Abstract
Villin, an actin-binding protein associated with the actin bundles that support microvilli, bundles, caps, nucleates, and severs actin in a calcium-dependant manner in vitro. We hypothesized that the severing activity of villin is responsible for its reported role in enhancing cell plasticity and motility. To test this hypothesis, we chose a loss of function strategy and introduced mutations in villin based on sequence comparison with CapG. By pyrene-actin assays, we demonstrate that this mutant has a strongly reduced severing activity, whereas nucleation and capping remain unaffected. The bundling activity and the morphogenic effects of villin in cells are also preserved in this mutant. We thus succeeded in dissociating the severing from the three other activities of villin. The contribution of villin severing to actin dynamics is analyzed in vivo through the actin-based movement of the intracellular bacteria Shigella flexneri in cells expressing villin and its severing variant. The severing mutations abolish the gain of velocity induced by villin. To further analyze this effect, we reconstituted an in vitro actin-based bead movement in which the usual capping protein is replaced by either the wild type or the severing mutant of villin. Confirming the in vivo results, villin-severing activity enhances the velocity of beads by more than two-fold and reduces the density of actin in the comets. We propose a model in which, by severing actin filaments and capping their barbed ends, villin increases the concentration of actin monomers available for polymerization, a mechanism that might be paralleled in vivo when an enterocyte undergoes an epithelio-mesenchymal transition.
INTRODUCTION
Villin is an actin-binding protein whose expression pattern is primarily restricted to the epithelial cells of the intestine and kidney proximal tubules where it localizes to microvilli (Bretscher and Weber, 1979; Matsudaira and Burgess, 1979; Robine et al., 1985). Microvilli are thin membrane extensions at the apical pole of epithelial cells, they largely increase the cell surface available for absorption and secretion. Microvilli have been extensively studied and shown to be a good model to understand morphogenesis and differentiation of cells. They are sustained by bundles of parallel actin filaments. Numerous actin-binding proteins among which is villin are responsible for the organization and the maintenance of the actin cytoskeleton in microvilli (Revenu et al., 2004).
Villin belongs to the gelsolin family. All proteins of this family are organized around a conserved module of ∼120 amino acids. This module is repeated three times in the proteins severin and fragmin that belong to lower eukaryotes (Dictyostelium and Physarum, respectively). Along evolution, a further duplication of the three modules occurred in gelsolin and villin, which contain six modules (Arpin et al., 1988; Bazari et al., 1988). These six modules named V1–V6 constitute the core of the villin protein (see Figure 1). Villin contains an additional C-terminal domain that is absent in gelsolin, called the headpiece (Arpin et al., 1988). The villin effects on actin have been analyzed in vitro and were shown to depend on the calcium concentration (Bretscher and Weber, 1980; Glenney et al., 1980, 1981; Mooseker et al., 1980). At low calcium levels (10–100 nM), villin bundles actin filaments. At micromolar calcium concentrations, villin starts to nucleate and cap the barbed ends of actin filaments. At higher calcium concentrations, villin is able to sever actin filaments, and this activity reaches its maximum efficiency above 100 μM calcium. Phosphorylation has also been shown to modulate the properties of villin. The in vitro phosphorylation of different tyrosines in villin enhances its severing and inhibits its bundling activities (Zhai et al., 2001, 2002). The domain responsible for the capping and severing activities of villin is included in the V1 and V2 modules where an F-actin binding site has been localized (Matsudaira et al., 1985; Janmey and Matsudaira, 1988). The second half of the villin core (V4–V6) is essential for its nucleation property (Friederich et al., 1999). The ability to bundle filaments is due to the presence of an additional F-actin–binding site in the headpiece (Glenney and Weber, 1981).
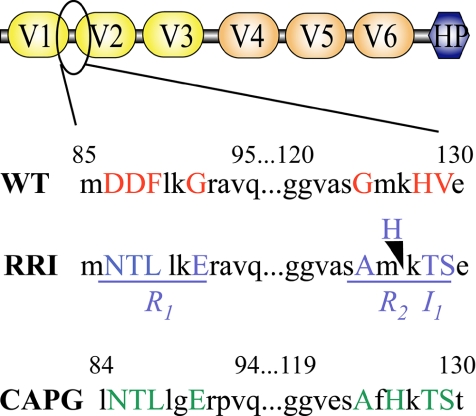
Figure 1.
The RRI mutations and their localization in villin domains. The scheme describes the domain structure organization of the villin protein. The mutations introduced in comparison with CapG are located in the circled region. This region is known to endow villin with a severing activity. The wild-type (WT) and the mutated (RRI) amino acid sequences of villin are shown as well as the corresponding sequence of CapG. The mutated amino acids are in capital letters. They are clustered in two regions (underlined, R1 and R2) with a histidine inserted (I1) in the second region.
Because villin is able to bundle actin filaments, it was thought to be involved in the formation of microvilli. Indeed, the overexpression of villin in fibroblastic-like cells CV1 is able to induce the formation of microvilli (Friederich et al., 1989), and the transcriptional silencing of villin in Caco-2 cells affects their distribution and length (Costa de Beauregard et al., 1995). These data obtained in cell cultures supported a role for villin in the formation and maintenance of microvilli. However, the knock out of the villin gene in mice (vil−/−) did not induce any detectable ultrastructural alteration of the microvilli of intestinal epithelial cells (Ferrary et al., 1999). Nevertheless, the intestinal epithelium of these mice exhibited a decreased cellular response to stresses and to the induction of lesions by dextran sodium sulfate, treatments that result in intracellular calcium rise (Ferrary et al., 1999). After lesions, enterocytes have to undergo an epithelio-mesenchymal transition (EMT) to reconstitute the epithelial barrier. The studies on the vil−/− mice suggested that the remodeling of the actin cytoskeleton needed in EMT was impaired in the epithelial cells knocked out for villin. In support of this hypothesis, the efficiency of cell motility and morphogenesis (Athman et al., 2003) as well as the infectious process of Shigella flexneri (Athman et al., 2005) were reported to be increased in villin expressing cells, confirming a new role for villin in actin cytoskeleton dynamics.
Our hypothesis is that the villin-severing activity is responsible for the enhancement of actin dynamics. By cutting actin filaments, villin would increase the number of free barbed and pointed ends and enhance the cycling of actin needed for actin based motility. To test this hypothesis, we designed a severing mutant of villin. We demonstrate that this mutant of villin has a strongly reduced severing activity while retaining the other known activities. Because only the severing activity of villin is affected, we used this mutant in vivo and in an in vitro motility assay to analyze the impact of villin severing on actin-based motility.
MATERIALS AND METHODS
Mutagenesis, Protein Expression, and Purification
Human villin cloned in the prokaryotic expression vector pGEX-2T (Friederich et al., 1999) was used as template for the mutation of putative sites responsible for villin-severing activity. All the mutations were introduced using the Quick-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA). Complementary primers containing the desired mutations were designed as described previously (Kumar et al., 2004a) for the mutations D61N, D86L, and A93G. For the RRI variant (86DDFLKG/NTLLKE, 125GMKHV/AMHKTS), the two regions and the insertion were introduced as follows. The primer sense ACC ACA CAG ATG AAT ACC TTG CTG AAG GAG CGG GCT GTG CAG CAC and the antisense GTG CTG CAC AGC CCG CTC CTT CAG CAA GGT ATT CAT CTG TGT GGT were used to mutate the amino acid sequence 86DDFLKG in NTLLKE. The primer sense GGG GGC GTG GCT TCT GCC ATG AAG ACC TCG GAG ACC AAC TCC and its complementary sequence GGA GTT GGT CTC CGA GGT CTT CAT GGC AGA AGC CAC GCC CCC were used to transform the amino acid sequence 125GMKHV in AMKTS. The primers sense GTG GCT TCT GCC ATG CAC AAG ACC TCG GAG ACC and antisense GGT CTC CGA GGT CTT GTG CAT GGC AGA AGC CAC were used to introduce an histidine in position 127. The whole gene was sequenced to ensure that only the desired mutations were introduced.
Wild-type (WT) villin and its variants were produced using standard procedures (Friederich et al., 1999). Briefly, villin and its variants, tagged with glutathione S-transferase (GST), were purified from the soluble fraction of Escherichia coli Bl21 bacteria using glutathione-Sepharose beads. The beads bound to GST-villin were digested with thrombin. Thrombin was then removed from the eluates containing villin and variants by an incubation with p-amino-benzamidine agarose beads (Sigma, St. Louis, MO). Proteins were dialyzed overnight against phosphate-buffered saline (PBS). They were then aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C.
Actin Polymerization and Depolymerization Assays Using Fluorescence Spectroscopy
Villin activities toward actin filaments were tested by the use of N-(1-pyrene)iodoacetamide–labeled actin (pyrene-actin). Monomeric pyrene-actin fluoresces weakly at 388 nm when excited at 365 nm. When polymerized, a strong fluorescence peak appears at this wavelength. Hence, if actin polymerizes, the fluorescence intensity increases and vice versa (Kouyama and Mihashi, 1981).
Actin was purified from rabbit skeletal muscle acetone powder according to the procedure from Spudich and Watt (1971) and kept in G-buffer (2 mM Tris, 0.2 mM CaCl2, 0.2 mM ATP, 1 mM dithiothreitol [DTT], pH 8.5) at −80°C. The labeling of actin with pyrene-iodoacetamide was obtained and quantified following standard procedures (Kouyama and Mihashi, 1981). Fluorescence measurements were performed at room temperature using a fluorescence spectroscope (Perkin-Elmer, Wellesley, MA) driven by the Flwinlab software. The excitation wavelength was set at 365 nm and the emission wavelength at 388 nm. All measurements were done on samples of 400 μl.
Severing of Actin Filaments
Actin (2 μM, 30% labeled) was polymerized overnight at 4°C in F-buffer (2 mM Tris, pH 7.2, 100 mM KCl, 2 mM MgCl2, 0.2 mM ATP, 1 mM DTT). At t = 0, F-actin was diluted to 100 nM by the addition of F-buffer containing CaCl2, villin, and its variants at specified concentrations. The fluorescence intensity was recorded over 330 s. The severing activity was estimated as described previously (Gettemans et al., 1995; Friederich et al., 1999). The decrease in fluorescence per minute of the linear range of the curves was considered as corresponding to 100% activity for WT villin and 0% activity for control experiments without severing protein.
Nucleation of Actin Polymerization
G-actin (2 μM, 10% labeled) was preincubated for 10 min on ice with 24 nM of villin or its variants in G-buffer containing 20 μM CaCl2. At t = 0, polymerization was induced by the addition of KCl and MgCl2 to reach the final concentrations of 100 and 2 mM, respectively. The fluorescence intensity was recorded over 30 min.
Elongation Assay To Determine the Affinity of Villin for the Barbed Ends of Actin Filaments
Actin filaments (1 μM final) were incubated for 5 min at room temperature with various concentrations of WT or RRI villin in F buffer containing 2.5 μM free Ca2+. Elongation was initiated by the addition to the actin filament mixture of 1 μM actin monomers (5% pyrene-labeled) bound to 2 μM profilin (human profilin 1 purified as described previously; Fedorov et al., 1994). The polymerization was followed by changes in pyrene fluorescence using a Xenius SAFAS (Safas SA, Monaco). The affinity of the proteins for the barbed ends of actin filaments was determined by the variation of the initial rate of elongation as a function of the concentration of villin, using the following equation:
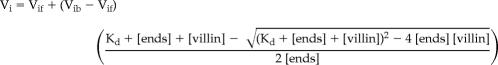 |
where Vi is the observed rate of elongation, Vif is the rate of elongation when all the barbed ends are free, Vib is the rate of elongation when all the barbed ends are capped, [ends] is the concentration of barbed ends, and [villin] is the concentration of villin. The data were modeled by Kaleidagraph v3.6 software (Synergy Software, Reading, PA).
Low-Speed Sedimentation Assay
Actin was polymerized in F buffer and then complemented to reach the final composition of the bundle buffer (5 mM KH2PO4, pH 7, 2 mM EGTA,1 mM DTT, 100 mM KCl, 1 mM MgCl2). F-actin (10 μM) was incubated for 1 h at room temperature with villin and its variants (2.5 μM) to allow bundling to occur. The proteins were centrifuged at 8000 × g for 15 min in order to pellet aggregates of filaments but not single filaments. The supernatant was separated from the pellet, and both were loaded on acrylamide gels. The gels were stained with Coomassie brilliant blue.
Electron Microscopy Analysis of Bundling Activity
To analyze bundling activity, F-actin was polymerized in F buffer and subsequently complemented to reach the composition of the bundle buffer. F-actin (3 μM) was incubated overnight at 4°C in the presence of 1.5 μM villin WT and variants. Actin filaments were incubated on carbon-coated grids in the presence of 2 mM phalloidin to stabilize filaments. They were fixed with 1% glutaraldehyde and negatively stained by incubation with 2% uranyl acetate. The observations were made with a Philips CM120 electron microscope (FEI, Eindoven, The Netherlands).
Expression of Villin and Variants in Mammalian Cells
Villin WT and mutants cDNA were cloned in the vector pEYFP-C1 (Clontech, Palo Alto, CA) within the restriction sites EcoRI and SalI. CV1 and LLCPK-1 cells were cultured in DMEM (Invitrogen, Carlsbad, CA) complemented with 10% fetal calf serum. CV1 cells were transfected by nucleofection (Amaxa, Gaithersburg, MD) according to the optimized procedure (solution V, program A33). LLCPK-1 cells were electroporated. At day 5 after transfection, cells were fixed in PFA and extracted with 0.5% Triton X-100. Actin filaments were stained using TRITC phalloidin. Cells were examined under an upright motorized microscope (Leica DMRA2, Wetzlar, Germany) equipped with an interlined CCD camera (Roper CoolSnap HQ, Tucson, AZ) and a piezo-electric motor (LVDT, Physik Instrument, Karlsruhe, Germany) mounted underneath the objective lens for rapid optical Z sectioning. The system was controlled by Metamorph software (Molecular Devices, Downingtown, PA). Stacks of images, taken with a 0.2-μm plane-to-plane distance, were restored using the Fast Iterative Constrained PSF-based algorithm (Sibarita, 2005).
For infection experiments, the yellow fluorescent protein (YFP) tag was replaced by mCherry (Shaner et al., 2004) using the restriction sites AgeI-BsrGI. Madin-Darby canine kidney (MDCK)-II cells were cultured as described above for LLCPK-1. The day before infection, MDCK-II cells were transfected with pmCherry-villin by nucleofection (Amaxa, solution L, program A24).
Infection by S. flexneri
The WT invasive strain of S. flexneri, M90T, and its variant expressing the green fluorescent protein (GFP; Rathman et al., 2000) were grown overnight in trypticase soy broth (TCS, Bio-Rad, Hercules, CA). On the day of infection, bacteria were subcultured to midexponential phase. MDCK cells transiently expressing mCherry-tagged WT or RRI villin were washed two times with PBS. S. flexneri was added at a multiplicity of infection of 200:1 in DMEM without serum and spun onto the cells at 2000 × g for 5 min at 20°C. The infected cells were then incubated at 37°C, 5% CO2. After 40 min, they were washed with DMEM containing 50 μg/ml gentamicin.
For immunofluorescence, WT M90T-infected cells were further incubated for 80 min. After an extensive wash with PBS, they were fixed in PFA and extracted with 0.5% Triton X-100. Actin filaments were stained with Alexa Fluor 488-phalloidin (Molecular Probes, Eugene, OR), and DNA was labeled with DAPI. The acquisitions were made with an epifluorescence microscope (Leica DM 6000B) coupled to a CCD camera (Roper CoolSnap HQ) and driven by the software Metamorph.
For videomicroscopy, GFP M90T infected cells were further incubated at 37°C for 20 min. 3D plus time sequences were acquired on an inverted microscope (Leica DMIRB2) with a set-up equivalent to the one described previously for rapid optical Z sectioning. The microscope was equipped with a thermoregulation system (Life Imaging Services, Reinach, Switzerland), allowing incubation of the cells at 37°C and 5% CO2. Illumination at 488 nm and 575 nm for GFP and mCherry, respectively, was performed with a monochromator. For each wavelength, stacks of four images were acquired every 5 s during 5 min, with a plane-to-plane distance of 1.5 μm. GFP bacteria-infecting cells expressing WT and RRI villin at equivalent levels or nontransfected cells were tracked with the Metamorph software. To evaluate the rate of disassembly, the average fluorescence intensity of a fixed area of the comet (diameter 5 pixels), located directly behind the bacterium in the initial frame analyzed, was monitored over 90 s.
In Vitro Actin-based Motility Assays
To reconstitute actin-based motility in vitro, polystyrene beads of 1 μm in diameter (Polysciences, Warrington, PA) were coated with the VCA domain of WASP as described previously and incubated in a motility medium (Bernheim-Groswasser et al., 2002; van der Gucht et al., 2005) containing 8 μM F-actin, 0.1 μM Arp2/3, 1 μM profilin, 5 μM ADF, and 0.1 μM villin WT or RRI. All the purified proteins were purchased from Cytoskeleton (Denver, CO) except villin that was produced as described above. Comet growth and bead movements were followed in phase-contrast microscopy. The velocity of the propelled beads was measured with the Metamorph software. To analyze the depolymerization of the actin array present in the comet, 10% Alexa 488–labeled actin (Molecular Probes) was used.
RESULTS
Design of a Severing Mutant of Villin
The severing activity of villin that requires high calcium concentration in vitro is endowed by a domain shared between modules V1 and V2. It highly overlaps with the domain responsible for the capping activity and with one of the F-actin–binding sites of villin. Several attempts to dissociate severing from the other activities of villin have failed using truncations (Friederich et al., 1999) or point mutations targeting potential regulatory residues (Zhai et al., 2002; Kumar et al., 2004a). We decided to use a rational biochemical approach to design mutations suppressing the severing activity of villin. We took advantage of the work of F. S. Southwick, where he succeeded to introduce a severing activity to the capping protein CapG (Southwick, 1995). CapG is, as villin, a member of the gelsolin family but fails to sever actin filaments. The comparison of the primary structures of CapG with the severing proteins of the family allowed the identification of two sets of divergent amino acids in the severing domain. These amino acids, once mutated, were able to confer a severing activity to CapG (Southwick, 1995). We took the reverse strategy and changed the same amino acids in villin to the CapG sequence. This lead to the introduction of four mutations in two regions (R1 and R2) and to the insertion (I1) of one histidine in the second region (Figure 1). To simplify the nomenclature, we will refer to this mutant as RRI.
To evaluate the potential impact of the RRI mutations on the severing activity of villin, we referred to the well-studied structure and severing mechanism of gelsolin (McGough et al., 2003; Burtnick et al., 2004). The corresponding R1 region in gelsolin is located at the C-terminal end of an α-helix in G1 (Supplementary Figure 1). On activation, this site appears to be precisely situated at the binding site of a calcium ion that is shared between G1 and actin (Choe et al., 2002; McGough et al., 2003). The binding of this shared calcium ion is thought to directly mediate the binding of gelsolin G1 to actin. This suggests that the R1 mutations introduced in RRI villin and present in CapG could reduce the efficiency of G1 to bind calcium. The corresponding R2 region is part of the linker between domains G1 and G2. It makes intimate contact with actin in the G1–G2:actin complex (Burtnick et al., 2004). To undergo the conformational changes from the inactive calcium-free structure of gelsolin G1–G3 to the calcium-activated actin-bound one, the G1–G2 linker has to stretch out along actin. This allows the G1 domain to move away from G2 and to be properly directed toward the region where it will disrupt an actin–actin interaction. Hence the two mutated regions in RRI villin are part of strategic domains in the last step of the activation process of gelsolin severing. The RRI mutations could thus impair the activation and positioning of the V1 domain whose proper insertion in between two actin protomers leads to actin filament severing.
The RRI mutations are located upstream of the minimal F-actin–binding peptide (amino acids 133-147) mapped in villin V2 (de Arruda et al., 1992) and we can thus expect that the binding of villin to filamentous actin will not be impaired. Moreover, a gelsolin construct consisting of G2–G3 and part of the G3–G4 linker is efficient in capping actin filaments (Sun et al., 1994), and structural analysis support an active role of the G3–G4 linker in obscuring the barbed end of a capped filament (Burtnick et al., 2004). We can thus expect that the RRI mutations will not impair the other activities of villin, specifically capping and bundling.
The RRI Mutations Most Strongly Impair Villin-severing Activity
The severing efficiency of the RRI mutant has been analyzed and compared with other mutants that have been previously described to reduce the severing activity of villin (Kumar et al., 2004a), namely D61N, D86L, and A93G and their four possible combinations (D61N, D86L; D61N, A93G; D86L, A93G; D61N, D86L, A93G). We also took into account the mutation E74L, which is described to strongly reduce both the severing activity as well as the capping ability of villin.
Severing activity was measured by analyzing the effect of villin and villin mutants on the depolymerization of actin filaments labeled with pyrene. The filaments were diluted below the critical concentration of the pointed ends (100 nM). This results in their depolymerization, which is analyzed by the decrease in fluorescence intensity. The rate of depolymerization is dependant on the number of pointed ends available. By cutting filaments, villin should increase the number of pointed ends and hence increase the depolymerization rate.
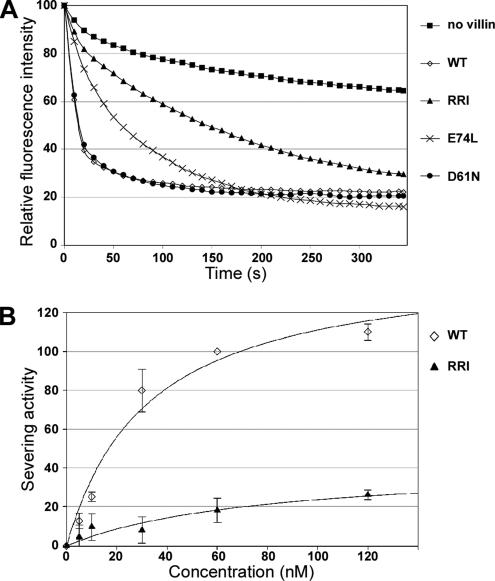
As presented in Figure 2A, a slow depolymerization is observed in the control experiment without villin, whereas the addition of WT villin induces a very fast depolymerization. In these conditions (60 nM villin and variants, 200 μM Ca2+) the three point mutations D61N, D86L, and A93G and their four combinations did not allow the detection of a significant difference with the severing activity of WT villin (only D61N is depicted for clarity). However, the mutations E74L and RRI reduced the severing activity of villin to 29 and 16%, respectively. The RRI mutations are thus the most efficiently reducing villin-severing activity. The dose–response analysis of the severing activity of RRI villin compared with WT villin (Figure 2B) confirms this very strong impairment.
Figure 2.
The RRI mutations are the most effective at disrupting the villin-severing activity. Pyrene-labeled actin filaments were diluted below the critical concentration of the pointed end (100 nM) in the presence of villin and its variants or in the absence of any severing protein (no villin). As the rate of depolymerization is proportional to the number of free pointed ends, the severing activity of villin and its variants was measured by following the decrease in fluorescence. (A) Depolymerization of F-actin in the presence of 200 μM calcium with 60 nM villin WT, RRI, E74L, and D61N. The graph represent the relative fluorescence intensity plotted as a function of time. The relative fluorescence is calculated to normalize the fluorescence of F-actin to 100 and the one of G-actin to 0. (B) Severing activity of WT (◇) and RRI (▲) villin plotted as a function of the concentration.
To further analyze the severing activity of the RRI variant, we checked the severing activity at lower calcium concentrations (Supplementary Figure 2). As expected, lowering the calcium concentration reduced the severing activity of WT and RRI villin. At 50 μM Ca2+, the RRI variant had no detectable severing activity. As WT villin, RRI villin is still sensitive to calcium.
Capping and Nucleation Activities Are Not Affected
Next, we determined if the RRI mutations were uniquely affecting villin-severing property without affecting the capping and nucleation activities.
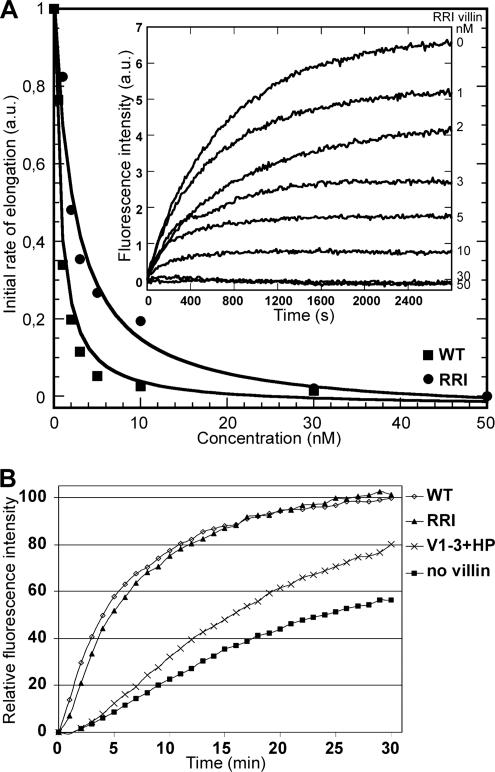
The barbed-end capping activity of villin is saturated at 2.5 μM Ca2+, whereas severing is not (Northrop et al., 1986). Actin filament elongation assays were performed in the presence of 2.5 μM Ca2+ in order to determine the affinity of WT and RRI villin for the barbed ends of actin filaments (Figure 3A). Seeds consisting of unlabeled actin filaments were incubated with various concentrations of WT or RRI villin before the addition of pyrene-labeled actin monomers bound to profilin. The increase in fluorescence observed corresponds to the elongation of pyrene-actin-profilin complexes at the barbed ends of the seeds, because profilin-actin does not bind to pointed ends (illustrated for RRI villin in Figure 3A, inset). The decrease in the initial rate of elongation as a function of the concentration of villin reflects the saturation of filament barbed ends by villin (Figure 3A). We determined Kd values of 0.8 nM for WT villin (Figure 3A, squares) and 1.8 nM for RRI villin (Figure 3A, circles) binding to actin filament barbed ends. This demonstrates that WT and RRI villin have equivalent capping activities in the nanomolar range. Moreover, this affinity is similar to the ones reported for CapG (Southwick, 1995) and gelsolin (Laine et al., 1998).
Figure 3.
The capping and nucleating activities are not affected in RRI villin. (A) To evaluate the capping activity, the elongation of actin filament barbed-ends in the presence of WT and RRI villin was analyzed. Preformed actin filaments (1 μM) were incubated with various concentrations of villin before the addition of 1 μM pyrene–actin monomers bound to 2 μM profilin. The initial rate of elongation was plotted as a function of the concentration of villin WT and RRI. The data were fit with Equation 1 (see Materials and Methods) considering 0.11 nM barbed ends, to determine equilibrium dissociation constant values (Kd) of around 0.8 nM for WT villin (■) and 1.8 nM for RRI villin (●). Inset, kinetics of actin filament barbed-ends elongation in the presence of various concentrations of RRI villin. (B) The nucleating activity was analyzed by evaluating the initial rate of polymerization of G-actin. Before inducing polymerization, pyrene–actin (2 μM) was incubated with villin WT and its variants (24 nM) in the presence of 20 μM calcium. No villin, polymerization of G-actin in the absence of nucleating protein; V1-3+HP, polymerization of G-actin in the presence of the nucleation mutant of villin V1-3+HP.
To evaluate the nucleation activity, we analyzed the initial rate of polymerization of labeled actin monomers (Figure 3B). Incubation with WT or RRI villin induces a strong and immediate polymerization as deduced from the rapid increase of fluorescence observed. The control (without villin) and the nucleation mutant V1-3+HP (Friederich et al., 1999) show a delay of 1–2 min followed by a slow increase of fluorescence. RRI behaves like WT villin, and the RRI mutations thus do not significantly impair villin nucleation activity.
RRI Villin Is Still Able To Bundle Filaments
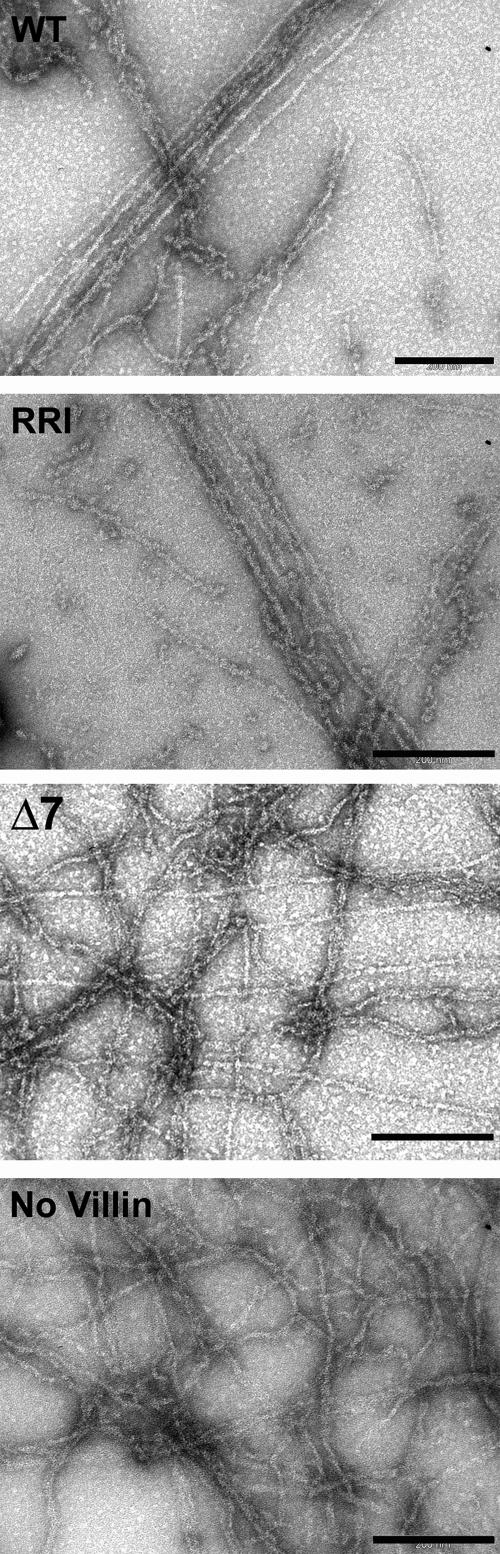
In vitro, at low calcium concentrations (<100 nM), villin is able to cross-link actin filaments in parallel bundles. This bundling ability is abolished by a deletion of the seven last C-terminal amino acids of villin, villin Δ7 (Friederich et al., 1992). The formation of elongated bundle-like filaments observed upon incubation of Alexa-labeled F-actin with villin WT or RRI in presence of EGTA was confirmed by a low-speed sedimentation assay of actin filaments incubated with villin and its severing and bundling mutants, RRI and Δ7. Most of the actin was pelleted with villin WT and RRI, whereas it remained in the supernatant in the absence of villin or with villin Δ7 (Supplementary Figure 3). To confirm that WT and RRI villin bundled actin filaments, they were processed for electron microscopy (Figure 4). In the presence of WT and RRI villin, straight actin filaments closely aligned in parallel bundles were observed. Actin filaments alone or incubated with the bundling mutant Δ7 appeared as single intermingled filaments with few disorganized aggregates but no bundles could be observed.
Figure 4.
The bundling activity is preserved in villin RRI. Electron micrographs of actin filaments (3 μM) negatively stained after incubation with villin and its variants (1.5 μM) in the presence of EGTA. Δ7, bundling mutant of villin; No villin, control with actin filaments alone. Bars, 200 nm.
The Morphogenic Effects of Villin Are Preserved Despite the Lack of Severing Activity
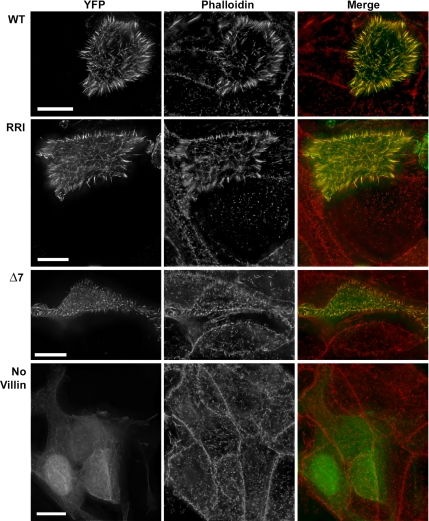
The overexpression of villin in CV1 cells is known to induce long microvilli on the dorsal face of the cells (Friederich et al., 1989). In LLCPK-1 cells, the overexpression of villin lengthens the existing microvilli (Arpin et al., 1994; Loomis et al., 2003). These morphogenic effects are abolished by the disruption of villin bundling activity (Friederich et al., 1992). In Figure 5, we illustrate the consequences of the overexpression in LLCPK-1 cells of YFP-tagged villin WT and its variants. As for WT villin, RRI villin-overexpressing cells have elongated microvilli on their apical surface. Actin filaments colocalize with villin WT and RRI in these structures. On the contrary, although the bundling mutant of villin, Δ7, is properly localized with actin in the microvilli, it does not induce detectable structural modifications. The expression of the YFP tag alone (control) does not change the morphology of the cells. The overexpression of villin and its variants in CV1 cells lead us to the same conclusions: villin WT and RRI but not Δ7 induced the appearance of microvilli on the dorsal face of the cells (data not shown). In conclusion, disrupting the severing activity in the RRI villin affects neither its localization in microvilli nor its morphogenic consequences.
Figure 5.
WT and RRI villin have the same localization and morphogenic effects in LLCPK-1 cells. LLCPK-1 cells were transiently transfected with cDNAs encoding wild-type villin (WT, upper row), the severing mutant (RRI, second row), and the bundling mutant (Δ7, third row) tagged with the fluorophore YFP or the YFP plasmid alone (No villin, last row). Cells were fixed and stained for actin with TRITC phalloidin. The acquisitions were made using 3D deconvolution microscopy. The pictures shown are the result of the projection of deconvolved Z-stacks in which the bottom of the cells were removed to avoid visualization of stress fibers. Bars, 10 μm.
WT But Not RRI Villin Enhances the Velocity of S. flexneri In Vivo
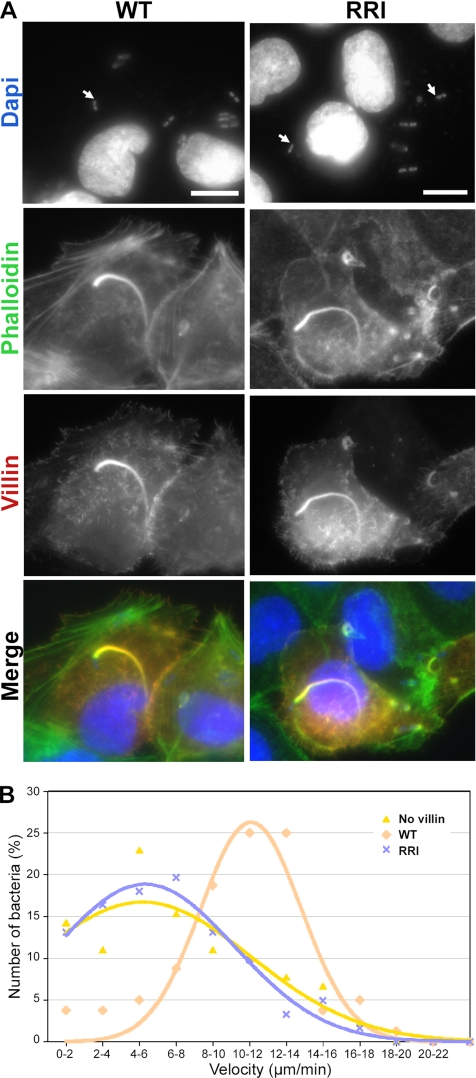
To test the impact of the loss of villin-severing activity in cells, we analyzed the movement of the enteroinvasive bacterium S. flexneri as a potential process where villin-severing activity might be acting. The motility of S. flexneri or Listeria monocytogenes is indeed widely accepted as a model to study actin dynamics supporting cell movement. Primary cultures of enterocytes cannot be readily transfected at present, but the polarized epithelial cells MDCK were shown, when transfected or not with the villin cDNA, to behave in a comparable way to primary cultures of enterocytes from WT or villin-deficient mice (Athman et al., 2003). MDCK cells were thus transiently transfected with mCherry-tagged WT and RRI villin and infected with S. flexneri. As S. flexneri enters the cell, it recruits the cell's cytoskeleton machinery to produce an actin tail. This comet allows the bacteria to move inside the cell (Bernardini et al., 1989). As previously described for WT villin (Athman et al., 2005), RRI villin is also incorporated in the comet where it colocalizes with actin (Figure 6A). The movement of GFP S. flexneri in these cells was analyzed by videomicroscopy (see Supplementary Video 1 available as supplementary data). All bacteria that show a directional movement in WT and RRI villin-transfected cells are propelled by a comet labeled with mCherry-villin. The velocities (Figure 6B) calculated from the movies give an average rate of 7.0 ± 4.3 μm/min in nontransfected cells (nControl = 92), 6.7 ± 4.0 μm/min in RRI villin expressing cells (nRRI = 61), and 10.3 ± 3.7 μm/min in WT villin expressing cells (nWT = 80). The increase in velocity reported for the bacteria in the presence of WT villin is highly significant (Wilcoxon test, p = 2.213E-07; Athman et al., 2005) and is lost in the presence of RRI villin (Figure 6B). Indeed, the velocities of bacteria moving in nontransfected cells are not significantly different from those in RRI villin expressing cells (Wilcoxon test, p = 0.7813). The fluorescence decay of the comets labeled with villin was not significantly different in the presence of WT or RRI villin, indicating that the rate of disassembly is not modified, whereas the comets were significantly longer in the presence of WT as compared with RRI villin (4.0 ± 1.8 and 2.7 ± 1.7 μm, respectively, nWT = 39 and nRRI = 26, t test, p < 0.01). The wide distribution of the velocities as well as the heterogeneity in the length and intensity of the comets analyzed limit the analysis of the actin dynamics in this system. Moreover, the activation status of villin and its mutant activities in cells cannot be controlled. This prompted us to use a simplified biomimetic system.
Figure 6.
The RRI mutations abolish the villin-dependent increase in velocity of S. flexneri. (A) As WT villin, RRI villin is localized in S. flexneri actin tails. MDCK cells were transiently transfected with mCherry-tagged WT and RRI villin and infected for 2 h with S. flexneri. Actin is stained with Alexa 488–phalloidin, and the bacteria and cells DNA were labeled with DAPI. Bars, 10 μm. (B) Distribution of bacteria as a function of their velocity in cells expressing WT villin (pink), RRI villin (blue), or no villin (yellow). The velocities were calculated from the video (see Supplementary Data) of GFP bacteria infecting MDCK cells transiently transfected with mCherry-tagged WT and RRI villin or nontranfected (no villin). The bacteria moved with an average rate of 7.0 ± 4.3 μm/min in nontransfected cells (nControl = 92), 6.7 ± 4.0 μm/min in RRI villin expressing cells (nRRI = 61), and 10.3 ± 3.7 μm/min in WT villin expressing cells (nWT = 80). The increased average velocity observed in the presence of WT villin in comparison with control or RRI expressing cells is highly significant (Wilcoxon test, p = 2.213E-7 and p = 2.042E-7, respectively).
Villin-severing Activity Enhances the Velocity of Beads in an In Vitro Motility Assay
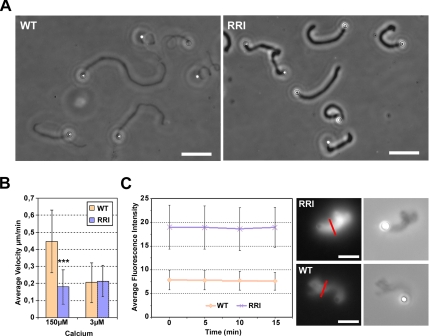
An in vitro assay has been used to reconstitute an actin-based movement of beads. A minimal set of proteins has previously been described as required for the polymerization of actin comets that propel bacteria (Loisel et al., 1999) and beads (van der Gucht et al., 2005) in the motility medium: an actin nucleator (Arp2/3) and its activator grafted around the beads (VCA), a capper (gelsolin or CapZ) and the depolymerizing/severing protein ADF/Cofilin. We replaced the capping protein of this assay by villin WT and RRI in the presence of 150 μM free calcium. In these conditions, WT villin is able to cap and sever actin filaments, whereas RRI villin is mostly able to cap filaments as shown in the pyrene assays. The presence of villin WT and RRI instead of the usual cappers allowed the formation of comets and the movement of the beads in the motility medium (Figure 7A). By phase contrast, the comets formed in the presence of the severing mutant of villin appeared darker and thus are denser than those formed in the presence of WT villin (refer also to Figure 7C). We analyzed the velocities of the beads induced by villin and its severing mutant. We measured the beads velocity by recording the lengthening of a comet over a given time. Indeed, as previously reported with this assay (Bernheim-Groswasser et al., 2002), the actin comets are terminated with a dense cloud at the end opposite to the bead. This dense part corresponds to the symmetry breaking event that precedes comet formation (van der Gucht et al., 2005) and is visible throughout the experiment. We thus took advantage of this effect to measure the velocity of the beads that coincides with the lengthening of the comet over time. In the presence of RRI villin, the velocity of the beads was reduced to <50% of the velocity induced by WT villin (Figure 7B). We decreased the free calcium concentration of the motility medium to 3 μM, conditions known to be saturating for the capping activity of villin whereas its severing activity is no longer detectable (Northrop et al., 1986). At 3 μM calcium, the velocity of the beads was not significantly higher for WT villin compared with RRI villin (Figure 7B). The average velocity of the beads remained about 0.2 μm/min in the presence of villin RRI but dropped from 0.45 to 0.2 μm/min in the presence of WT villin. The severing activity of villin is thus responsible for an enhancement of actin-based motility in vitro.
Figure 7.
The severing activity of villin enhances actin based motility in vitro. Actin-based motility was reconstituted using recombinant proteins. (A) Villin WT and RRI are able to replace the capping protein used in the standard motility medium as we observe the growth of comets. Bars, 20 μm. (B) Average velocity of the beads in the presence of WT (pink) and RRI (blue) villin with 150 μM and 3 μM free calcium. In the presence of 150 μM calcium, the velocity of the beads is significantly enhanced with WT villin compared with RRI villin (t test, nWT = 43 nRRI = 70, p < 0.0001). The reduction of the calcium concentration to 3 μM abolishes the discrepancy between velocities. (C) Evolution over time of the average fluorescence intensity of comets grown with Alexa 488–labeled actin in the presence of WT (pink) and RRI (blue) villin. The fluorescence intensity is the result of the average intensity per pixel of segments of given length drawn perpendicularly to the comets. An example (red line) of the way the measures were done is illustrated on the left pictures showing Alexa 488–actin comets in the presence of WT (lower picture) and RRI (upper picture) villin. Corresponding phase-contrast pictures are given on the right. Bars, 5 μm.
Villin Does Not Sever Actin Branches in the Body of the Comets
We have just shown that the severing activity of villin has a direct impact on the velocity of beads; therefore, we investigated whether this severing activity is occurring in the comets. The villin present in the medium could sever the branches stabilized in the comets and hence liberate numerous short actin filaments with free pointed ends. If this is the case, the amount of actin found in the comet should decrease over time. To analyze this possibility, we monitored the change in the fluorescence intensity of comets made by Alexa 488–labeled actin. We followed over time the average and maximal fluorescence intensity of segments drawn perpendicular to the comets. First, the comets formed with villin RRI showed a significantly higher fluorescence intensity than the ones formed with WT villin (Figure 7C), indicating that they contain more actin. Hence, the increased density observed in phase-contrast microscopy of the comets formed with RRI villin (Figure 7A) is due to a higher content of actin. Second, neither the presence of WT villin nor the one of RRI villin allowed the detection of a decrease of the fluorescence intensity over time. The same results were obtained by evaluating the maximal fluorescence intensity of the segments (data not shown). In conclusion, the difference in actin dynamics reported in presence of WT and RRI villin does not seem to be due to severing of the actin branches in the comet body once formed. The enhancement of actin dynamics reported would then only be due to the severing by WT villin of filaments free in the medium and elongating at the bead surface.
DISCUSSION
We succeeded in dissociating the severing activity from the three other activities of villin. This result contrasts with a recent report where the same mutations were introduced in a truncated gelsolin protein (aa 1-160), however, without affecting its severing activity (Zhang et al., 2006). There are several explanations for this discrepancy: villin and gelsolin are structurally similar; however, their specific activities are not regulated via identical mechanisms. Therefore the same mutations in the two proteins may have two different effects. In addition the gelsolin mutations were made on a truncated protein that may not mimic the behavior of a full-length protein with the same mutations. This highlights the importance of the various domains in the regulation of the proteins different activities. The severing mutant characterized in our work has nucleation and capping and bundling activities similar to that of WT villin and localizes properly in cells. The use of this mutant in functional assays in vivo and in vitro allows us to demonstrate that villin-severing activity is responsible for an enhancement of actin-based motility.
Villin-severing Activity Enhances Actin-based Motility
Villin increases the velocity of the bacteria S. flexneri in vivo (Athman et al., 2005). We not only confirm this result in MDCK cells by reporting a 45% rise in the average velocity of the bacteria in presence of WT villin but also show that the severing mutant of villin generated in this study abolishes this increase. The fact that the disassembly rate of the comets was not affected, whereas length and velocities were modified, is in favor of a role for villin in an enhancement of the polymerization at the surface of the bacteria. Similar results were obtained for L. monocytogenes motility after overexpression of gelsolin in infected cells (Laine et al., 1998). Nevertheless, a decrease, due to gelsolin, of the rate of Listeria actin tails disassembly has recently been observed after reduction of the calcium concentration (Larson et al., 2005). This was not accompanied by an impairment of the velocity of the bacteria. Playing on the regulation of gelsolin by calcium seems thus to have different effects than the ones observed previously by Laine et al. (1998) and in this work for villin.
Numerous parameters that cannot be evaluated are influencing actin polymerization and the movement of the bacteria in the cell. Among them, phosphorylation and phosphoinositides are known to modulate the activity of villin and likely of the RRI mutant. Moreover, the heterogeneity of the cytoplasm and the multiple obstacles that the bacteria have to face are probably responsible for the wide range of velocities reported (Figure 6B and Goldberg and Theriot, 1995) and for the variability observed in the length and intensity of the comet tails. We thus decided to mimic this movement in a simplified model system in order to control these parameters, especially the calcium-dependent biochemical activities of villin, and to validate that the effect reported in vivo is directly due to the lack of severing activity in RRI villin. The in vitro motility assay that we used generates the actin-based movement of beads. Despite slight differences with the in vivo situation (lower velocities, no detectable disassembly of the comets rear), the in vitro motility assay strongly strengthens the cell analysis as it results in a twofold increase in the speed of the beads in the presence of WT villin without influencing the disassembly of the comets. This confirms that the severing activity of villin by itself accounts for the enhanced motility of the bacteria. Apart from the fact that we cannot rule out contributions of other, uncharacterized activities of villin that may be affected by the mutations, our results strongly support the relevance of villin-severing activity in vivo.
What Mechanism Can Be Proposed from the In Vitro–reconstituted Motility?
This is the first report where villin is used to reconstitute an actin-based movement in vitro. We show that villin is able to replace the capping protein commonly used in this motility assay. The comparison of villin and its severing mutant RRI demonstrates a cooperation between the capping and severing activities in inducing and accelerating the actin-based movement. The comets formed in cell-free assays are most probably made of an Arp2/3 branched actin array (Svitkina and Borisy, 1999; Cameron et al., 2001). The site of polymerization is located at the interface between the bead and the comet where the Arp2/3 complex is activated. A capping protein, villin in our case, is required to limit the elongation process in space and to concentrate the force production toward the bead. Villin has a higher affinity for barbed end than for side binding on actin filaments (Northrop et al., 1986). Hence, as soon as the filaments elongate away from the bead, they are capped by villin, and all filaments that do not face the bead surface are expected to be barbed-end capped.
To account for the increased velocity reported, the severing activity of villin must somehow raise the concentration of actin monomers available for polymerization and hence accelerate the polymerization rate, which is directly proportional to the concentration of actin subunits (for review see Pollard and Borisy, 2003). As gelsolin, villin severs actin filaments and rapidly caps the newly formed barbed ends (Northrop et al., 1986). Severing by villin thus creates a net increase of free pointed ends. Consequently, in our assay, the villin-severing activity induces the appearance in the medium of an increased number of filaments capped at their barbed ends and free at their pointed ends. This increases net depolymerization and leads to a higher concentration of actin monomers. Actin polymerization is enhanced at the only free barbed-ends available, at the interface between the beads and the comets (illustrated in Figure 8A). Both in vitro and in vivo, there is no difference in the disassembly rate of the comets, whereas the increase in the velocities proves that actin cycling is enhanced by villin severing. Villin severing would thus not occur in the dense array constituting the comets but in a subset of other actin filaments. The different mechanisms controlling the disassembly of the structurally distinct actin arrays present in cells are indeed not well understood and are starting to be addressed (Brieher et al., 2006).
Figure 8.
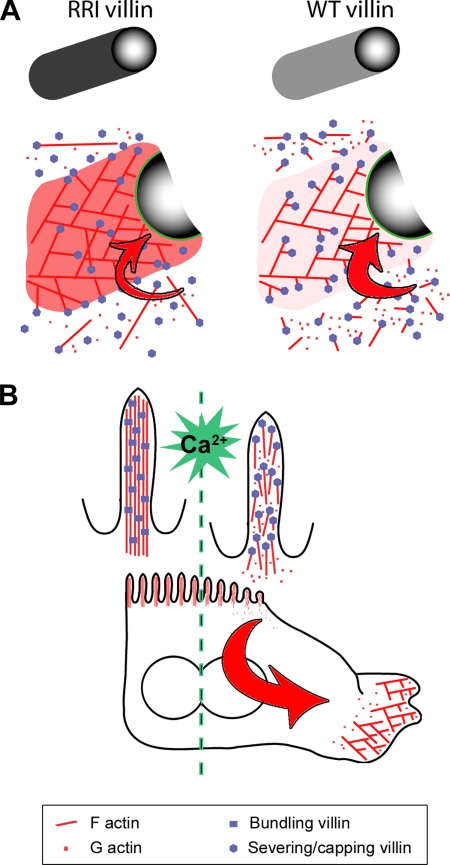
Model for the enhancement of actin dynamics by villin-severing activity. (A) Model depicting the consequence of the villin-severing activity on the motility of beads or bacteria. At high calcium concentration, in the presence of RRI villin that lacks severing activity, the branched actin array in the comet as well as filaments in solution are capped at their barbed ends except at the bead/bacterium surface. This maintains a pool of actin monomers free to polymerize at the only barbed ends available: at the bead/bacterium surface (flux of monomers depicted by a red arrow). In the presence of WT villin, the severing activity followed by capping creates a higher number of free pointed ends in solution, which increases the concentration of actin monomers and the polymerization rate at the bead surface (enlarged red arrow). We propose that WT villin cannot only cap but also sever the new branches elongating apart from the bead and hence induce the formation of an actin array with shortened branches. This effect would account for the reduced density of actin in the comets grown with WT versus RRI villin. Moreover, by releasing short actin filaments, severing of the new branches contributes with the severing of filaments in solution to the increased number of free pointed ends. Altogether, villin-severing activity results in an enhancement of the velocity of the beads/bacteria. (B) Proposed role of villin in an enterocyte acquiring a motile phenotype. In a polarized enterocyte, villin is bundling actin filaments in microvilli. As the cell undergoes a calcium-triggered epithelio-mesenchymal transition, villin activity switches to severing. Filaments from the bundles are cut in small-barbed end-capped filaments and depolymerize from their pointed ends. This results in the depolarization of the cell and creates a flux of actin monomers (red arrow) available for polymerization. It enhances the rapid growth of a lamellipodium and augments the movement of the cell as described above for the model of bead/bacterium motility.
In the in vitro assay, villin could increase the number of filaments available for depolymerization by severing the filaments present in solution and/or in the comet. If a new branch elongating apart from the bead surface is severed by villin, a short actin filament is released in the medium, whereas the branch is directly capped by RRI villin. Thus, the severing of elongating branches by WT villin contributes to the creation of a higher number of filaments with pointed ends free for depolymerization around the beads. Moreover, this could also account for the difference in density of the comets grown in the presence of WT and RRI villin (Figure 8A). The comets produced with WT villin will have shorter branches than the one produced with RRI villin and will thus be less dense in actin as observed (Figure 7, A and C). Because we excluded the possibility of villin introducing cuts in the comet body by measuring over time the fluorescence intensity of labeled actin comets (Figure 7C), villin would thus not have access to the dense actin array in the comet body. If the reported difference in density is indeed due to the creation of shorter branches in the presence of WT villin, we have to postulate that filaments elongating at the bead surface are more accessible to villin than filaments buried in the comet body.
Among the proteins used in this in vitro motility assay is the protein ADF/Cofilin. ADF/Cofilin used at high concentrations (5 μM) strongly accelerates the symmetry breakage of the actin gel growing around the beads, hence the appearance of a comet, and highly increases the velocity of bacteria or beads (Loisel et al., 1999; van der Gucht et al., 2005). Its property on actin filaments—depolymerizing and/or severing—is still controversial (Carlier et al., 1997; Zebda et al., 2000; Ichetovkin et al., 2002). Even if villin is able to sever filaments, replacing ADF by villin failed in producing motility: a short comet could only very rarely be observed with 100 nM villin in the absence of ADF but never at higher villin concentrations. The concentrations of villin allowing for the formation of comets in the presence of ADF are restricted to a very narrow range, as was already observed for CapZ (Loisel et al., 1999). The velocity of the beads picks at 50–100 nM villin and the movement is already blocked at 300 nM (data not shown). Consequently, at the concentrations used for ADF, the capping activity of villin overcomes the nucleation process by Arp2/3 and blocks the system as it has already been described for CapZ and gelsolin (Loisel et al., 1999; Pantaloni et al., 2000; van der Gucht et al., 2005).
Villin-severing Activity in Cell Movement
What clues does this work provide to the understanding of villin cellular function ? We demonstrated previously that villin enhances the motility and morphogenesis of cells (Athman et al., 2003). The increase in free barbed ends and the reduced F-actin content observed are in favor of a severing mechanism. As for villin, cell motility and dynamics have been positively correlated to cellular gelsolin content. Cells deficient in gelsolin exhibit defects in migration (Witke et al., 1995; Chen et al., 1996) and in cytoskeletal remodeling (Hartwig, 1992; Witke et al., 1995; Lu et al., 1997). In accordance with our observations for villin, increased velocity in fibroblasts expressing gelsolin correlates with reduced F-actin fraction and increased actin turnover (McGrath et al., 2000). Finally, villin as well as gelsolin were reported to accelerate the motility of S. flexneri (this work and Athman et al., 2005) and L. monocytogenes (Laine et al., 1998), respectively.
This set of data suggests a regulation of the actin cycle by the F-actin–severing activity of proteins like villin. However, because these proteins have multiple properties toward actin, at least in vitro, it remained to prove that the observed phenotypes are effectively due to the severing activity. This work is to our knowledge the first demonstration of a direct role of the severing activity of villin or a related protein in the enhancement of actin dynamics sustaining motility in vitro and in vivo.
We believe that this role of the severing activity of villin has a relevance in vivo for cell shape changes and movement. By creating pointed ends in an environment where filaments barbed ends are all capped, as in a lamella away from the extreme leading edge, villin could accelerate depolymerization and enhance actin polymerization rate at the leading edge. In vivo, on the contrary to our in vitro motility assay, villin can be dissociated from the barbed ends by several mechanisms as binding to phosphoinositides (Janmey and Matsudaira, 1988; Kumar et al., 2004b). Villin severing can then also contribute, in cooperation with other severing proteins such as gelsolin and ADF/Cofilin (Chan et al., 2000; Zebda et al., 2000), to the rapid creation of free barbed ends when a burst of polymerization is necessary as for the extension of a lamellipodium.
Finally, the restriction of villin expression to epithelial cells that develop a brush border hints at a particular role of villin in these specialized cells (Figure 8B). Intestinal epithelial cells move in response to signals or stresses in various situations, during embryogenesis, wound healing, or metastatic process. They are not adapted to move in their apico-basal polarized state but need to undergo an EMT to acquire a motile “fibroblastic-like” morphology (Nusrat et al., 1992). This transition requires the remodeling of the actin cytoskeleton. Gelsolin is absent from the microvilli (Yin et al., 1981), where villin is associated with the actin bundles. In response to signaling (calcium, phosphorylation; for review see Revenu et al., 2006), villin could efficiently shift from bundling to severing and rapidly break down this apically concentrated F-actin structure. It would contribute to the depolarization of the cell, increase the concentration of monomers available for polymerization of a new actin structure, and as reported here for bead or bacteria velocity, enhance the efficiency of movement. Our work thus supports a functional role of severing by villin in the EMT of enterocytes.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Rania Zaarour and Danijela Vignjevic for careful reading of the manuscript and helpful comments. We thank Graça Raposo and Ahmed El Marjou for their help in the electron microscopy and protein production respectively. We also gratefully thank Laurent Blanchoin and Paul Janmey for their comments and help on the capping assays. We finally acknowledge Jean-Baptiste Sibarita, Vincent Fraisier, and François Waharte for their advices in imaging and for deconvolution. Irit Paz and Philippe Sansonetti kindly gave us S. flexneri strains, and Roger Y. Tsien allowed us to use the mCherry fluorescent protein. This work was supported by funds from the French Ministry of Research, Action Concertée Incitative Biologie du Développement et Physiologie Intégrative. C.R. is supported by a Ph.D. fellowship from the Centre National de la Recherche Scientifique.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0423 on December 20, 2006.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Arpin M., Friederich E., Algrain M., Vernel F., Louvard D. Functional differences between L- and T-plastin isoforms. J. Cell Biol. 1994;127:1995–2008. doi: 10.1083/jcb.127.6.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin M., Pringault E., Finidori J., Garcia A., Jeltsch J. M., Vandekerckhove J., Louvard D. Sequence of human villin: a large duplicated domain homologous with other actin–severing proteins and a unique small carboxy-terminal domain related to villin specificity. J. Cell Biol. 1988;107:1759–1766. doi: 10.1083/jcb.107.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman R., Fernandez M. I., Gounon P., Sansonetti P., Louvard D., Philpott D., Robine S. Shigella flexneri infection is dependent on villin in the mouse intestine and in primary cultures of intestinal epithelial cells. Cell Microbiol. 2005;7:1109–1116. doi: 10.1111/j.1462-5822.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- Athman R., Louvard D., Robine S. Villin enhances hepatocyte growth factor-induced actin cytoskeleton remodeling in epithelial cells. Mol. Biol. Cell. 2003;14:4641–4653. doi: 10.1091/mbc.E03-02-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazari W. L., Matsudaira P., Wallek M., Smeal T., Jakes R., Ahmed Y. Villin sequence and peptide map identify six homologous domains. Proc. Natl. Acad. Sci. USA. 1988;85:4986–4990. doi: 10.1073/pnas.85.14.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim-Groswasser A., Wiesner S., Golsteyn R. M., Carlier M. F., Sykes C. The dynamics of actin-based motility depend on surface parameters. Nature. 2002;417:308–311. doi: 10.1038/417308a. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc. Natl. Acad. Sci. USA. 1979;76:2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980;20:839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Brieher W. M., Kueh H. Y., Ballif B. A., Mitchison T. J. Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J. Cell Biol. 2006;175:315–324. doi: 10.1083/jcb.200603149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick L. D., Koepf E. K., Grimes J., Jones E. Y., Stuart D. I., McLaughlin P. J., Robinson R. C. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell. 1997;90:661–670. doi: 10.1016/s0092-8674(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Burtnick L. D., Urosev D., Irobi E., Narayan K., Robinson R. C. Structure of the N-terminal half of gelsolin bound to actin: roles in severing, apoptosis and FAF. EMBO J. 2004;23:2713–2722. doi: 10.1038/sj.emboj.7600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. A., Svitkina T. M., Vignjevic D., Theriot J. A., Borisy G. G. Dendritic organization of actin comet tails. Curr. Biol. 2001;11:130–135. doi: 10.1016/s0960-9822(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Laurent V., Santolini J., Melki R., Didry D., Xia G. X., Hong Y., Chua N. H., Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. Y., Bailly M., Zebda N., Segall J. E., Condeelis J. S. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J. Cell Biol. 2000;148:531–542. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Murphy-Ullrich J. E., Wells A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J. Cell Biol. 1996;134:689–698. doi: 10.1083/jcb.134.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H., Burtnick L. D., Mejillano M., Yin H. L., Robinson R. C., Choe S. The calcium activation of gelsolin: insights from the 3A structure of the G4–G6/actin complex. J. Mol. Biol. 2002;324:691–702. doi: 10.1016/s0022-2836(02)01131-2. [DOI] [PubMed] [Google Scholar]
- Costa de Beauregard M. A., Pringault E., Robine S., Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409–421. doi: 10.1002/j.1460-2075.1995.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Arruda M. V., Bazari H., Wallek M., Matsudaira P. An actin footprint on villin. Single site substitutions in a cluster of basic residues inhibit the actin severing but not capping activity of villin. J. Biol. Chem. 1992;267:13079–13085. [PubMed] [Google Scholar]
- Fedorov A. A., Pollard T. D., Almo S. C. Purification, characterization and crystallization of human platelet profilin expressed in Escherichia coli. J. Mol. Biol. 1994;241:480–482. doi: 10.1006/jmbi.1994.1522. [DOI] [PubMed] [Google Scholar]
- Ferrary E., et al. In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. J. Cell Biol. 1999;146:819–830. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E., Huet C., Arpin M., Louvard D. Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell. 1989;59:461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Friederich E., Vancompernolle K., Huet C., Goethals M., Finidori J., Vandekerckhove J., Louvard D. An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell. 1992;70:81–92. doi: 10.1016/0092-8674(92)90535-k. [DOI] [PubMed] [Google Scholar]
- Friederich E., Vancompernolle K., Louvard D., Vandekerckhove J. Villin function in the organization of the actin cytoskeleton. Correlation of in vivo effects to its biochemical activities in vitro. J. Biol. Chem. 1999;274:26751–26760. doi: 10.1074/jbc.274.38.26751. [DOI] [PubMed] [Google Scholar]
- Gettemans J., De Ville Y., Waelkens E., Vandekerckhove J. The actin-binding properties of the Physarum actin-fragmin complex. Regulation by calcium, phospholipids, and phosphorylation. J. Biol. Chem. 1995;270:2644–2651. doi: 10.1074/jbc.270.6.2644. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr., Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc. Natl. Acad. Sci. USA. 1980;77:6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr., Kaulfus P., Weber K. F actin assembly modulated by villin: Ca++-dependent nucleation and capping of the barbed end. Cell. 1981;24:471–480. doi: 10.1016/0092-8674(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr., Weber K. Calcium control of microfilaments: uncoupling of the F-actin-severing and -bundling activity of villin by limited proteolysis in vitro. Proc. Natl. Acad. Sci. USA. 1981;78:2810–2814. doi: 10.1073/pnas.78.5.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Theriot J. A. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc. Natl. Acad. Sci. USA. 1995;92:6572–6576. doi: 10.1073/pnas.92.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H. Mechanisms of actin rearrangements mediating platelet activation. J. Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichetovkin I., Grant W., Condeelis J. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr. Biol. 2002;12:79–84. doi: 10.1016/s0960-9822(01)00629-7. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Matsudaira P. T. Functional comparison of villin and gelsolin. Effects of Ca2+, KCl, and polyphosphoinositides. J. Biol. Chem. 1988;263:16738–16743. [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur. J. Biochem. 1981;114:33–38. [PubMed] [Google Scholar]
- Kumar N., Tomar A., Parrill A. L., Khurana S. Functional dissection and molecular characterization of calcium-sensitive actin-capping and actin-depolymerizing sites in villin. J. Biol. Chem. 2004a;279:45036–45046. doi: 10.1074/jbc.M405424200. [DOI] [PubMed] [Google Scholar]
- Kumar N., Zhao P., Tomar A., Galea C. A., Khurana S. Association of villin with phosphatidylinositol 4,5-bisphosphate regulates the actin cytoskeleton. J. Biol. Chem. 2004b;279:3096–3110. doi: 10.1074/jbc.M308878200. [DOI] [PubMed] [Google Scholar]
- Laine R. O., Phaneuf K. L., Cunningham C. C., Kwiatkowski D., Azuma T., Southwick F. S. Gelsolin, a protein that caps the barbed ends and severs actin filaments, enhances the actin-based motility of Listeria monocytogenes in host cells. Infect. Immun. 1998;66:3775–3782. doi: 10.1128/iai.66.8.3775-3782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L., Arnaudeau S., Gibson B., Li W., Krause R., Hao B., Bamburg J. R., Lew D. P., Demaurex N., Southwick F. Gelsolin mediates calcium-dependent disassembly of Listeria actin tails. Proc. Natl. Acad. Sci. USA. 2005;102:1921–1926. doi: 10.1073/pnas.0409062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel T. P., Boujemaa R., Pantaloni D., Carlier M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- Loomis P. A., Zheng L., Sekerkova G., Changyaleket B., Mugnaini E., Bartles J. R. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 2003;163:1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Witke W., Kwiatkowski D. J., Kosik K. S. Delayed retraction of filopodia in gelsolin null mice. J. Cell Biol. 1997;138:1279–1287. doi: 10.1083/jcb.138.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P., Jakes R., Walker J. E. A gelsolin-like Ca2+-dependent actin-binding domain in villin. Nature. 1985;315:248–250. doi: 10.1038/315248a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J. Cell Biol. 1979;83:667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A. M., Staiger C. J., Min J. K., Simonetti K. D. The gelsolin family of actin regulatory proteins: modular structures, versatile functions. FEBS Lett. 2003;552:75–81. doi: 10.1016/s0014-5793(03)00932-3. [DOI] [PubMed] [Google Scholar]
- McGrath J. L., Osborn E. A., Tardy Y. S., Dewey C. F., Jr., Hartwig J. H. Regulation of the actin cycle in vivo by actin filament severing. Proc. Natl. Acad. Sci. USA. 2000;97:6532–6537. doi: 10.1073/pnas.100023397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Graves T. A., Wharton K. A., Falco N., Howe C. L. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J. Cell Biol. 1980;87:809–822. doi: 10.1083/jcb.87.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J., Weber A., Mooseker M. S., Franzini-Armstrong C., Bishop M. F., Dubyak G. R., Tucker M., Walsh T. P. Different calcium dependence of the capping and cutting activities of villin. J. Biol. Chem. 1986;261:9274–9281. [PubMed] [Google Scholar]
- Nusrat A., Delp C., Madara J. L. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J. Clin. Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D., Boujemaa R., Didry D., Gounon P., Carlier M. F. The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins. Nat. Cell Biol. 2000;2:385–391. doi: 10.1038/35017011. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Borisy G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Rathman M., Jouirhi N., Allaoui A., Sansonetti P., Parsot C., Tran Van Nhieu G. The development of a FACS-based strategy for the isolation of Shigella flexneri mutants that are deficient in intercellular spread. Mol. Microbiol. 2000;35:974–990. doi: 10.1046/j.1365-2958.2000.01770.x. [DOI] [PubMed] [Google Scholar]
- Revenu C., Athman R., Robine S., Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat. Rev. Mol. Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- Revenu C., Louvard D., Robine S. A dual role for the actin binding protein villin, as a key player in the maintenance and plasticity of the intestinal epithelial cell architecture. In: Naim H. Y., Zimmer K.-P., editors. The Brush Border Membrane from Molecular Cell Biology to Clinical Pathology, Vol. Perspectives in Paediatric Gastroenterology, Fulda 2005. Heilbronn, Germany: SPS Publications; 2006. pp. 260–274. [Google Scholar]
- Robine S., Huet C., Moll R., Sahuquillo-Merino C., Coudrier E., Zweibaum A., Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc. Natl. Acad. Sci. USA. 1985;82:8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Sibarita J. B. Deconvolution microscopy. Adv. Biochem. Eng. Biotechnol. 2005;95:201–243. doi: 10.1007/b102215. [DOI] [PubMed] [Google Scholar]
- Southwick F. S. Gain-of-function mutations conferring actin-severing activity to human macrophage cap G. J. Biol. Chem. 1995;270:45–48. doi: 10.1074/jbc.270.1.45. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- Sun H. Q., Wooten D. C., Janmey P. A., Yin H. L. The actin side-binding domain of gelsolin also caps actin filaments. Implications for actin filament severing. J. Biol. Chem. 1994;269:9473–9479. [PubMed] [Google Scholar]
- Svitkina T. M., Borisy G. G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gucht J., Paluch E., Plastino J., Sykes C. Stress release drives symmetry breaking for actin-based movement. Proc. Natl. Acad. Sci. USA. 2005;102:7847–7852. doi: 10.1073/pnas.0502121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Sharpe A. H., Hartwig J. H., Azuma T., Stossel T. P., Kwiatkowski D. J. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J. Cell Biol. 1981;91:901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebda N., Bernard O., Bailly M., Welti S., Lawrence D. S., Condeelis J. S. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J. Cell Biol. 2000;151:1119–1128. doi: 10.1083/jcb.151.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L., Kumar N., Panebra A., Zhao P., Parrill A. L., Khurana S. Regulation of actin dynamics by tyrosine phosphorylation: identification of tyrosine phosphorylation sites within the actin-severing domain of villin. Biochemistry. 2002;41:11750–11760. doi: 10.1021/bi0263762. [DOI] [PubMed] [Google Scholar]
- Zhai L., Zhao P., Panebra A., Guerrerio A. L., Khurana S. Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton. J. Biol. Chem. 2001;276:36163–36167. doi: 10.1074/jbc.C100418200. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Vorobiev S. M., Gibson B. G., Hao B., Sidhu G. S., Mishra V. S., Yarmola E. G., Bubb M. R., Almo S. C., Southwick F. S. A CapG gain-of-function mutant reveals critical structural and functional determinants for actin filament severing. EMBO J. 2006;25:4458–4467. doi: 10.1038/sj.emboj.7601323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.