and patients often have ongoing local symptoms related to mechanical dysfunction associated with prior treatment. When these findings are accompanied by rising CEA, they are generally assumed to relate to local recurrence in the absence of other CT abnormalities. In the absence of CEA elevation or symptoms, the very same findings will be assumed to be most likely post-treatment scarring and warrant no more than a follow-up CT at some later time-point to exclude progressive growth. In patients with hepatic metastases on CT that would be potentially suitable for resection, abnormality at the primary resection site create a major dilemma and usually mandates biopsy to exclude extra-hepatic disease that would make hepatic resection inappropriate. All these combinations of clinical, biochemical and CT findings can be associated with a range of true disease distributions including absence of disease, local recurrence alone, hepatic metastasis alone, both local and systemic disease and systemic metastasis involving liver and extra-hepatic sites. Clearly the prognosis, the need and potential for salvage therapies, and the planning of the chosen therapy are vitally dependent on accurately confirming the presence of residual disease and its extent.
There is substantial evidence demonstrating that FDG PET is more accurate than diagnostic CT scanning in detecting recurrent colorectal cancer, particularly for extra-hepatic sites but even for hepatic lesions [51, 52]. In a series of patients from the Peter MacCallum Cancer Centre, we found [31] that in patients who were suspected to have recurrent disease, based on symptoms, tumour markers, clinical or radiological findings, and for whom salvage therapy was being contemplated, FDG PET directly influenced management in 60 (59%) of 102 patients. The discrepant PET results could be validated in 57 patients and were correct for both the presence and the extent of malignant disease in 52 (91%) of these patients. Relapse was confirmed in 49 (98%) of 50 evaluable patients with positive PET findings. Significantly, planned surgery was abandoned in 26 (60%) of 43 patients because of incremental PET findings. The performance of CT in this group of patients was so poor as to raise questions whether it added significant information after the PET had characterised the extent of disease.
Again, these data arose from patients selected on the basis of a prior diagnostic CT and utilising a stand-alone PET. In the era of PET/CT, the better differentiation of physiological FDG uptake from pathological accumulations, the improved attenuation-correction and reconstruction algorithms, and the more precise localisation of abnormalities, are likely to more accurately define appropriate further investigation strategies, or to allow definitive treatment selection. There is evidence that PET/CT is more accurate than stand-alone PET in colorectal cancer restaging [7, 53]. Based on these findings, diagnostic CT may be unnecessary in the case of disseminated disease, or be prescribed differently depending on the sites of abnormality identified. For example, patients with isolated liver metastases could have a detailed evaluation of hepatic vascular anatomy to be correlated with the sites of metabolic abnormality and thereby better plan surgical resection. This could include a respiratory-gated static image of the liver combined with a contrast-enhanced CT, or MRI of the liver [51]. Conversely, isolated local recurrence may benefit from a different prescription, or even modality, to optimally display regional anatomical relations for treatment planning purposes. Thus, by more accurately excluding disease at sites of residual structural abnormality, PET/CT could allow patient reassurance and avoid unnecessary salvage therapies. More timely detection of local recurrence may also improve the likelihood of successful salvage treatment. Finally, earlier and more precise definition of the extent of systemic metastases may improve selection of patients for surgery or chemotherapy and may lead to improved outcomes by more timely commencement of treatment.
Conclusion
The examples discussed above potentially lend themselves to randomised trials wherein patients could be assigned to arms where either diagnostic CT or PET/CT is performed as the initial cancer staging procedure. By prospectively tracking treatment plans before and after each subsequent investigation, it would be feasible to track the incremental diagnostic information and the cumulative cost of investigations required to achieve a definitive treatment plan. In addition to these outcomes, it would also be possible to assess the time taken to formulate a final management plan, the types of treatments delivered, and the subsequent disease course in each in group, including survival. The two examples discussed are but two of many potential clinical scenarios that a PET/CT-first paradigm could be advocated. In our opinion, evaluation of pulmonary masses incidentally identified on chest x-ray, primary staging of rectal cancer and of locally advanced breast cancer, primary staging and restaging of ovarian cancer, restaging of head and neck cancer, evaluation of carcinoma of unknown primary, assessment of patients suggestive of a para-neoplastic syndrome or with elevated tumour markers, are also situations that may benefit from this approach.
References
- 1.Jones LA, Chilton JA, Hajek RA, Iammarino NK, Laufman L. Between and within: international perspectives on cancer and health disparities. J Clin Oncol. 2006;24:2204–8. doi: 10.1200/JCO.2005.05.1813. [DOI] [PubMed] [Google Scholar]
- 2.Bosanquet N, Sikora K. The economics of cancer care in the UK. Lancet Oncol. 2004;5:568–74. doi: 10.1016/S1470-2045(04)01569-4. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 4.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 5.Beyer T, Townsend DW, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–79. [PubMed] [Google Scholar]
- 6.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, Altman H, Keidar Z, Israel O. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–9. [PubMed] [Google Scholar]
- 7.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–803. [PubMed] [Google Scholar]
- 8.Branstetter BF, Blodgett TM, Zimmer LA, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005;235:580–6. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 9.Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–9. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 10.Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–87. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 11.Israel O, Mor M, Guralnik L, et al. Is 18F-FDG PET/CT useful for imaging and management of patients with suspected occult recurrence of cancer? J Nucl Med. 2004;45:2045–51. [PubMed] [Google Scholar]
- 12.Schaefer NG, Hany TF, Taverna C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging—do we need contrast-enhanced CT? Radiology. 2004;232:823–9. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 13.Schroder W, Baldus SE, Monig SP, et al. Lymph node staging of esophageal squamous cell carcinoma in patients with and without neoadjuvant radiochemotherapy: histomorphologic analysis. World J Surg. 2002;26:584–7. doi: 10.1007/s00268-001-0271-5. [DOI] [PubMed] [Google Scholar]
- 14.van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–93. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 15.MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287–93. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- 16.MacManus MP, Wong K, Hicks RJ, Matthews JP, Wirth A, Ball DL. Early mortality after radical radiotherapy for non-small-cell lung cancer: comparison of PET-staged and conventionally staged cohorts treated at a large tertiary referral center. Int J Radiat Oncol Biol Phys. 2002;52:351–61. doi: 10.1016/s0360-3016(01)02673-6. [DOI] [PubMed] [Google Scholar]
- 17.Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s—meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- 18.Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–89. [PubMed] [Google Scholar]
- 19.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–24. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 20.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–56. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 21.Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 22.Delgado-Bolton RC, Fernandez-Perez C, Gonzalez-Mate A, Carreras JL. Meta-analysis of the performance of 18F-FDG PET in primary tumor detection in unknown primary tumors. J Nucl Med. 2003;44:1301–14. [PubMed] [Google Scholar]
- 23.Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med. 2003;44:717–24. [PubMed] [Google Scholar]
- 24.Isasi CR, Moadel RM, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat. 2005;90:105–12. doi: 10.1007/s10549-004-3291-7. [DOI] [PubMed] [Google Scholar]
- 25.Hicks RJ, MacManus MP. 18F-FDG PET in candidates for radiation therapy: is it important and how do we validate its impact? J Nucl Med. 2003;44:30–2. [PubMed] [Google Scholar]
- 26.Miles KA, Griffiths MR, Fuentes MA. Standardized perfusion value: universal CT contrast enhancement scale that correlates with FDG PET in lung nodules. Radiology. 2001;220:548–53. doi: 10.1148/radiology.220.2.r01au26548. [DOI] [PubMed] [Google Scholar]
- 27.Keith CJ, Miles KA, Griffiths MR, Wong D, Pitman AG, Hicks RJ. Solitary pulmonary nodules: accuracy and cost-effectiveness of sodium iodide FDG-PET using Australian data. Eur J Nucl Med Mol Imaging. 2002;29:1016–23. doi: 10.1007/s00259-002-0833-2. [DOI] [PubMed] [Google Scholar]
- 28.Lejeune C, Al Zahouri K, Woronoff-Lemsi MC, et al. Use of a decision analysis model to assess the medicoeconomic implications of FDG PET imaging in diagnosing a solitary pulmonary nodule. Eur J Health Econ. 2005;6:203–14. doi: 10.1007/s10198-005-0279-0. [DOI] [PubMed] [Google Scholar]
- 29.Lejeune C, Bismuth MJ, Conroy T, et al. Use of a decision analysis model to assess the cost-effectiveness of 18F-FDG PET in the management of metachronous liver metastases of colorectal cancer. J Nucl Med. 2005;46:2020–8. [PubMed] [Google Scholar]
- 30.Dendukuri N, Rahme E, Belisle P, Joseph L. Bayesian sample size determination for prevalence and diagnostic test studies in the absence of a gold standard test. Biometrics. 2004;60:388–97. doi: 10.1111/j.0006-341X.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalff V, Hicks RJ, Ware RE, Hogg A, Binns D, McKenzie AF. The clinical impact of (18)F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med. 2002;43:492–9. [PubMed] [Google Scholar]
- 32.Hicks RJ, Kalff V, MacManus MP, et al. (18)F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med. 2001;42:1596–604. [PubMed] [Google Scholar]
- 33.MacManus MP, Hicks RJ, Ball DL, et al. F-18 fluorodeoxyglucose positron emission tomography staging in radical radiotherapy candidates with nonsmall cell lung carcinoma: powerful correlation with survival and high impact on treatment. Cancer. 2001;92:886–95. doi: 10.1002/1097-0142(20010815)92:4<886::aid-cncr1397>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.MacManus MR, Hicks R, Fisher R, et al. FDG-PET-detected extracranial metastasis in patients with non-small cell lung cancer undergoing staging for surgery or radical radiotherapy—survival correlates with metastatic disease burden. Acta Oncol. 2003;42:48–54. doi: 10.1080/0891060310002230. [DOI] [PubMed] [Google Scholar]
- 35.Hicks RJ, Kalff V, MacManus MP, et al. The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42:1605–13. [PubMed] [Google Scholar]
- 36.Ware RE, Matthews JP, Hicks RJ, et al. Usefulness of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with a residual structural abnormality after definitive treatment for squamous cell carcinoma of the head and neck. Head Neck. 2004;26:1008–17. doi: 10.1002/hed.20097. [DOI] [PubMed] [Google Scholar]
- 37.Cachin F, Prince HM, Hogg A, Ware RE, Hicks RJ. Powerful prognostic stratification by [18F]fluorodeoxyglucose positron emission tomography in patients with metastatic breast cancer treated with high-dose chemotherapy. J Clin Oncol. 2006;24:3026–31. doi: 10.1200/JCO.2005.04.6326. [DOI] [PubMed] [Google Scholar]
- 38.Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47:14–22. [PubMed] [Google Scholar]
- 39. Simcock B, Neesham D, Quinn M, Drummond E, Milner A, Hicks RJ, The impact of PET/CT in the management of recurrent ovarian cancer. Gynecol Oncol 2006; Apr 17 [Epub ahead of print]
- 40.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 41.Stephens MR, Lewis WG, Brewster AE, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus. 2006;19:164–71. doi: 10.1111/j.1442-2050.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 42.Kaklamanos IG, Walker GR, Ferry K, Franceschi D, Livingstone AS. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–61. doi: 10.1245/aso.2003.03.078. [DOI] [PubMed] [Google Scholar]
- 43.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–43. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 44.Duong CP, Demitriou H, Weih L, et al. Significant clinical impact and prognostic stratification provided by FDG-PET in the staging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33:759–69. doi: 10.1007/s00259-005-0028-8. [DOI] [PubMed] [Google Scholar]
- 45.Bar-Shalom R, Guralnik L, Tsalic M, et al. The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2005;32:918–24. doi: 10.1007/s00259-005-1795-y. [DOI] [PubMed] [Google Scholar]
- 46.Leong T, Everitt C, Yuen K, et al. A prospective study to evaluate the impact of FDG-PET on CT-based radiotherapy treatment planning for oesophageal cancer. Radiother Oncol. 2006;78:254–61. doi: 10.1016/j.radonc.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Panebianco V, Grazhdani H, Iafrate F, et al. 3D CT protocol in the assessment of the esophageal neoplastic lesions: can it improve TNM staging? Eur Radiol. 2006;16:414–21. doi: 10.1007/s00330-005-2851-5. [DOI] [PubMed] [Google Scholar]
- 48.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 49.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–74. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 50.Johnson FE, Longo WE, Ode K, et al. Patient surveillance after curative-intent surgery for rectal cancer. Int J Oncol. 2005;27:815–22. [PubMed] [Google Scholar]
- 51.Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—meta-analysis. Radiology. 2005;237:123–31. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 52.Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104:2658–70. doi: 10.1002/cncr.21569. [DOI] [PubMed] [Google Scholar]
- 53.Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027–34. doi: 10.1097/01.sla.0000146145.69835.c5. discussion 1035–6. [DOI] [PMC free article] [PubMed] [Google Scholar]