Abstract
PET scanning is an emerging technology for the clinical evaluation of many disease processes in man. The vast majority of clinical positron emission tomography (PET) studies are performed using a single tracer, fluorodeoxyglucose. Despite the excellent diagnostic performance of this tracer, it has recognised limitations. New tracers offer the potential to both address these limitations, and to establish new applications for PET. Small animal PET is a logical technique for validating new tracers relevant to human diseases. However, interspecies differences in the handling of chemicals may significantly influence the handling of novel tracers. This requires caution in extrapolating findings in animals to expectations of performance in man. Already there are several examples where biodistribution studies in mice would not have predicted the clinical utility of existing PET tracers. Nevertheless, application of a systematic approach to tracer development is likely to speed transition of new tracers from animals into man.
Keywords: PET, tracer development, fluorine-18, choline, proliferation, fluoro-ethyl-tyrosine, fluorocholine, 3 ′-deoxy-3 ′-fluorothymidine
Introduction
Positron emission tomography (PET) is a form of molecular imaging that relies on derangement of physiological and biochemical processes for the detection of many diseases. Long recognised as being a useful technique in neurology and cardiology, recent growth has been largely driven by its role in oncology. This growth has been based almost exclusively on the imaging characteristics of a single tracer, fluorine-18 fluorodeoxyglucose (FDG), that simply visualises a basic biochemical function of most living cells; their ability to utilise glucose as an energy substrate. Disease processes can either up-regulate or down-regulate glucose metabolism. Despite the excellent diagnostic performance of FDG, it has recognised limitations. Broadly, these relate, first, to the non-specificity of glucose-metabolic changes and, second, to lack of contrast between physiological and pathological uptake, limiting sensitivity.
Lack of specificity of glucose metabolism as a target for disease detection is particularly relevant for the detection of cancer. Since enhanced glycolytic metabolism is a characteristic of many cancers but also of active inflammatory processes, cancer staging can be compromised by apparently focal accumulations of FDG due to infectious diseases, granulomatous lesions and inflammatory healing responses related to previous interventions or therapies. Although pattern recognition can play an important role in differentiating these processes [1], biopsy may be necessary to confirm the nature of FDG PET abnormalities definitively. Even when cancer is known to be present, high uptake in adjacent tissues due to physiological processes can reduce lesion contrast and thereby impair the sensitivity of FDG PET for the detection of disease. The most obvious example of this is in the brain where high glucose use by the normal cortex can mask the presence of brain tumours. Low FDG-avidity can also occur with certain tumours, reducing contrast and, therefore, sensitivity even in the absence of high background activity. Although both its specificity and sensitivity are imperfect, the overall diagnostic accuracy of FDG in cancer is so good in most clinical situations [2] there is little room for improvement with respect to new tracers, which have to match and then improve on this already high sensitivity and specificity. Nevertheless, new tracers offer the potential to address both these limitations and also to establish new applications for PET, particularly in niche clinical applications.
The ability of PET to go beyond the anatomical information provided by imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT), or even the physiological information obtained from standard nuclear medicine procedures, down to the biochemical level is one of its major advantages [3]. In an era where molecular profiling is identifying specific and mechanistically important alterations in diseased cells, a logical progression of PET tracer development is to go beyond probing of basic biochemical processes to looking at more specific features of cancer biology.
The ten commandments of tracer development
The process of developing new tracers for human use is complex, but can be broken into logical steps. On one hand, there are phases related to identifying and meeting a clinical need, and on the other, phases involved with the development of the tracer itself. Successful tracer development requires that these elements go hand in hand.
For clinical development of new tracer there are five major steps:
(1) Definition of clinical need
(2) Establishment of an appropriate pre-clinical model for testing
(3) Evaluation in an appropriate clinical setting
(4) Comparison with existing standards
(5) Documentation of safety and efficacy.
For the design and production of new tracers another five steps are involved:
(1) Identification of an appropriate target
(2) Selection of precursor ligands
(3) Development of labelling strategies
(4) Optimisation of labelling and QC
(5) Documentation of drug master file (dosimetry, toxicity etc.).
These 10 steps are all required to develop a new tracer to the point of clinical application but cannot occur as independent processes. The most important step in our opinion is the recognition of clinical need based on existing limitations of available tracers and shortfalls in other imaging modalities. From this need and an understanding of disease causation, identification of potential imaging targets can ensue. Target discovery is a complex and, sometimes, serendipitous process but, in line with the process of drug discovery, is becoming increasingly sophisticated and technology-driven. In particular, it is now often based on genomic and proteomic analysis of diseased tissues. Once a target is selected, then appropriate models for testing potential ligands need to be established. Ideally, these are cells over-expressing the particular target and that can be both grown in culture for in vitro studies and induced in animals, either through direct implantation, by physical means, or by genetic modification, allowing in vivo testing. The selection of precursor ligands is driven by the target identified but also by an understanding of available labelling techniques. Many biological interactions relate to stereochemistry and therefore, initial precursor selection can occur from libraries tested in silico before subsequent in vitro and in vivo testing. The development of labelling strategies leads to production of a radioligand suitable for further testing in the chosen model system. Demonstration of targeting is sought along with further information about biodistribution. If the radiotracer appears promising, further work can proceed to optimise labelling and produce the compound in sufficient quantity and sufficient purity to enable testing in human subjects. The ideal means of confirming the potential utility of a new tracer is to test it in an appropriate population with the disease for which the tracer was developed. These preliminary human studies are consistent with recently promulgated ‘micro-dosing’ principles for tracer compounds [4]. If biodistribution studies look encouraging and the disease process can be visualised, further work to improve the synthesis yields, and to obtain more complete toxicity data in animals, is worthwhile. On the clinical validation side, subsequent comparison with existing standards is pertinent to demonstrate the incremental benefits of the new agent compared to existing diagnostic tests. The final phase of development relates to the documentation of clinical efficacy and safety, and of a regulatory approval file relating to the production and quality assurance of the tracer.
The role of small animal PET in tracer development
As mentioned above, increasing understanding of molecular biology enables identification of novel potential targets for the diagnosis and treatment of diseases. These targets can be as specific as a mutation in a key gene or reflect downstream consequences as generic as substrate utilisation by cells. Small animal PET is being increasingly used by academic institutions and pharmaceutical companies as a platform technology for translational research and drug development. The ability to extrapolate from animals to human studies makes PET a logical technique for both pre-clinical testing of new therapeutic drugs and for the validation of new tracers that might be relevant to the evaluation of human diseases. Development of appropriate animal models for testing targeted tracers is an important step in this process.
Manipulation of cells and tissues to produce animal models that mimic human diseases provides a useful system to test new tracers. However, interspecies differences in the handling of chemicals may significantly influence the biodistribution and kinetics of novel tracers, requiring caution in predicting behaviour of these tracers in man. At a pragmatic level, a short feedback loop between studies in mice and then in man is important. The marked variability in the biodistribution of fluorine-18 3 ′-deoxy-3 ′-fluorothymidine (FLT) in various animal models is but one salutary example of the limitations in evaluating new tracers in animal models. While unrestrained proliferation has been described as a hallmark of cancer and is a target of several targeted anti-cancer therapeutics, due to differences in blood thymidine levels and in the metabolism of FLT, both uptake and excretion of this tracer show significant differences in biodistribution in different species. Thus they may not necessarily provide the same information, nor necessarily reflect the process that they purport to evaluate [5]. For example, dogs have low uptake of FLT in the liver [6], whereas intense uptake is seen in man. Nevertheless, the cellular uptake of FLT provides evaluation of cellular proliferation, primarily through reflecting thymidine kinase 1 (TK1) activity. In animal and human clinical studies, FLT uptake has been found to correlate with the immunohistochemical proliferation marker ki-67 [7].
Limitations of animal imaging
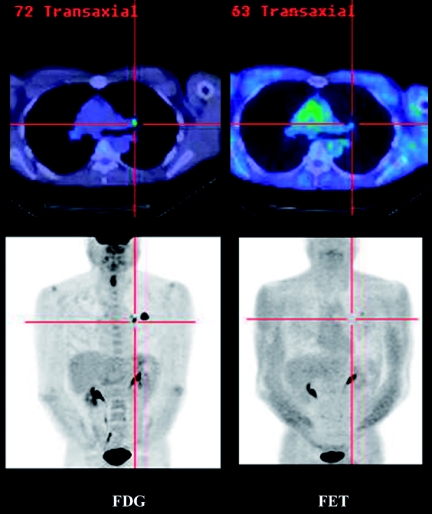
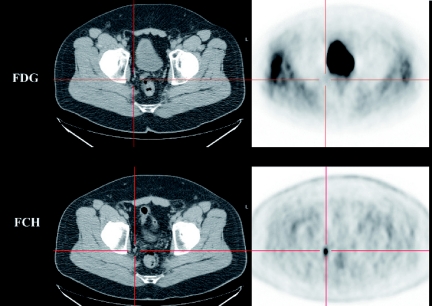
Our experience of various other tracers in mice and man have indicated that such interspecies differences also exist for amino-acid analogues like flourine-18 fluoro-ethyl-tyrosine (FET) and tracers of cell membrane formation like fluorine-18 fluorocholine (FCH). The uptake of FET in most human xenografts that we have evaluated demonstrate excellent tumour-to-background contrast whereas in solid tumours in man, relatively low contrast compromises the sensitivity of this agent for tumour detection relative to FDG (Fig. 1). On the other hand, uptake of the choline analogue, FCH, in almost all xenograft models that we have evaluated is very poor but this tracer shows excellent uptake in some tumours that can have relatively low FDG-avidity, like prostate cancer (Fig. 2). The effects of micro-environmental factors and species-specific antigenic characteristics are likely to further increase the complexity of assessing tracers that rely on metabolic or immunogenic targeting. SAP is an exciting tool but needs to be used in full recognition of both its strengths and weaknesses.
Figure 1.
A comparison of FDG and FET uptake in a primary lung cancer demonstrates substantially higher contrast with FDG and therefore, despite theoretically higher specificity for malignancy, the lower sensitivity decreases confidence in the veracity of a negative result.
Figure 2.
A comparison of FDG and FCH uptake in nodal metastases demonstrates higher contrast and therefore, greater sensitivity of FCH in this case.
Examples of tracers in current development
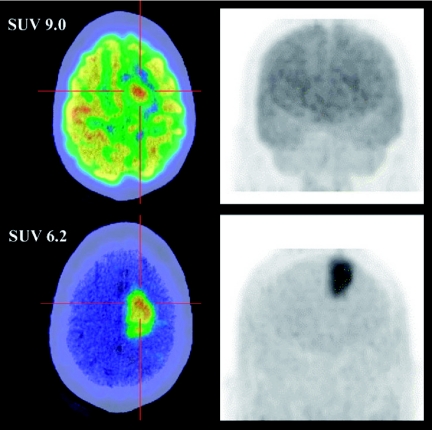
Based on established principles of PET metabolic imaging using tracers of substrate metabolism, development of radiolabelled amino acids has been seen as a means to address the limitations of FDG with respect to specificity for cancer and also for sensitivity of lesion detection in the brain where high background uptake can mask tumoral radiotracer accumulation. Up-regulation of amino acid transport and enhanced protein synthesis is a hallmark of cancer. Carbon-11 methionine has been shown to have similar sensitivity to FDG for detection of various cancers, including head and neck and lung cancer, but a somewhat higher specificity due to a reduced tendency to be accumulated in inflammatory lesions. As such, it is one of the few radiopharmaceuticals to compete favourably with FDG in terms of diagnostic accuracy in cancer [8]. Unfortunately the short physical half-life of C-11 makes this an impractical tracer for routine clinical applications. FET was developed by German researchers as an alternative cancer-imaging agent [9] and in comparative studies with C-11 methionine was shown to have similar characteristics [10] but has the practical advantages of using F-18 as the imaging radionuclide. This agent demonstrates very low uptake in the normal brain and relatively higher uptake in brain tumours; it provides more sensitive detection of disease and better characterisation of the extent of tumoral involvement than does FDG (Fig. 3).
Figure 3.
A comparison of FDG and FET in malignant brain tumour demonstrates substantially higher contrast with FET than FDG, despite lower absolute uptake as reflected by the standardised uptake value (SUV).
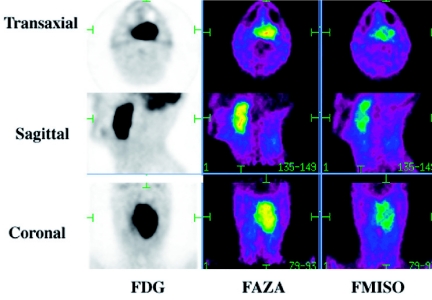
Extensive efforts have been made to develop PET tracers to enable the non-invasive imaging of hypoxia. Fluorine-18 fluoromisonidazole (FMISO) is the most extensively studied agent both in animal models and humans. In particular, demonstration of the ability of FMISO to stratify prognosis in patients receiving conventional radiotherapy and targeted therapy of hypoxia has been demonstrated [11]. However, FMISO is a relatively lipophilic compound and demonstrates relatively low uptake in hypoxic tissue relative to normal tissue, and slow clearance from normal tissues requiring delayed scanning with consequences on contrast and image quality. This has led to the development of other hypoxic tracers with more favourable imaging properties. Fluorine-18 fluoro-azomycin arabinoside (FAZA) is one such agent [12]. Preliminary results have been obtained at our institution with the use of FAZA to image hypoxia within head and neck squamous cell carcinomas (Fig. 4). These suggest that FAZA is likely to be a superior agent for hypoxia imaging.
Figure 4.
A comparison of FAZA and FMISO shows comparable lesions but higher contrast on FAZA, aiding clinician confidence in the images and region-of-interest assignment.
Fluorine-18 fluoromethyl-dimethyl-2-hydroxyethyl-ammonium or fluorocholine (FCH) is an interesting new tracer. Tumour tissues have a requirement for increased synthesis of phosphatidylcholine, an important constituent of cell membranes. Increased rates of transmembrane transport and subsequent phosphorylation of choline by the enzyme choline kinase in tumours has been demonstrated. Carbon-11 labelled choline has been used to successfully image a variety of tumours including prostate cancer, brain tumours, and oesophageal cancer and lung cancer but because of the short half-life of carbon-11 (20 min), fluorinated choline analogues, like FCH, are potentially more suitable for clinical studies. Preliminary studies suggest that FCH may be more sensitive than FDG for detection of nodal and bone metastases in prostate cancer [13]. High uptake in the liver and kidneys is a limitation of this tracer in detecting primary or secondary tumours in these organs.
Future directions
Due to the favourable imaging characteristics and ready availability of fluorine-18, there has been an increasing focus on fluorinated tracers. However, a wide range of both cyclotron and generator produced isotopes are becoming available. These include yttrium-86, gallium-68 and iodine-124. These tracers lend themselves to complexing with biological macromolecules, such as peptides [14] and antibodies. The availability of long-lived positron emitting radionuclides offers particular opportunities for translational research in the labelling of larger molecular species, including monoclonal antibodies, which can have slow accumulation in tissues [15].
Conclusion
The tale of a tracer’s journey from concept to clinical utility is long and littered with failures, not least because of the great success of the existing yardstick, FDG. Nevertheless, there remain many opportunities for new tracers to complement existing diagnostic methods and potentially to supplant them in some situations. Tracer development is not solely the domain of radiochemists delivering products for testing by nuclear medicine specialists. It is a multidisciplinary effort combining the skills and expertise of clinicians and scientists from many specialties. The commonality of goal needs to be delivering better diagnosis and, thereby, better health.
References
- 1.Hicks RJ, MacManus MP, Matthews JP, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys. 2004;60:412–8. doi: 10.1016/j.ijrobp.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 3.Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–81. [PubMed] [Google Scholar]
- 4.Bergstrom M, Grahnen A, Langstrom B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur J Clin Pharmacol. 2003;59:357–66. doi: 10.1007/s00228-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 5.Krohn KA, Mankoff DA, Muzi M, Link JM, Spence AM. True tracers: comparing FDG with glucose and FLT with thymidine. Nucl Med Biol. 2005;32:663–71. doi: 10.1016/j.nucmedbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 7.Vesselle H, Grierson J, Muzi M, et al. In vivo validation of 3 ′-deoxy-3 ′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–23. [PubMed] [Google Scholar]
- 8.Kubota K, Matsuzawa T, Ito M, et al. Lung tumor imaging by positron emission tomography using C-11 L-methionine. J Nucl Med. 1985;26:37–42. [PubMed] [Google Scholar]
- 9.Wester HJ, Herz M, Weber W, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40:205–12. [PubMed] [Google Scholar]
- 10.Weber WA, Wester HJ, Grosu AL, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl- 11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27:542–9. doi: 10.1007/s002590050541. [DOI] [PubMed] [Google Scholar]
- 11.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 12.Piert M, Machulla HJ, Picchio M, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–13. [PubMed] [Google Scholar]
- 13.DeGrado TR, Coleman RE, Wang S, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res. 2001;61:110–7. [PubMed] [Google Scholar]
- 14.Hofmann M, Maecke H, Borner R, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–7. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 15.Pagani M, Stone-Elander S, Larsson SA. Alternative positron emission tomography with non-conventional positron emitters: effects of their physical properties on image quality and potential clinical applications. Eur J Nucl Med. 1997;24:1301–27. doi: 10.1007/s002590050156. [DOI] [PubMed] [Google Scholar]