Abstract
Ghrelin is an acylated peptidyl gastric hormone acting on the pituitary and hypothalamus to stimulate appetite, adiposity, and growth hormone release, through activation of growth hormone secretagogue receptor (GHSR)-1a receptor. Moreover, ghrelin features several activities such as inhibition of apoptosis, regulation of differentiation, and stimulation or inhibition of proliferation of several cell types. Ghrelin acylation is absolutely required for both GHSR-1a binding and its central endocrine activities. However, the unacylated ghrelin form, des-acyl ghrelin, which does not bind GHSR-1a and is devoid of any endocrine activity, is far more abundant than ghrelin in plasma, and it shares with ghrelin some of its cellular activities. Inhere we show that both ghrelin and des-acyl ghrelin stimulate proliferating C2C12 skeletal myoblasts to differentiate and to fuse into multinucleated myotubes in vitro through activation of p38. Consistently, both ghrelin and des-acyl ghrelin inhibit C2C12 proliferation in growth medium. Moreover, the ectopic expression of ghrelin in C2C12 enhances differentiation and fusion of these myoblasts in differentiation medium. Finally, we show that C2C12 cells do not express GHSR-1a, but they do contain a common high-affinity binding site recognized by both acylated and des-acylated ghrelin, suggesting that the described activities on C2C12 are likely mediated by this novel, yet unidentified receptor for both ghrelin forms.
INTRODUCTION
Ghrelin (GHR) is a circulating peptidyl hormone, octanoylated on Ser3, mainly produced by the stomach, which, by acting on the hypothalamus and the pituitary, induces a strong release of growth hormone (GH) and stimulates food intake and adiposity (Kojima et al., 1999; Kohno et al., 2003; Reimer et al., 2003). GHR exerts these activities through binding and activation of growth hormone secretagogue receptor (GHSR)-1a, a G protein-coupled receptor identified previously as the receptor for synthetic growth hormone secretagogues (GHSs) (Howard et al., 1996). In addition to its endocrine activities, GHR features several activities in the cardiovascular system in vivo, as it improves cardiac performances after heart damage (Nagaya et al., 2001, 2004). Moreover, GHR acts as a vasodilator, enhancing nitric oxide bioactivity in metabolic syndrome patients (Tesauro et al., 2005). In vitro, GHR inhibits apoptosis of cardiomyocytes and endothelial cells as well as apoptosis of preadipocytic and preosteoblastic cells, through activation of extracellular signal-regulated kinase (ERK)-1/2 and phosphoinositide 3-kinase/Akt pathways (Baldanzi et al., 2002; Kim et al., 2004; Kim et al., 2005). In addition, GHR induces differentiation of osteoblasts, adipocytes, and neurons by stimulating proliferation of their precursors (Choi et al., 2003; Kim et al., 2005; Zhang et al., 2005), although overexpression of GHR in preadipocytes strongly stimulates their proliferation, impairing rather than promoting adipocytic differentiation (Zhang et al., 2004). Conversely, GHR stimulates differentiation of immature Leydig cells by inhibiting their proliferation in vivo (Barreiro et al., 2004). GHR is also involved in regulation of cell growth, although it either stimulates or inhibits proliferation in different cell types. Indeed, GHR stimulates proliferation of preosteoblastic cells (Fukushima et al., 2005; Maccarinelli et al., 2005; Delhanty et al., 2006), neuron precursor of the dorsal ganglion (Zhang et al., 2005), primary oral keratinocytes (Groschl et al., 2005), HEL erythroleukemic cell line (De Vriese et al., 2005), zona glomerulosa cells (Andreis et al., 2003; Mazzocchi et al., 2004), GH3 rat pituitary cell line (Nanzer et al., 2004), 3T3-L1 preadipocytes (Kim et al., 2004; Zhang et al., 2004), pancreatic adenocarcinoma cells (Duxbury et al., 2003), H9C2 cardiomyocyte cell line (Pettersson et al., 2002), and several prostate cancer cell lines (Jeffery et al., 2002). Conversely, GHR inhibits cell proliferation of cell lines derived from several carcinomas, including prostate (Cassoni et al., 2004), thyroid (Volante et al., 2003), mammary (Cassoni et al., 2001), and lung (Ghè et al., 2002), as well as immature Leydig cells (Barreiro et al., 2004) and splenic T-cells costimulated by anti-CD3 antibodies (Xia et al., 2004).
Des-acyl ghrelin (D-GHR), the unacylated form of GHR, whose concentration in plasma and tissues is higher than that of GHR, does not bind GHSR-1a and is devoid of any central activity on GH release, appetite and adiposity. These observations initially suggested that D-GHR might act as a reservoir of inactive GHR. However, an increasing body of evidence indicates that D-GHR shares with GHR many biological activities and common binding sites on several peripheral tissues and cell types. Indeed, both GHR and D-GHR inhibit apoptosis and recognize common binding sites in H9c2 cardiomyocytes (Baldanzi et al., 2002); inhibit proliferation and recognize common binding sites in breast and prostate carcinoma cells (Jeffery et al., 2002; Cassoni et al., 2001); stimulate proliferation of preosteoblastic as well as GH3 pituitary cells (Fukushima et al., 2005; Maccarinelli et al., 2005; Nanzer et al., 2004; Delhanty et al., 2006); stimulate differentiation of osteoblasts in vitro (Delhanty et al., 2006); and adipogenesis in vivo (Choi et al., 2003), and activate ERK-1/2 and Akt signaling pathways, which mediate their antiapoptotic and proliferative responses.
In most, but not all, of the cells where D-GHR activity was investigated, GHSR-1a is not expressed, strongly suggesting that such pleiotropic activities of both GHR and D-GHR may be mediated by a yet unidentified receptor. In summary, these data indicate that D-GHR shares a subset of biological activities with ghrelin in peripheral tissues through an unidentified receptor distinct from GHSR-1a.
In vivo, GHR treatment has been reported to ameliorate chronic heart failure- and cancer-induced cachexia, whereas its plasma concentration is increased in cachectic patients (Nagaya et al., 2001, 2005; Granado et al., 2005). However, no studies have addressed whether GHR may act directly on the muscle. Intriguingly, binding sites for hexarelin, a synthetic GHS, have been observed in skeletal muscle (Papotti et al., 2000). Based on these observations, we investigated GHR and D-GHR biological activities in skeletal muscle myoblasts.
Skeletal muscle satellite cells are mononucleated myoblasts, which, upon muscle diseases or direct injury, are activated to undergo proliferation and eventually differentiate to form new muscle fibers to allow muscle regeneration. In vivo, differentiation of skeletal muscle involves first the growth factor-sustained expansion of the population of skeletal myoblasts and then cell cycle exit and initiation of terminal differentiation, which involves expression of contractile proteins and formation of multinucleated syncitia by myocytes fusion. The extracellular signals triggering growth arrest and the molecular mechanisms involved in the induction of myoblasts differentiation and fusion still remain to be fully elucidated.
In vitro, muscle differentiation steps can be reproduced with myoblastic satellite-derived cell lines, such as the C2C12 murine myoblast cells used in this study. C2C12 myoblasts proliferate in presence of 10% fetal calf serum (FCS) (growth medium; GM), and undergo differentiation when cultured in 2% horse serum (differentiation medium; DM).
Herein, we provide data demonstrating that both GHR and D-GHR act on skeletal myoblasts by inhibiting cell proliferation and by promoting muscle differentiation and fusion.
MATERIALS AND METHODS
Reagents
Synthetic ghrelin-(1-28), or GHR; Tyr4-ghrelin-(1-28), or Tyr4-GHR; truncated ghrelin-(9-28), or GHR-(9-28); des-acyl ghrelin-(1-28), or D-GHR; and motilin were provided by NeoMPS (Strasbourg, France). The anti-myosin heavy chain (MHC; MF-20) and anti-myogenin antibodies were kind gifts of Dr. Mara Brancaccio (University of Torino, Torino, Italy). Anti-phospho-Akt, anti-Akt, anti-phospho-ERK-1/2, anti-ERK-1/2 antibodies, and p38 MAPK assay kit were from Cell Signaling Technology (Beverly, MA). All reagents were from Sigma, unless otherwise indicated.
Cell Cultures
C2C12 myoblasts were grown at low density in a proliferative medium (GM) consisting in DMEM supplemented with 10% FCS (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml antimycotic. To induce differentiation, cells were allowed to become confluent, and the medium was switched to DM consisting in DMEM supplemented with 2% horse serum, penicillin, streptomycin, and antimycotic as described above.
Western Blot
After the indicated treatments, cells were washed in ice-cold phosphate-buffered saline (PBS) and solubilized with a lysis buffer containing 25 mM HEPES, pH 8, 135 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM ZnCl2, 50 mM NaF 50, 1% NP-40, 10% glycerol, 0.05 mg/ml leupeptin, 0.005 mg/ml pepstatin, 200 μM phenylmethylsulfonyl fluoride, and 1 mM Na3VO4. Lysates were stirred at 4°C for 15 min and centrifuged at 13,000 × g for 15 min at 4°C. Protein concentration was determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA). Proteins (20–50 μg protein/lane) were separated by 5–12% SDS-PAGE and transferred to polyvinylidene difluoride filters (Hybond-P; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Membranes were incubated with the primary antibodies, washed with Tris-buffered saline/0.1% Tween, incubated with the appropriate secondary antibody (PerkinElmer Life and Analytical Sciences, Boston, MA), visualized with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life and Analytical Sciences), acquired with VersaDoc 3000 (Bio-Rad), and analyzed with Quantity One software (Bio-Rad). Equal protein loading was further controlled by Ponceau red staining. After anti-phospho-Akt and anti-phospho-ERK-1/2, membranes were stripped with ReBlot Plus (Chemicon International, Temecula, CA) and reblotted with the corresponding total protein antibodies.
Immunofluorescence
Cells were plated on 24-well plates and treated as indicated. At the end of the treatments, cells were fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and incubated with anti-MHC followed by incubation with the secondary antibody and 4,6-diamidino-2-phenylindole (DAPI), and visualized by fluorescence microscopy (Axiovert 40; Carl Zeiss, Jena, Germany). Each treatment was in triplicate, and each experiment was repeated at least two times. Images were acquired (10 fields/well) and analyzed to determine differentiation and fusion indexes.
Differentiation Index and Fusion Index
To quantify the differentiation and fusion of C2C12 cells after treatments, we calculated the differentiation index as the percentage of MHC-positive cells above total nuclei and the fusion index as the average number of nuclei in MHC-positive cells with at least three nuclei above total number of nuclei, respectively.
Cell Proliferation
C2C12 cells were starved overnight in 0.2% FCS and then maintained for 24 h with or without GHR and D-GHR in GM to evaluate the inhibition of proliferation. At the end of treatments, cells were incubated with 2 μCi/ml [3H]thymidine (GE Healthcare) for 3 h, washed with PBS, treated with 5% trichloroacetic acid (TCA) to precipitate proteins and DNA, and finally lysed by adding 0.5 M NaOH and 0.5% SDS. Positive control for proliferation was GM, whereas negative control was 0.2% FCS. The amount of incorporated [3H]thymidine was evaluated by beta-counter (Tri-Carb 2800TR; Perkin Elmer) analysis. The data presented here are the average of triplicate assays, and similar results were obtained in at least three independent experiments.
p38 Kinase Assay
The ability of GHR and D-GHR to activate p38 was assayed by a specific p38 nonradioactive kinase assay kit from Cell Signaling Technology, according to the protocol provided by the supplier. Briefly, after the indicated treatments, cells were solubilized with a lysis buffer, the phosphorylated p38 was immunoprecipitated, and an in vitro kinase assay was performed using activating transcription factor (ATF)-2 as a substrate. Phosphorylated ATF-2 was finally detected by Western blotting.
Generation of the Ghrelin-expressing Lentiviral Vector (MA1-GHR)
Total RNA from mouse stomach, mechanically triturated in liquid nitrogen, was extracted by TRIzol (Invitrogen), following the manufacturer's instructions. The RNA obtained was retrotranscribed, and the cDNA was used to clone the total ghrelin in the lentiviral vector MA1 (pCCL.sin.PPT.polyA. CTE.eGFP.minhCMV.hPGK.WPRE), a kind gift from Prof. L. Naldini (HSR-Tiget, Milan, Italy), containing a synthetic bidirectional promoter that simultaneously promotes the transcription of two divergent mRNA sequences, one sequence of which encoded for an enhanced green fluorescent protein (EGFP) (Amendola et al., 2005). The generated construct has been transfected in myoblasts to verify the ability of this MA1-GHR vector to afford in vitro the expression of the ghrelin gene. Cells were transfected with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions.
Radioimmunoassay (RIA) Analysis
The ability of MA1-GHR vector to afford the expression of the GHR gene in C2C12 myoblasts and the secretion of the hormone in culture medium was assayed by a specific RIA kit from Phoenix Pharmaceuticals (Belmont, CA), according to the protocol provided by the supplier.
GHSR-1a Expression
Total RNA from cultured cells was extracted by Nucleospin RNA II (Macherey- Nagel, Düren, Germany) following the manufacturer's instructions, whereas RNA from mouse brain, mechanically triturated in liquid nitrogen, was extracted by TRIzol (Invitrogen). The RNA obtained was retrotranscribed with SuperScript reverse transcriptase II (Invitrogen). The quality of cDNAs has been assessed by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification, and then reverse transcription-polymerase chain reaction (RT-PCR) of GHSR-1a was performed using DNAzyme EXT polymerase (Finnzymes, Espoo, Finland) and the following primers: GHSR-1a exon 1-for 5′-AGTATCGGCCCTGGAACTT-3′, GHSR-1a exon 1-rev 5′-ACGCTCGACACCCATACCAT-3′, GHSR-1a exon 2-for 5′-TGGTGTTTGCTTTCATCCTC-3′, GHSR-1a exon 2-rev 5′-CGGGAACTCTCATCCTTCAGA-3′, GHSR-1a complete-for 5′-AAGGTGGTGGTCACCAAGG-3′, and GHSR-1a complete-rev 5′-CGGTACTTCTTGGACATGATG-3′.
Ghrelin Binding Assay
Tyr4-GHR was radioiodinated (125I-Tyr4-GHR; specific activity 2000 Ci/mmol) by using a lactoperoxidase method by GE Healthcare and used as a radioligand in the binding studies. Tyr4-GHR has been reported to be a reliable probe for labeling GHS-R in tissue or cell membranes and to retain the same GH-releasing potency of the native peptide (Muccioli et al., 2001, 2004; Baldanzi et al., 2002).
Binding of 125I-Tyr4-GHR to crude C2C12 myoblast membranes (30,000 × g pellet), and saturation binding analysis were determined as described previously (Muccioli et al., 2001, 2004). IC50 values of specific radioligand binding were determined by radioligand ghrelin displacement curves with increasing concentrations of unlabeled GHR, D-GHR, GHR-(9-28) fragment, or motilin. The maximal number of binding sites (Bmax), the dissociation constant (Kd), and the IC50 values were calculated with the iterative curve-fitting Prism 4 program (GraphPad Software Inc., San Diego, CA).
Statistical Analysis
Where appropriate, data are presented as the mean ± SEM, and the statistical significance was assessed using Student's t test.
RESULTS
Ghrelin and Des-Acyl Ghrelin Promote Differentiation and Fusion of C2C12 Myoblasts in Growth Medium
C2C12 myoblasts, a skeletal muscle satellite-derived cell line, is a common model to investigate cellular and molecular mechanisms of muscle differentiation. Upon culture in 2% horse serum, C2C12 cells exit the cell cycle, differentiate, and fuse into multinucleated skeletal myotubes expressing contractile proteins (Blau et al., 1985). The extracellular signals triggering growth arrest and the molecular mechanisms involved in the induction of myoblasts differentiation and fusion still remain to be elucidated.
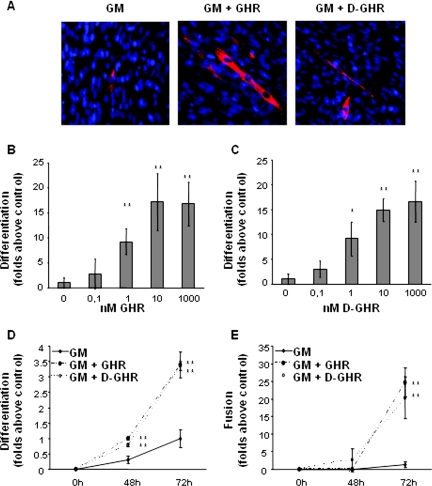
GHR and D-GHR induce muscle differentiation and fusion of proliferating C2C12 myoblasts in GM (10% FCS), as shown by immunofluorescence microscopy with anti-MHC antibodies. Figure 1A shows typical immunofluorescence images obtained from C2C12 skeletal muscle cells cultured for 72 h in GM in presence or absence of either 10 nM GHR or 10 nM D-GHR. Cells positive for MHC, a marker for terminal differentiation, are red stained, whereas nuclei are blue stained (DAPI). In a representative field of C2C12 cells maintained in GM, only a single MHC-positive cell is visible, but no multinucleated tubes are present, indicating a minimal spontaneous differentiation tendency. In representative fields of C2C12 cells in GM treated with 10 nM GHR or D-GHR, respectively, both single-nucleated MHC-positive cells and multinucleated myotubes are clearly visible.
Figure 1.
GHR and D-GHR induce differentiation and fusion of C2C12 myoblasts in GM. Cells were treated either with GHR or D-GHR in GM and fixed for staining with anti-MHC antibody and DAPI. (A) Representative immunofluorescences of C1C12 myoblasts treated for 72 h with 10 nM GHR or D-GHR stained with anti-MHC antibody and DAPI are shown. (B and C) Dose–response activity of GHR and D-GHR in inducing differentiation of C2C12 myoblasts after 96 h of treatment. (D and E) Differentiation and fusion indexes after 48 and 72 h of treatment with 10 nM GHR and D-GHR. **p < 0.01 versus control.
The extent of differentiation of skeletal muscle cells was measured by differentiation and fusion indexes, reflecting, respectively, MHC expression and multinucleated myotubes formation. Differentiation index is calculated as the number of MHC-positive cells, expressed as percentage of total number of cells counted by DAPI-stained nuclei. Fusion index is calculated as the average number of nuclei contained in MHC-positive cells with at least three nuclei, compared with the total number of nuclei.
Differentiation index of C2C12 myoblasts is significantly increased in a concentration-dependent manner upon 96 h of treatment with rising concentrations of either GHR or D-GHR in GM. Maximal response was observed at 10 nM, whereas minimum significant differentiation was already observed at 1 nM (Figure 1, B and C). Differentiation was already evident and significant upon 48 and 72 h of treatment (Figure 1D).
In addition, the differentiating activity of GHR and D-GHR is not limited to stimulating MHC expression; it also induces myocyte fusion to form multinucleated syncitial myotubes. Fusion index of myocytes cultured in presence of either 10 nM GHR or D-GHR was increased up to 20- to 25-fold after 72 h of treatments, compared with untreated control myoblasts in GM (Figure 1E). Thus, these data clearly show that both GHR and D-GHR activate a complete differentiation program in C2C12 skeletal myoblasts driving both expression of contractile proteins and cellular events leading to the formation of multinucleated myotubes.
Ghrelin and Des-Acyl Ghrelin Induce the Expression of Early and Late Markers of Skeletal Muscle Differentiation in C2C12 Myoblasts
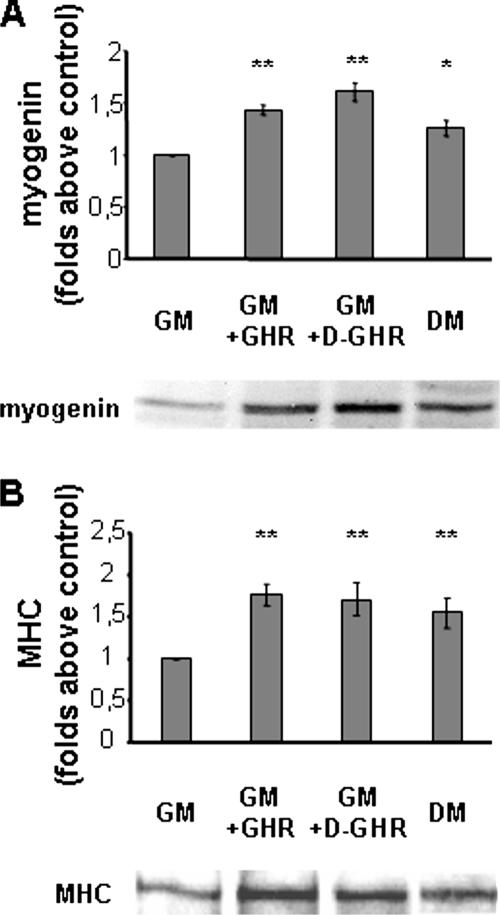
To consolidate the observation that indeed GHR and D-GHR activate a differentiating program in skeletal myoblasts, we have verified the ability of both GHR and D-GHR to induce the expression of myogenin and MHC proteins, as detected by Western blot. While MHC is a late differentiation marker, myogenin is a helix-loop-helix transcription factor whose expression is induced early in differentiation, preceding cell cycle exit (Andres and Walsh, 1996; Zhang et al., 1999).
C2C12 cells cultured in GM were treated with 10 nM GHR or D-GHR or switched to DM for either 24 or 72 h. Expression of myogenin and MHC was measured by Western blot of whole cell lysates, and the intensity of the bands was quantified. Figure 2A shows that upon 24-h treatment with both GHR and D-GHR the expression of myogenin is significantly increased compared with control cells in GM, at similar extent of the expression induced by DM. Moreover, upon 72-h treatment, when multinucleated myotubes are formed, the expression of the terminal differentiation marker MHC was significantly induced (Figure 2B). These results confirm that GHR and D-GHR are able to promote both early and late steps of skeletal muscle differentiation in GM.
Figure 2.
Western blot analysis of myogenic markers expression. C2C12 myoblasts were incubated in GM for 24 or 72 h with 10 nM GHR or D-GHR. The content of myogenin and MHC was measured by Western blot of whole cell lysates and the intensity of the bands quantified. Cells differentiated in DM were considered as positive control. (A) Myogenin expression. (B) MHC expression. Representative Western blots are shown at the bottom of each panel. **p < 0.01 and *p < 0.05 versus control.
Ghrelin and Des-Acyl Ghrelin Inhibit Proliferation of C2C12 Myoblasts
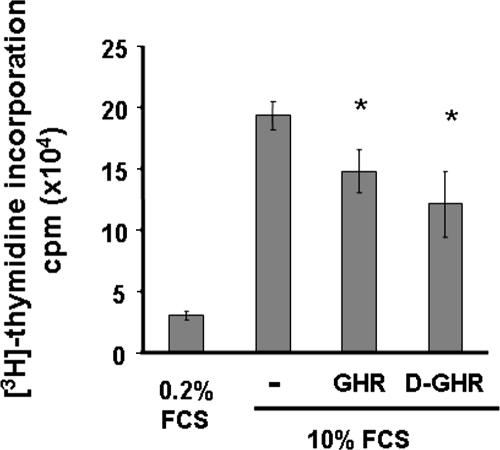
Proliferating C2C12 myoblasts exit cell cycle upon switching from GM to DM, to initiate terminal differentiation. Because both GHR and D-GHR stimulate differentiation of proliferating myoblasts, we have investigated their ability to inhibit cell proliferation, measured as inhibition of DNA synthesis.
Growing C2C12 myoblasts were starved overnight in 0.2% FCS to synchronize their cell cycles, and then they were maintained for 24 h in GM with or without 10 nM GHR and D-GHR. As positive control of inhibition of proliferation, cells were maintained in 0.2% FCS. Either GHR or D-GHR in GM inhibit DNA synthesis of C2C12 myoblasts of ∼25% compared with control cells (Figure 3).
Figure 3.
Effect of GHR and D-GHR on basal incorporation of [3H]thymidine into DNA by C2C12 myoblasts. DNA synthesis was estimated by incorporation of [3H]thymidine after a 24-h incubation with or without 10 nM GHR or D-GHR in GM. *p < 0.05 versus control.
Ghrelin and Des-Acyl Ghrelin Induce Differentiation and Fusion of C2C12 by Activation of p38
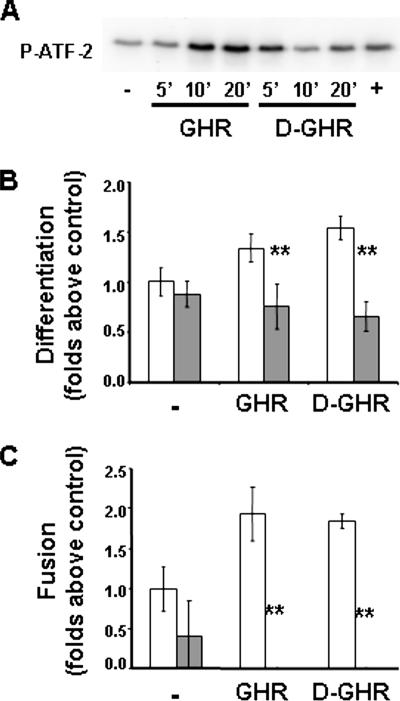
Overexpression of a constitutively activated form of MKK6, activating endogenous p38, stimulates muscle differentiation even in the presence of antimyogenic cues (Wu et al., 2000). Because both GHR and D-GHR stimulate the differentiation of proliferating C2C12 myoblasts, we investigated whether GHR and D-GHR induce the activation of p38 (Figure 4A). Indeed, GHR and D-GHR stimulate activation of p38, as measured by its specific kinase activity in vitro on purified ATF-2, suggesting that this pathway may be involved in the differentiative signaling triggered by these factors. To verify such hypothesis, we have investigated whether pharmacological inhibition of p38 impairs GHR and D-GHR differentiative activity in C2C12. Indeed, cell pretreatment with 5 μM SB203580 for 15 min significantly inhibited differentiation up to ∼40% (Figure 4B), and abolished fusion (Figure 4C), induced by 72-h treatment with 10 mM GHR and D-GHR.
Figure 4.
GHR and D-GHR induce differentiation and fusion of C2C12 myoblasts by activation of p38. (A) Kinase activity of p38 on ATF-2 induced by 1 μM GHR and D-GHR in C2C12 myoblasts. Cells were starved in 0.2% FCS overnight and then treated for the indicated times. Treatment with 10 μg/ml lysophosphatidic acid for 5 min was used as positive control (+). (B and C) Inhibition of p38 reduces 10 mM GHR- and D-GHR–induced differentiation and fusion of C2C12. Cells were pretreated with 5 μM SB302580 for 15 min, treated with GHR or D-GHR in GM, and fixed after 72 h for staining with anti-MHC antibody and DAPI to determine the differentiation and fusion indexes. Empty bars, without SB302580; filled bars, with SB302580. **p < 0.01 versus control.
Ghrelin and Des-Acyl Ghrelin Enhance Differentiation and Fusion of C2C12 Myoblasts in Differentiation Medium
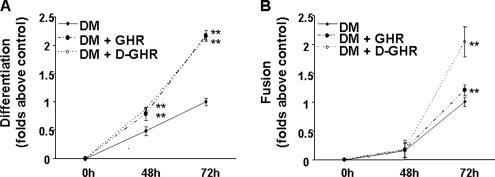
To provide further evidence that GHR and D-GHR may participate in the regulation of muscle differentiation, we investigated whether they affect DM-induced differentiation and fusion of C2C12 myoblasts. Indeed, after 72-h treatment with either GHR or D-GHR in DM, both differentiation index (Figure 5A) and fusion index (Figure 5B) increased up to twofold, compared with nontreated cells in DM.
Figure 5.
GHR and D-GHR enhance differentiation and fusion of C2C12 myoblasts in differentiation medium. Cells were treated with 10 nM GHR or D-GHR in DM and fixed after 48 and 72 h for staining with anti-MHC antibody and DAPI in order to determine the differentiation index. ** p < 0.01 versus control.
Furthermore, we artificially generated a ghrelin autocrine loop by ectopic expression of ghrelin gene in C2C12 myoblasts. The murine ghrelin gene was subcloned in a lentiviral vector under the control of a bidirectional promoter, which simultaneously promotes the expression of EGFP (MA1-GHR). Lentiviral vector expressing EGFP alone was used as control. After transfection with MA1-GHR vector, ghrelin secretion, as assayed in culture medium by RIA, was twofold compared with control cells (data not shown).
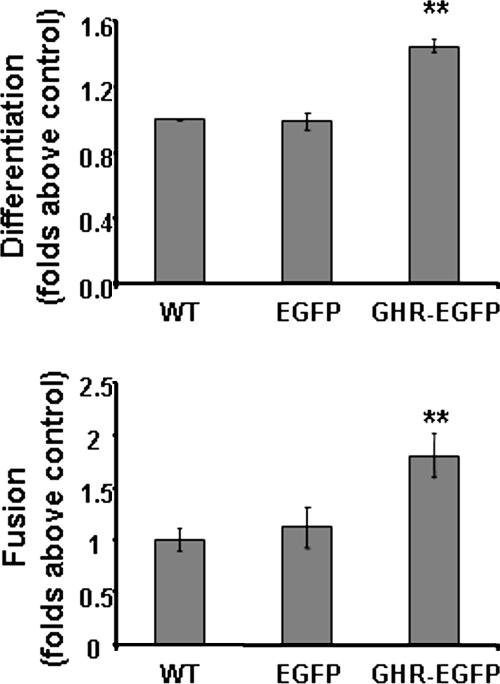
C2C12 cells transiently expressing either EGFP alone or EGFP and GHR were induced to differentiate in DM. After 72 h from transfection, differentiation index of ghrelin-overexpressing cells is increased by ∼45% compared with EGFP-expressing cells. Similarly, fusion index is also increased by ∼80% compared with control cells. Untransfected cells feature differentiation and fusion indexes similar to those of cells expressing EGFP alone, indicating that the viral construct does not affect differentiation and fusion by itself (Figure 6).
Figure 6.
Increase of differentiation and fusion of C2C12 myoblasts overexpressing murine GHR. Cells transfected with a lentiviral vector expressing both GHR and EGFP under control of a bidirectional promoter, a lentiviral vector expressing EGFP only, and C2C12 wild-type cells were induced to differentiate switching the GM to DM. Cells were fixed after 72 h in DM and stained with anti-MHC antibody and DAPI to determine differentiation index (top) and fusion index (bottom). **p < 0.01 versus control.
C2C12 Skeletal Muscle Cells Do Not Express GHSR-1a, but They Contain High-Affinity Binding Sites That Are Recognized by Both Ghrelin and Des-Acyl Ghrelin
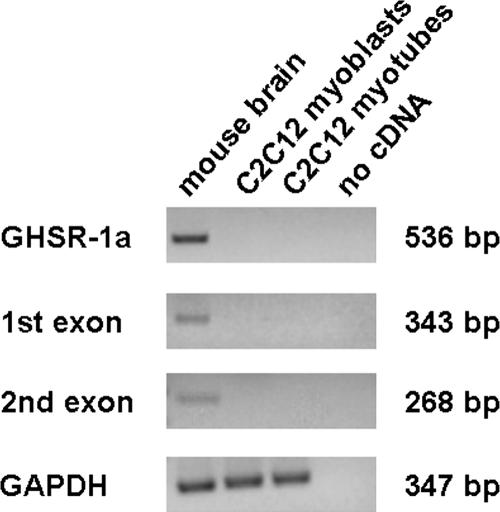
We previously showed that GHSR-1a, the only known GHR receptor, is not expressed in cardiomyocytes and endothelial cells where GHR and D-GHR inhibit cell death. Thus, we have assayed by RT-PCR the expression of GHSR-1a in C2C12 myoblasts and in differentiated myotubes, by using cDNA from whole mouse brain as positive control. PCR reactions were performed using primers amplifying either the first exon, common to both GHSR-1a and GHSR-1b, or the second exon, specific of GHSR-1a. In addition, to avoid false positives due to genomic contamination, we also used intron-spanning primers, to amplify the complete GHSR-1a. No expression was detected in both undifferentiated and differentiated C2C12 myocytes (Figure 7), suggesting that GHR and D-GHR activities in C2C12 skeletal muscle cells are not mediated by GHSR-1a.
Figure 7.
Expression of GHSR-1a, by RT-PCR, in C2C12 myoblasts and myotubes. Mouse brain was used as positive control for amplification of complete receptor, first exon, and second exon. Qualitative controls for samples were performed using mouse GAPDH.
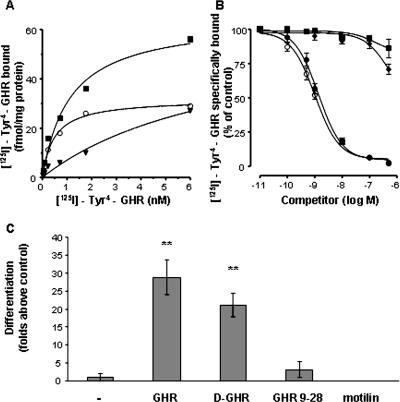
To further investigate the identity of the receptor mediating the effects of GHR and D-GHR on C2C12 skeletal muscle cells, we performed binding studies of radiolabeled GHR (125I-Tyr4-GHR) to membranes of C2C12 myoblasts. Binding experiments with increasing concentrations of 125I-Tyr4-GHR (0.035–6 nM) revealed the existence of saturable and high-affinity binding sites in C2C12 cells (Figure 8A) with an apparent Kd and a Bmax value (mean ± SEM of 3 independent experiments) of 0.39 ± 0.04 nM and 26.3 ± 2.0 fmol/mg protein, respectively. Binding of radiolabeled GHR was displaced in a dose-dependent manner and with equal efficacy by unlabeled GHR and D-GHR. The IC50 values calculated from competition binding curves, expressed as nanomolar concentrations (mean ± SEM of three independent experiments), were 1.20 ± 0.09 for GHR and 1.32 ± 0.08 for D-GHR. In contrast, motilin or GHR-(9–28), a synthetic truncated GHR derivative, was able, at the maximal concentration tested, to displace only 14–37% of the specifically bound radiolabeled GHR (Figure 8B). Accordingly, neither GHR-(9-28) nor motilin were able to induce C2C12 myoblasts differentiation (Figure 8C). These findings provide evidence that GHR and D-GHR may act directly as factors modulating cell proliferation and differentiation on C2C12 myoblasts through binding to a specific receptor that is distinct from GHSR-1a.
Figure 8.
C2C12 myoblasts contain a GHR receptor distinct from GHSR-1a. (A) Specific binding (○) was determined by incubation of crude membranes with increasing concentrations of radiolabeled GHR (125I-Tyr4-GHR) in the absence (total binding, ■) or in the presence (nonspecific binding, ▾) of 1 μM unlabeled GHR. Data are the average of triplicate determinants. Similar results were obtained in at least two other independent experiments. (B) Displacement curves of radiolabeled GHR by unlabeled GHR (○), D-GHR (●), GHR-(9-28) fragment (♦), and motilin (■). Values are the mean ± SEM of three independent experiments. (C) Cells were fixed after 72 h treatment with 10 mM GHR, D-GHR, GHR-(9-28) fragment, and motilin in GM and stained with anti-MHC antibody and DAPI to determine the differentiation index. **p < 0.01 versus control.
DISCUSSION
Skeletal muscle satellite cells are quiescent mononucleated myoblasts, located between the sarcolemma and the basal membrane of terminally differentiated adult muscle fibers. On muscle diseases or direct injury, quiescent satellite cells are activated to undergo proliferation and eventually differentiate to allow muscle regeneration.
Skeletal muscle regeneration involves, sequentially, satellite cell proliferation, commitment to terminal differentiation, cell fusion into multinucleated syncitia, and muscle fiber formation.
Such mechanisms leading to muscle regeneration are poorly understood; they seem to recapitulate the embryonic program of differentiation, although the extracellular factors regulating such processes may be different.
Satellite cell differentiation into skeletal muscle can be subdivided into temporally separable events, coordinated by the expression of proteins of the muscle regulatory factors family, such as myogenin, and of cyclin-dependent kinase inhibitor of the p21 family (Andres and Walsh, 1996), resulting in cell cycle exit and commitment to terminal differentiation. Later on, expression of muscle contractile proteins, such as MHCs and myosin light chains (MLCs), are hallmarks of phenotypic differentiation. Finally, fusion of myocytes into multinucleated myotubes is the terminal step of muscle differentiation.
The growing interest in skeletal muscle regeneration is associated to the opening of new therapeutic strategies for several muscular degenerative pathologies such as dystrophies, muscular atrophy, and cachexia associated to aging, cancer, chronic heart failure, and acquired immunodeficiency syndrome as well as the treatments of skeletal muscle injury after trauma.
Although GHR is a circulating hormone mainly secreted by the stomach, it is also synthesized in a number of tissues, suggesting both endocrine and paracrine effects (Gnanapavan et al., 2002).
The evidence that 1) GHR up-regulation is specifically associated to either congestive heart failure (CHF)- or cancer-induced cachexia (Nagaya et al., 2001, Shimizu et al., 2003) and that its administration strongly prevents CHF-associated cachexia (Nagaya et al., 2004); 2) GHR, D-GHR, and GHSs inhibit apoptosis of cardiac myocytes (Filigheddu et al., 2001; Baldanzi et al., 2002); and 3) skeletal muscle features high binding sites for synthetic GHSs (Papotti et al., 2000), lead us to speculate that GHR and D-GHR may act directly also on skeletal muscle. Indeed, we observed that both GHR and D-GHR stimulate tyrosine phosphorylation of several proteins and activate ERK-1/2 and Akt (data not shown), indicating that both factors could exert a biological activity on these cells.
Here, we show that nanomolar concentrations of both GHR and D-GHR induce the differentiation of proliferating skeletal myoblasts in a concentration-dependent manner and promote their fusion into multinucleated syncitia in vitro. The cellular and molecular mechanisms by which GHR and D-GHR elicit these responses are not known. Cell cycle withdrawal is a prerequisite for myogenic terminal differentiation (Walsh and Perlman, 1997). Indeed, the ability of GHR and D-GHR to reduce DNA synthesis of proliferating C2C12 myoblasts is highly consistent with their prodifferentiative activity. However, inhibition of cell proliferation is not sufficient to elicit muscle differentiation. For example, myostatin inhibits both proliferation and differentiation of C2C12 myoblasts, through down-regulation of MyoD and myogenin expression (Joulia et al., 2003). Conversely, GHR and D-GHR, beyond inhibiting cell proliferation, induce the expression of myogenin, which is required for the complete program of differentiation of skeletal myoblasts to proceed (Zhang et al., 1999). To our knowledge this is the first evidence for an extracellular factor able to induce muscle differentiation of proliferating skeletal myoblasts in GM.
In proliferating C2C12 myoblasts, activation of p38 pathway obtained by overexpression of constitutively active MKK6 is sufficient to induce myogenin expression, cell cycle exit, and skeletal muscle terminal differentiation (Wu et al., 2000). Thus, we investigated whether GHR and D-GHR prodifferentiative activity is mediated by p38. Consistently, inhibition of p38 by cell treatment with SB203580 resulted in the partial albeit significant inhibition of GHR and D-GHR-induced differentiative activity. In addition, we also showed that both GHR and D-GHR activate p38. Altogether, these data demonstrate that GHR and D-GHR act as antiproliferative and prodifferentiative factors by stimulating the p38 pathway.
The lack of expression of GHSR-1a in either C2C12 myoblasts and skeletal muscle tissue (Gnanapavan et al., 2002) as well as the activity exerted by D-GHR suggest that GHR- and D-GHR–differentiating activities are mediated by a yet unidentified receptor, common to both acylated and unacylated peptide and distinct from GHSR-1a. Indeed, here we showed that C2C12 cells feature high-affinity common binding sites for both GHR and D-GHR. Such binding sites are specific, because they do not recognize either N-terminal truncated ghrelin or motilin, which are unable to induce differentiation. These studies also demonstrate that the N-terminal portion of the GHR peptide is required for binding and induction of C2C12 muscular differentiation. Together, these data provide further evidence for novel GHR receptor subtypes, which do not discriminate between the acylated and unacylated peptide. Although evidence for common GHR and D-GHR receptors have been reported in several cells, including a cardiomyocyte-derived cell line (Baldanzi et al., 2002), this is the first evidence for their expression in skeletal muscle.
We also verified whether the ghrelin gene is up-regulated in C2C12 myoblasts induced to differentiate in DM. However, no difference of ghrelin expression was detected by real-time RT-PCR between proliferating and differentiating cells (data not shown), suggesting that GHR gene product is not involved in DM-induced skeletal muscle differentiation in vitro.
By showing that GHR and D-GHR stimulate terminal differentiation of skeletal myoblasts in vitro, we may raise the hypothesis that the function of GHR gene may be involved in skeletal muscle differentiation in vivo. However, the lack of a consistent phenotype in GHR knockout mice, suggests that GHR function is not required for myogenesis during development. Consistently, we have not detected any GHR expression in somites or related structures during embryonic development by in situ hybridization (data not shown). However, although not essential for embryo development, GHR might be involved in the complex process of myogenesis in the adulthood, i.e., in regenerative processes of skeletal muscle. This hypothesis is consistent with the data showing that FGF6 is not required for muscle development, but is required in the adult for damage-induced muscle regeneration (Floss et al., 1997).
Upon muscular injury, skeletal myoblasts are activated to terminally differentiate through an autocrine/paracrine loop. We may speculate that GHR would contribute to skeletal muscle plasticity, promoting the differentiation and fusion of myoblasts in the damaged muscles. If this hypothesis would be proved, the activation of the receptor mediating GHR and D-GHR differentiative activity as well as the overexpression of the hormone may provide novel therapeutic strategies for the reduction or retardation of several skeletal muscle pathologies, including dystrophies, atrophies, and cachexia.
ACKNOWLEDGMENTS
We acknowledge Giulio Cossu, Marco Crescenzi, and Pier Lorenzo Puri for scientific input, valuable discussions, and helpful comments about skeletal muscle differentiation; Luigi Naldini, Mario Amendola, and Chiara Ambrogio for the assistance with lentiviral vector design and infection, and Anna Rapa for skilled help with RIA assay. This work was supported by grants to A.G. (Telethon GGP030386, Regione Piemonte A224/2004 and 37B1.2/2003; Fondazione Cariplo; and MIUR-PRIN 2005) and to G.M. (Regione Piemonte Grant A58-2004).
Abbreviations used:
- D-GHR
des-acyl ghrelin
- DM
differentiation medium
- EGFP
enhanced green fluorescent protein
- GHR
ghrelin
- GHSR
growth hormone secretagogue receptor
- GM
growth medium
- MHC
myosin heavy chain.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0402) on January 3, 2007.
REFERENCES
- Amendola M., Venneri M. A., Biffi A., Vigna E., Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat. Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- Andreis P. G., Malendowicz L. K., Trejter M., Neri G., Spinazzi R., Rossi G. P., Nussdorfer G. G. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 2003;536:173–179. doi: 10.1016/s0014-5793(03)00051-6. [DOI] [PubMed] [Google Scholar]
- Andres V., Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldanzi G., et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro M. L., Gaytan F., Castellano J. M., Suominen J. S., Roa J., Gaytan M., Aguilar E., Dieguez C., Toppari J., Tena-Sempere M. Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology. 2004;145:4825–4834. doi: 10.1210/en.2004-0732. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Cassoni P., Ghè C., Marrocco T., Tarabra E., Allia E., Catapano F., Deghenghi R., Ghigo E., Papotti M., Muccioli G. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur. J. Endocrinol. 2004;150:173–184. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- Cassoni P., Papotti M., Ghe C., Catapano F., Sapino A., Graziani A., Deghenghi R., Reissmann T., Ghigo E., Muccioli G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 2001;86:1738–1745. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- Choi K., Roh S. G., Hong Y. H., Shrestha Y. B., Hishikawa D., Chen C., Kojima M., Kangawa K., Sasaki S. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology. 2003;144:754–759. doi: 10.1210/en.2002-220783. [DOI] [PubMed] [Google Scholar]
- De Vriese C., Gregoire F., De Neef P., Robberecht P., Delporte C. Ghrelin is produced by the human erythroleukemic HEL cell line and involved in an autocrine pathway leading to cell proliferation. Endocrinology. 2005;146:1514–1522. doi: 10.1210/en.2004-0964. [DOI] [PubMed] [Google Scholar]
- Delhanty P. J., van der Eerden B. C., van der Velde M., Gauna C., Pols H. A., Jahr H., Chiba H., van der Lely A. J., van Leeuwen J. P. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006;188:37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- Duxbury M. S., Waseem T., Ito H., Robinson M. K., Zinner M. J., Ashley S. W., Whang E. E. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 2003;309:464–468. doi: 10.1016/j.bbrc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Filigheddu N, et al. Hexarelin protects H9C2 cardiomyocytes from doxorubicin-induced cell death. Endocrine. 2001;14:113–119. doi: 10.1385/ENDO:14:1:113. [DOI] [PubMed] [Google Scholar]
- Floss T., Arnold H. H., Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima N, et al. Ghrelin directly regulates bone formation. J. Bone Miner. Res. 2005;20:790–798. doi: 10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- Ghè C., Cassoni P., Catapano F., Marrocco T., Deghenghi R., Ghigo E., Muccioli G., Papotti M. The antiproliferative effect of synthetic peptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology. 2002;143:484–491. doi: 10.1210/endo.143.2.8654. [DOI] [PubMed] [Google Scholar]
- Gnanapavan S., Kola B., Bustin S. A., Morris D. G., McGee P., Fairclough P., Bhattacharya S., Carpenter R., Grossman A. B., Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87:2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- Granado M., Priego T., Martin A. I., Villanua M. A., Lopez-Calderon A. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005;289:E1007–E1014. doi: 10.1152/ajpendo.00109.2005. [DOI] [PubMed] [Google Scholar]
- Groschl M., Topf H. G., Bohlender J., Zenk J., Klussmann S., Dotsch J., Rascher W., Rauh M. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 2005;51:1–10. doi: 10.1373/clinchem.2004.040667. [DOI] [PubMed] [Google Scholar]
- Howard H. D, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–976. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Jeffery P. L., Herington A. C., Chopin L. K. Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J. Endocrinol. 2002;172:R7–R11. doi: 10.1677/joe.0.172r007. [DOI] [PubMed] [Google Scholar]
- Joulia D., Bernardi H., Garandel V., Rabenoelina F., Vernus B., Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 2003;286:263–275. doi: 10.1016/s0014-4827(03)00074-0. [DOI] [PubMed] [Google Scholar]
- Kim M. S, et al. The mitogenic and antiapoptotic actions of ghrelin in 3T3–L1 adipocytes. Mol. Endocrinol. 2004;18:2291–2301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- Kim S. W., Her S. J., Park S. J., Kim D., Park K. S., Lee H. K., Han B. H., Kim M. S., Shin C. S., Kim S. Y. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3–E1 cells. Bone. 2005;37:359–369. doi: 10.1016/j.bone.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Kohno D., Gao H. Z., Muroya S., Kikuyama S., Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca(2+) signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Maccarinelli G., Sibilia V., Torsello A., Raimondo F., Pitto M., Giustina A., Netti C., Cocchi D. Ghrelin regulates proliferation and differentiation of osteoblastic cells. J. Endocrinol. 2005;184:249–256. doi: 10.1677/joe.1.05837. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G., Neri G., Rucinski M., Rebuffat P., Spinazzi R., Malendowicz L. K., Nussdorfer G. G. Ghrelin enhances the growth of cultured human adrenal zona glomerulosa cells by exerting MAPK-mediated proliferogenic and antiapoptotic effects. Peptides. 2004;25:1269–1277. doi: 10.1016/j.peptides.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Muccioli G., Papotti M., Locatelli V., Ghigo E., Deghenghi R. Binding of 125I-labelled ghrelin to membranes from human hypothalamus and pituitary gland. J. Endocrinol. Invest. 2001;24:RC7–RC9. doi: 10.1007/BF03343831. [DOI] [PubMed] [Google Scholar]
- Muccioli G., Pons N., Ghè C., Catapano F., Granata R., Ghigo E. Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type growth hormone secretagogue receptor. Eur. J. Pharmacol. 2004;498:27–35. doi: 10.1016/j.ejphar.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Nagaya N., Itoh T., Murakami S., Oya H., Uematsu M., Miyatake K., Kangawa K. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- Nagaya N., Moriya J., Yasumura Y., Uematsu M., Ono F., Shimizu W., Ueno K., Kitakaze M., Miyatake K., Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- Nagaya N, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- Nanzer A. M., Khalaf S., Mozid A. M., Fowkes R. C., Patel M. V., Burrin J. M., Grossman A. B., Korbonits M. Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur. J. Endocrinol. 2004;151:233–240. doi: 10.1530/eje.0.1510233. [DOI] [PubMed] [Google Scholar]
- Papotti M., Ghè C., Cassoni P., Catapano F., Deghenghi R., Ghigo E., Muccioli G. Growth hormone secretagogue. GHS binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 2000;85:3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Muccioli G., Granata R., Deghenghi R., Ghigo E., Ohlsson C., Isgaard J. Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J. Endocrinol. 2002;175:201–209. doi: 10.1677/joe.0.1750201. [DOI] [PubMed] [Google Scholar]
- Reimer M. K., Pacini G., Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Nagaya N., Isobe T., Imazu M., Okumura H., Hosoda H., Kojima M., Kangawa K., Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin. Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- Tesauro M., Schinzari F., Iantorno M., Rizza S., Melina D., Lauro D., Cardillo C. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation. 2005;112:2986–2992. doi: 10.1161/CIRCULATIONAHA.105.553883. [DOI] [PubMed] [Google Scholar]
- Volante M., Allia E., Fulcheri E., Cassoni P., Ghigo E., Muccioli G., Papotti M. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am. J. Pathol. 2003;162:645–654. doi: 10.1016/S0002-9440(10)63858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Perlman H. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Wu Z., Woodring P. J., Bhakta K. S., Tamura K., Wen F., Feramisco J. R., Karin M., Wang J. Y., Puri P. L. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q., Pang W., Pan H., Zheng Y., Kang J. S., Zhu S. G. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul. Pept. 2004;122:173–178. doi: 10.1016/j.regpep.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wong C., Liu D., Finegold M., Harper J. W., Elledge S. J. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Hu Y., Lin T. R., Fan Y., Mulholland M. W. Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides. 2005;26:2280–2288. doi: 10.1016/j.peptides.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhao L., Lin T. R., Chai B., Fan Y., Gantz I., Mulholland M. W. Inhibition of adipogenesis by ghrelin. Mol. Biol. Cell. 2004;15:2484–2491. doi: 10.1091/mbc.E03-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]