Abstract
The control of cell adhesion is an important mechanism by which Eph receptors regulate cell sorting during development. Activation of EphA4 in Xenopus blastulae induces a reversible, cell autonomous loss-of-adhesion and disruption of the blastocoel roof. We show this phenotype is rescued by Nckβ (Grb4) dependent on its interaction with EphA4. Xenopus p21Cdc42/Rac-activated kinase xPAK1 interacts with Nck, is activated in embryo by EphA4 in an Nck-dependent manner, and is required for EphA4-induced loss-of-adhesion. Ectopic expression of xPAK1 phenocopies EphA4 activation. This does not require the catalytic activity of xPAK1, but it does require its GTPase binding domain and is enhanced by membrane targeting. Indeed, membrane targeting of the GTPase binding domain (GBD) of xPAK1 alone is sufficient to phenocopy EphA4 loss-of-adhesion. Both EphA4 and the xPAK1-GBD down-regulate RhoA-GTP levels, and consistent with this, loss-of-adhesion can be rescued by activated Cdc42, Rac, and RhoA and can be epistatically induced by dominant-negative RhoA. Despite this, neither Cdc42 nor Rac activities are down-regulated by EphA4 activation or by the xPAK1-GBD. Together, the data suggest that EphA4 activation sequesters active Cdc42 and in this way down-regulates cell–cell adhesion. This novel signaling pathway suggests a mechanism for EphA4-guided migration.
INTRODUCTION
Development of the vertebrate body plan involves several stages during which embryonic cells must migrate to form new structures. The regulation of these migration events is complex, being controlled at the levels of cell–cell adhesion, cytoskeletal dynamics, intercellular signaling, and genetic reprogramming. However, the fundamental problem posed by cell migration is very simple: how does a cell find its way to its target site and recognize that site once there? In greater part, the answer seems to be by regulating cell adhesion (Steinberg, 1996; Tepass et al., 2002). The large family of Eph tyrosine kinase (TK) receptors and their membrane-bound ephrin ligands have been recognized as key regulators of cell migration, adhesion, and targeting in a broad range of tissues (for reviews, see Cowan and Henkemeyer, 2002; Kullander and Klein, 2002; Tepass et al., 2002; Murai and Pasquale, 2003; Pasquale, 2005). These receptor–ligand combinations seem to control cell migration and cell targeting predominantly by regulating cell adhesion. The paradigm for these receptors is the contact-mediated repulsion of Eph-expressing axon growth cones by ephrin-expressing cells. During this rapid repulsion event, the growth cone cytoskeleton is seen to collapse, linking Eph signaling directly with cytoskeletal dynamics. But Eph and ephrin signaling has also been widely implicated in the establishment or maintenance of tissue boundaries, especially during development, e.g., hindbrain segment boundaries, and in targeted cell migration, e.g., cranial neural crest migration. Ectopic expression of EphA4 or B2 and ephrin B2 in separate populations of zebrafish blastomeres abolishes cell mixing, hence simulating cell sorting (Mellitzer et al., 1999; Cooke et al., 2005). This sorting depends on bidirectional signaling, that is, the so-called forward signaling from the Eph receptor and reverse signaling from ephrin B ligands.
Many intracellular factors have been shown to interact with both the Eph receptors and ephrin ligands (for review, see Kullander and Klein, 2002; Murai and Pasquale, 2003; Huot, 2004; Poliakov et al., 2004). Quite strikingly, many of the factors have the potential either directly or indirectly to regulate the cytoskeleton via the Rho GTPases (Noren and Pasquale, 2004). The Rho family of GTPases, Cdc42, Rac, and Rho, have been shown to regulate, respectively, filopodia, lamellipodia/ruffles, and stress fiber formation in mammalian cell culture (for review, see Hall, 1998; Jaffe and Hall, 2005), and Rho activation is correlated with growth cone collapse (for review, see Luo, 2000). Ephexin1 (Eph-interacting exchange protein), a guanine nucleotide exchange factor (GEF) for Cdc42, Rac, and Rho, interacts through its tandem Dbl and pleckstrin homology domains with EphA4. Despite this interaction being constitutive, receptor activation seems to restrict Ephexin's GEF activity to Rho, causing an imbalance in GTPase activation and growth cone collapse (Shamah et al., 2001; Sahin et al., 2005). In neurons, intersectin, another GEF, interacts with the EphB2 receptor and in cooperation with the Wiskot–Aldrich Syndrome Protein (WASP) activates Cdc42 (Irie and Yamaguchi, 2002), whereas EphB signaling causes translocation of the GEF kalirin to synapses where it activates Rac and its effector PAK (Penzes et al., 2003). A group of proteins of unknown function including AF-6 bind the PDZ-homology domain of some Eph receptors (for review, see Kullander and Klein, 2002). However, the largest group of known Eph-interacting factors contains Src homology (SH)2 domains that interact with receptor phosphotyrosines. This group includes Abelson Abl/Arg and Src family tyrosine kinases and the near ubiquitous SH3–SH2 adapter Nck (for review, see Kullander and Klein, 2002; Murai and Pasquale, 2003). Nck has also been implicated in transducing signals from a range of other receptors, including epidermal growth factor (EGF) receptor (EGFR) and platelet-derived growth factor (PDGF) receptor, physically and functionally linking these receptors to the p21-activated kinase PAK1 (Bokoch et al., 1996; Galisteo et al., 1996; Lu et al., 1997). PAK1 is activated not only by interaction with GTP-bound Cdc42 and Rac but also by a GTPase-independent interaction with filamin (Vadlamudi et al., 2002), and it has been implicated in regulating the actin cytoskeleton, in activating myosin II, and in mitogen-activated protein-kinase signaling (Bisson et al., 2003; Bokoch, 2003; Maruta et al., 2003). The SH3 domains of Nck have also been shown to regulate the nucleation of actin polymerization via WASP-family verprolin homologous protein in conjunction with activated Rac GTPase (Eden et al., 2002) and via WASP (Rivera et al., 2004).
Despite the long list of proteins shown to interact with the Eph receptors, there is still little understanding of the biological importance of these interactions and in particular of the mechanisms by which these receptors regulate cell migration, adhesion and repulsion. Studies have been hampered by the lack of relatively simple model systems in which Eph receptor activity can be modulated and the physiological effects precisely defined. However, Xenopus embryos express several Eph receptors and ephrin ligands, and these receptors and ligands have been shown to be important for development (Winning and Sargent, 1994; Jones et al., 1995; Scales et al., 1995; Tanaka et al., 2003; Lee et al., 2006). Activation of EphA4 in early Xenopus embryos induces a loss of blastomere adhesion (loss-of-adhesion), which provides a unique, simple and reliable physiological readout of receptor activity (see Figure 1A; Winning et al., 1996, 2001, 2002). The normal epithelial structure of the blastocoel roof is disrupted in these embryos, leading to an occlusion of the blastocoel. The loss-of-cell adhesion phenotype in Xenopus was shown to require catalytic receptor activity and to be independent of the Ras–GTPase pathway. The phenotype is suppressed by ectopic expression of C-cadherin, the major adhesion receptor in early embryos and can be fully reversed by transferring embryos to lower ionic strength medium, which enhances adhesion through adherens junctions. Thus, the EphA4 phenotype is probably due in greater part to a down-regulation of normal cell–cell adhesion via adherens junctions. More recently, this mutant phenotype was shown to be associated with a loss of microvilli and of apical/basolateral polarity (Winning et al., 2001) and to be rescued by activated RhoA (Winning et al., 2002). This suggested that EphA4 activation suppressed Rho activity, but no signaling pathway was identified. Here, we delineate a novel signaling pathway from EphA4 that passes via Nckβ (Grb4) and xPAK1, leading to loss-of-blastomere adhesion. We further show that by recruiting xPAK1 to the plasma membrane, EphA4 activation does not suppress active Cdc42 but must rather sequester or mask it. This positions xPAK1 as an upstream modulator of active Cdc42, rather than a downstream effector.
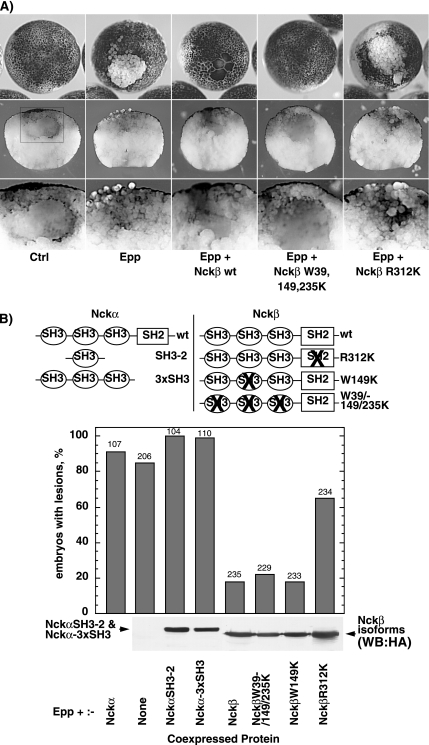
Figure 1.
Nckβ overexpression rescues the Epp loss-of-adhesion phenotype. (A) Example of a lesion caused by loss of animal pole blastomere adhesion resulting from the expression of Epp. Examples of rescue of this phenotype by the coexpression of Nckβ forms are also shown. The middle panels show corresponding manually cross-sectioned embryos, and the boxed regions are shown at higher magnification in the bottom panels. (B) The structure of Nckα and Nckβ and their mutant forms is shown above a histogram of the percentage of embryos displaying loss-of-adhesion lesions. Scoring was on the basis of visible lesions; no correction for lesion size was made. The ratio of Epp to Nck RNA injected was maintained at 1-4. The numbers above the histogram columns refer to the total number of embryos scored. The bottom panel shows the relative Nckβ and Nckα SH3-2 and 3xSH3 expression levels determined by Western analysis using an antibody against the HA epitope tag. Nckα wild type (wt) was not tagged and hence could not be detected in this way.
MATERIALS AND METHODS
Plasmid Constructions
The Xenopus EphA4 receptor, the chimeric receptor Epp, kinase dead EppK (K652A), the human EGF receptor, and the mouse Ephexin constructions were kindly provided by T. Sargent (National Institutes of Health, Bethesda, MD) and J. Scales (Department of Biology, University of Wisconsin Eau-Claire, Eau-Claire, WI) (Winning et al., 1996). Point mutations in Epp were introduced by polymerase chain reaction (PCR) by using mutated primers. The cDNA encoding full-length xPAK1 (amino acids 1-527) or ΔN159, an xPAK1 mutant in which the N-terminal control region (amino acids 1-159) had been deleted, were subcloned into pT7TS (provided by P. Krieg, Department of Cell Biology and Anatomy, University of Arizona, Tuscon, AZ) in-frame with an HA1-epitope (Wilson et al., 1984) as described previously (Bisson et al., 2003). Lysine 281, flanking the ATP binding domain, was mutated to alanine to inactivate the catalytic domain, producing kinase dead (KD) forms of both the full-length and ΔN159-xPAK1 (Bisson et al., 2003). Point mutations in KD-xPAK1 were introduced by PCR by the use of mutated primers (see Figure 5A), and deletion of amino acids 66-77 was accomplished by removing a BsaAI to EcoRV fragment and reclosing pT7TSxPAK1 cDNA by blunt end ligation. GST-PAK-GBD 61-85 (amino acids) and GST-PAK-GBD 61-123 were constructed by amplifying xPAK1 and subcloning at BamHI and XhoI sites in pGEX-4T3. PAK-GBD 61-85 and PAK-GBD 61-123 constructs were made by amplifying glutathione S-transferase (GST)-GTPase binding domain (GBD) in the constructs mentioned above and subcloning in pT7TS-HA at EcoRI and SpeI sites. The cDNA segments encoding the second SH3 domain (a.a. 61-195), the three SH3 (a.a. 1-274), and the full length (a.a. 1-377) of the Nckα were PCR amplified from the construct pRK5-Nck (Galisteo et al., 1996) and subcloned EcoRI/SpeI in pT7TS-HA. HA-Nckβ wt and mutants (Chen et al., 2000) were recovered from the pRK5 vector by digestion with XbaI and subcloned in pT7TS at the SpeI site. Cdc42, Rac1, and RhoA GTPases constructs were provided by N. Lamarche-Vane. All constructions were then validated by DNA sequencing.
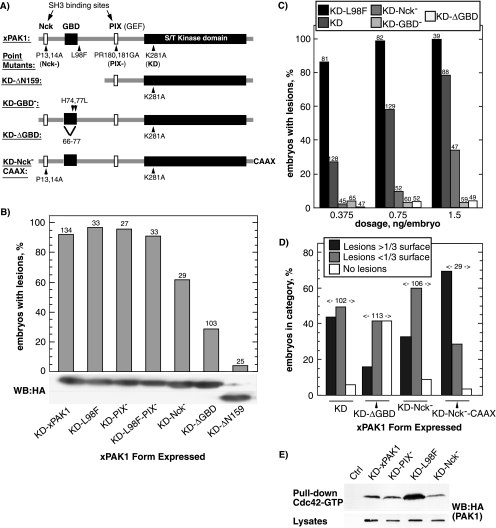
Figure 5.
The xPAK1 loss-of-adhesion phenotype requires the GBD and is enhanced by the Nck binding site and by CAAX targeted membrane recruitment. (A) The structure of the xPAK1 mutants used. Amino acid mutations and the extent of deletion mutations are indicated as is the C-terminal CAAX extension. (B) Each xPAK1 mutant was injected and loss-of-adhesion lesions were scored. RNA injections were adjusted to give a high penetrance of the KD-xPAK1–induced phenotype, and this same amount of each mutant RNA was then injected. Bottom panel shows relative protein expression levels as determined by Western analysis by using an antibody to the HA epitope-tag. Two regions of the same Western analysis are shown. (C) Dose–response relationship for increasing amounts of injected KD-L98F, KD−, KD-Nck−, KD-GBD−, and KD-ΔGBD-xPAK1 RNAs. Scoring was regardless of lesion size. (D) Comparison of lesion size. Equal RNA amounts of each mutant were injected, and the sizes of loss-of-adhesion lesions were categorized relative to the total animal pole surface. The numbers above the histogram columns refer to the total number of embryos scored. (E) KD-L98F xPAK1 displays an increased affinity for GTP-Cdc42. HA-tagged xPAK1 constructs were expressed in embryo, whole extracts applied to Sepharose-bound GST-Cdc42 fusion protein precharged with GTP, and revealed by Western blotting by using the HA epitope tag.
Embryo Injections and Manipulations
Capped RNA was transcribed from the linearized plasmids by using T7 or SP6 polymerase mMESSAGE-mMACHINE kits (Ambion, Austin, TX), and 1-4 nl of RNA in water was injected into one blastomere of two- to four-cell stage Xenopus embryos. We injected 0.2–0.5 ng of EphA4 and related RNAs per embryo, and 0.5–1.5 ng per embryo of xPAK1-related constructs, except GBD constructs, for which 0.07–0.4 ng per embryo was injected, 0.1–0.5 ng of GTPase constructs, and 0.5–1.5 ng of all other RNAs. For antibody coinjections, affinity-purified anti-xPAK1 antibody raised against a.a. 1-64 of xPAK1 as described previously (Bisson et al., 2003) or anti-TAF1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was dialyzed against phosphate-buffered saline (PBS), concentrations were determined by spectrophotometry, and 10 pg was injected in two-cell stage embryos. In the same embryos 0.7 ng of Epp mRNA was injected at the 4-cell stage, in the same side. Due to variations in the sensitivity of embryos from different females to the induction of loss of blastomere adhesion, adjustments were made to the amounts of RNA injected for each source of embryos. The penetrance of the loss-of-adhesion phenotype was also adjusted in the same way to achieve an appropriate range of effect for the experimental constructs studied. As appropriate, experimental results were normalized to reference constructs Epp or xPAK1 on each given batch of embryos before data from repeat experiments were combined (all experiments were repeated several times on different embryo batches). For coinjections, the ratio of Eph-related to coinjected RNA was maintained at 1-4 unless mentioned otherwise, and RNAs were injected as a single solution. For cell adhesion assays, embryos were incubated at 19°C in 1× MMR (0.1M NaCl, 2 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM HEPES, and 0.1 mM EDTA, pH 7.8), whereas for xPAK1 activation assays embryos were incubated in 0.1× MMR to inhibit the loss-of-adhesion phenotype.
Embryos were observed at several time points after injection by using a Leica MZFLIII fluorescence binocular microscope, and images were recorded using a Dage 3CCD camera connected to a CG7 (Scion, Frederick, MD) image grabber board. Embryos were immediately fixed in 3.7% formaldehyde in 0.1× MMR for 1 h or more before being manually sectioned.
Phalloidin Staining and Confocal Microscopy Imaging
Animal caps were removed from loss-of-adhesion or control blastula embryos in 1× MMR and were fixed for 30 min in 3.7% formaldehyde, 0.25% glutaraldehyde, 0.1% Triton X-100 in PBS. Caps were washed twice, stained for 2 h in 0.005 U per microliter of Alexa Fluor 488 phalloidin (Invitrogen, Carlsbad, CA), and washed again twice. Caps were mounted in PBS and visualized by confocal microscopy by using an MRC 1024 (Bio-Rad, Hercules, CA) coupled to a TE-200 inverted microscope (Nikon, Tokyo, Japan).
Coimmunoprecipitation
For Epp–Nckβ binding, 293T kidney cells were transfected with 5 μg of Epp-FLAG and 5 μg of HA-Nckβ or pCDNA3 empty vector by using a standard CaPO4/chloroquine procedure as described previously (Poitras et al., 2003b). Cells were starved for 16 h in serum-free medium and induced for 5 min with 50 ng/ml human EGF (Invitrogen) before being lysed as described below. For PAK1–Nckβ binding, 293T kidney cells were transfected with 5 μg of HA-Nckβ and 5 μg of PAK-FLAG or pCDNA3 empty vector. After 24 h, cells were lysed with 300 μl (for 60-mm dishes) of ice-cold lysis buffer (25 mM Tris, pH 7.5, 1 mM EDTA, 0.1 mM EGTA, 5 mM MgCl2, 1 mM dithiothreitol [DTT], 150 mM NaCl, 10% glycerol, 1% IGEPAL, 2 mM NaVO4, 50 IU/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 2 μg/ml leupeptin) and left 15 min on ice. Extracts were cleared at 15 000 rpm for 10 min at 4°C. Lysates were incubated with 10–20 μl of anti-FLAG M2 affinity gel (Sigma Diagnostics Canada, Mississauga, Ontario, Canada) for 2 h at 4°C on a stir plate. Beads were washed three times with 1 ml of lysis buffer and once with lysis buffer without detergent. Precipitates were resolved on a 7 or 10% SDS-PAG, and the immunoprecipitated proteins were detected with an anti-hemagglutinin (HA) antibody (12CA5) or anti-FLAG antibody (Sigma Diagnostics Canada) and developed using a horseradish peroxidase-coupled second antibody in conjunction with the ECL-Plus reagent kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
xPAK1 Kinase Assay and Embryo Extracts
Embryos were injected with the indicated mixture of in vitro-transcribed capped messages (0.35 ng of EphA4 or EGFR, 0.7 ng of xPAK1 or KD-xPAK1, and 0.7 ng of Nck or green fluorescent protein [GFP] RNAs, the last to maintain a constant amount of RNA injected) to express the appropriate proteins. They were then incubated for 6–8 h in 0.1× MMR. XPAK1 was recovered by immunoprecipitation via its N-terminal HA-1 epitope tag for use in kinase assays as follows. Total protein extracts from injected and uninjected embryos were first prepared by homogenizing embryos (20 embryos/100 μl) in MGE (20 mM 4-morpholinepropanesulfonic acid, pH 7.0, 10% glycerol, 0.5 mM EDTA, 5 mM EGTA, 1 mM NaVO4, 5 mM tetrasodium diphosphate decahydrate, 50 mM NaF, 80 mM β-glycerophosphate, 1% Triton X-100, 1 mM benzamidine, 1 mM DTT, and 1 mM PMSF), by repeated rapid aspiration with a micropipette, and they were then centrifuged at 120,000 × g for 15 min. The clear supernatant was collected and rapidly frozen and stored at −80°C. For each kinase assay, 25 μl of this embryo supernatant was diluted to 60 μl with MIKI (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 1 mM EGTA, 1 mM MgCl2, 1 mM NaVO4, 1% Triton X-100, and 1 mM PMSF), 5 μl of anti-HA-1 ascites (12CA5) was added, and after 60 min incubation on ice, 10 μl of a 50% protein A-Sepharose slurry (GE Healthcare) in MIKI was added, and the incubation was continued for another 30 min. The slurry was then washed three times in 500 μl of MIKI, resuspended in 1 volume (20 μl) of D2X (50 mM HEPES, pH 7.4, 50 mM β-glycerol-phosphate, 50 mM MgCl2, 0.2 mM NaVO4, 4 mM DTT, and 75 μM [γ-32P]ATP [5000 dpm/pmol]) containing 50 ng of myelin basic protein (Invitrogen), and incubated for 30 min at 30°C. After electrophoresis on 12% polyacrylamide-SDS, relative substrate incorporation was quantified on a STORM 860 PhosphorImager (GE Healthcare).
Interaction of xPAK1 Mutants with Cdc42
To determine relative affinities of xPAK mutants for Cdc42-GTP (Figure 5E), 2 μg of immobilized GST-Cdc42 (kindly provided by A. Hall, MRC Laboratory for Molecular Cell Biology, University College London, London, UK) was incubated with 100 μM GTP in GTPase exchange buffer (50 mM Tris, pH 7.5, 5 mM EDTA, and 0.5 mg/ml bovine serum albumin [BSA]) for 10 min at 30°C, and then the MgCl2 concentration was adjusted to 10 mM. The GST-Cdc42-GTP was incubated for 1 h at 4°C with total protein extracts of X. laevis embryos expressing the xPAK1 constructs. The immobilized protein complexes were washed three times with 500 μl of PBS (145 mM NaCl, 10 mM Na-phosphate buffer, pH 7.4) and three times with Tris-buffered saline (10 mM Tris-HCl, pH 7.4, and 150 mM NaCl). Bound xPAK1 was visualized by Western blot by using a xPAK1 polyclonal antibody (Bisson et al., 2003).
GTPase Binding Assay
[35S]methionine-labeled Rac1 or Cdc42 were prepared by coupled in vitro transcription/translation in rabbit reticulocytes lysates (Promega, Madison, WI), and 100 μl was purified through G-25 Sephadex in exchange buffer (50 mM Tris, pH 7.4, 5 mM EDTA, 100 mM NaCl, and 0.5 mg/ml BSA) to remove the excess of GTP/GDP. GDP or GTP (100 μM final; Sigma Diagnostics Canada) was added, and extracts were incubated at 30°C for 30 min. MgCl2 was then added to 10 mM on ice. Sepharose-immobilized GST-PAK-GBD 61-85, GST-PAK-GBD 61-123, or GST was incubated with 300 μl of Rac1-GTP/GDP or Cdc42-GTP/GDP for 60 min at 4°C, and the immobilized protein complexes were washed five times with 500 μl of washing buffer (50 mM Tris, pH 7.4, 5 mM EDTA, 100 mM NaCl, 10 mM MgCl2, and 100 μM GTP or GDP). Sepharose beads were resuspended in SDS-PAGE loading buffer, and proteins were fractionated on 12% SDS gels. Gels were fixed, dried, and analyzed by phosphorimaging (Storm 860; GE Healthcare).
GTPase Activity Assay
Xenopus embryos were injected with Epp/EppK, or xPAK1-GBD 61-123/61-85 mRNA with 5–40 pg of HA-tagged Cdc42, Rac1, or RhoA mRNA to determine GTP-bound GTPases levels specifically in the affected region of the embryo. GTPase expression levels were adjusted such that they did not affect the loss-of-adhesion phenotype in the injected embryos. Binding assays were performed as described previously (Benard et al., 1999; Habas et al., 2003). Samples were resolved on 12% SDS-PAGE and immunoblotted with an anti-HA (12CA5) antibody. Active HA-GTPase-GTP levels were normalized to the total expression level of HA-GTPase. The mean of two to four experiments is shown.
RESULTS
The Xenopus EphA4 loss of blastomere adhesion phenotype is unique in providing a simple, direct, and easily accessible in vivo model in which to study signaling downstream of the EphA4 receptor. To define the pathway by which EphA4 regulates cell adhesion in early Xenopus embryos, we first sought to block this phenotype by expressing dominant-negative forms of potential downstream effectors. The chimeric receptor Epp (Winning et al., 1996), consisting of the extracellular domain of the EGFR and the intracellular domain of EphA4, has the great advantage of being unable to reverse signal to adjacent ephrin-expressing cells (Cowan and Henkemeyer, 2002). As with the wild-type EphA4 receptor, overexpression of Epp (activated EphA4) in Xenopus embryos is sufficient to induce receptor autoactivation, leading to loss of cell adhesion (Winning et al., 1996) (Figure 1A). This approach, thus, avoids cell nonautonomous effects.
EphA4-induced Loss-of-Adhesion Is Specifically Blocked by Nckβ
In mammals, the EphB1 receptor has been shown to bind the SH3–SH2 adapter protein Nckα (Holland et al., 1997; Stein et al., 1998; Becker et al., 2000), suggesting that other Eph receptors might also signal via adapters of the Nck family. Overexpression of the wild-type Nck has been observed to have a dominant-negative function in other systems. For example, in Drosophila and in mammalian tissue culture, overexpression of wild-type Dock (DNck) or Nckβ has been found to block receptor signaling (Rao and Zipursky, 1998; Chen et al., 2000). However, when Nckα or its truncation mutants were ectopically expressed in Xenopus embryos, they did not rescue of the Epp-induced loss-of-adhesion phenotype (Figure 1B). In contrast, coexpression of wild-type Nckβ with Epp gave almost completely rescue (Figure 1, A and B). Neither Nckα nor Nckβ induced a loss-of-adhesion when expressed alone (data not shown). Inactivation of the Nckβ SH2 domain by point mutation prevented rescue of Epp loss-of-adhesion (Figure 1, A and B). In contrast, inactivation of the SH3 domains did not affect rescue. Because the SH2 domain of Nckβ was required to rescue the Epp phenotype, this rescue probably occurred via a blockade of one or more phosphotyrosines on the activated receptor.
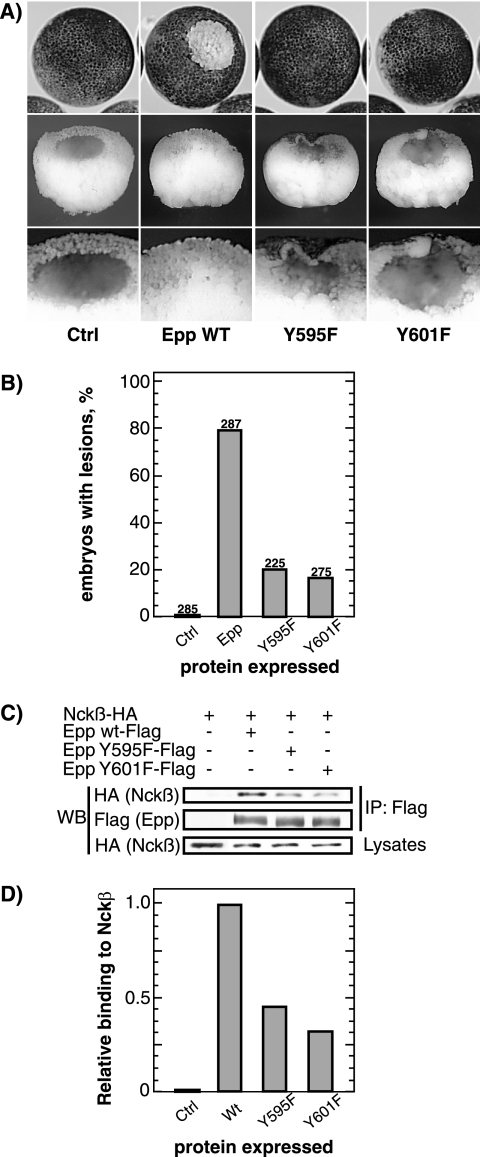
Although the Ncks have been shown to directly interact with numerous tyrosine kinase receptors, including EphB1 (Holland et al., 1997; Stein et al., 1998; Becker et al., 2000), an interaction between EphA4 and Nckβ has never been demonstrated. When Epp and Nckβ were coexpressed, Nckβ was found to coimmunoprecipitate with the receptor (Figure 2, C and D). Detailed protein sequence analysis of EphA and EphB receptors led us to identify two conserved tyrosine residues within the juxtamembrane domain of Xenopus EphA4 at positions 595 and 601. Independent mutation of these residues to phenylalanine in each case rescued the loss-of-adhesion phenotype to a significant degree (Figure 2, A and B). These mutations also significantly reduced the interaction of Nckβ with activated EphA4 (Epp) (Figure 2, C and D).
Figure 2.
Tyrosines 595 and 601 on EphA4 are essential for the loss-of-adhesion phenotype and for Nckβ interaction. (A) Example of a lesion caused by loss of animal pole blastomere adhesion resulting from the expression of Epp and block of this phenotype by independent mutagenesis of two conserved tyrosines in the juxtamembrane domain. (B) Histogram of the percentage of embryos displaying loss-of-adhesion lesions. Scoring was on the basis of visible lesions, no correction for lesion size was made. The same amount of RNA was injected for wild-type (wt) or mutant Epp. The numbers above the histogram columns refer to the total number of embryos scored over three independent experiments. (C) Interaction of xEpp with Nckβ. HA-Nckβ and xEpp-FLAG (wt or mutants) were transfected in 293T cells, and total protein extracts were immunoprecipitated with anti-FLAG antibody, Western blotted, and probed with anti-HA antibody. (D) The data in C were quantified and are shown normalized to the wt Epp–Nckβ interaction levels.
EphA4 Activates xPAK1 in a Nck-dependent Manner In Embryo
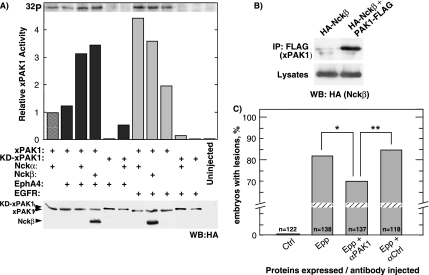
The above-mentioned experiments suggested that Nckβ provided an essential link in the pathway by which EphA4 regulates blastomere adhesion. PAK1, an important regulator of cell motility, was previously shown to be recruited to activated PDGF and EGF receptors via Nckα, leading to its catalytic activation (Bokoch et al., 1996; Galisteo et al., 1996; Lu et al., 1997). We therefore asked whether Xenopus PAK1 (Faure et al., 1997; Islam et al., 2000; Bisson et al., 2003; Poitras et al., 2003b) might also be an intracellular target of EphA4 signaling. Only when either Nckα or Nckβ were coexpressed with EphA4 and xPAK1 was a significant increase in xPAK1 activity observed (Figure 3A). Consistent with previous data (Galisteo et al., 1996), a Nck-dependent increase in xPAK1 activity was also observed when it was coexpressed with EGFR (Figure 3A). As expected, when wild-type xPAK1 was replaced by its kinase dead (KD-xPAK1) form (K281A), no significant xPAK1 activity was detected, whether in the presence or absence of ectopic Nck. The small degree of EGFR-dependent activation of xPAK1 observed in the absence of exogenous Nck could be explained by endogenous Nck, both Nckα and β being maternal products (Tanaka et al., 1997; Gupta and Mayer, 1998; Jean, unpublished data). Although mammalian Nckα has been shown to directly interact with PAK (Bokoch et al., 1996; Galisteo et al., 1996; Lu et al., 1997), an interaction between Nckβ and PAK has not yet been demonstrated. When HA-Nckβ and xPAK1-FLAG were coexpressed, Nckβ was found to coprecipitate with xPAK1 (Figure 3B).
Figure 3.
xPAK1 is activated by EphA4 in embryo. (A) Assay of xPAK1 kinase activity in extracts from embryos coexpressing xPAK1 or a catalytically inactive xPAK1 (KD-xPAK1), the receptors EphA4 or hEGFR and the two Nck isoforms. Relative xPAK1 and Nckβ expression were determined by Western analysis by using an antibody to the HA epitope-tag. Nckα was not epitope tagged and so was not detected in this assay. (B) Interaction of xPAK1 with Nckβ. HA-Nckβ and xPAK1-FLAG were transfected in 293T cells, and total protein extracts were immunoprecipitated with anti-FLAG antibody, Western blotted, and probed with anti-HA antibody. (C) xPAK1 is required for loss-of-adhesion. Histogram showing the percentage of embryos displaying loss-of-adhesion lesions when injected with Epp alone or when coinjected with an affinity-purified anti-xPAK1 (αPAK1) antibody or a control antibody (αCtrl). Scoring was on the basis of visible lesions, no correction for lesion size was made. Statistical analysis showed that differences were significant; *p = 0.0199 and **p = 0.0106.
We next asked whether xPAK1 was required downstream of EphA4 for the loss-of-adhesion phenotype. xPAK1 is an abundant maternal protein and hence its activity in embryo is not easily manipulated. We, therefore, attempted to attenuate this activity by preinjecting anti-xPAK1 antibody. Affinity purified antibodies were injected into embryos at the two-cell stage, and the corresponding blastomeres were subsequently injected with mRNA encoding activated EphA4 (Epp) at the four-cell stage. Embryos injected with the anti-xPAK1 antibody showed a statistically significant decrease in the penetrance of the loss-of-adhesion phenotype compared with embryos injected only with Epp RNA or with Epp RNA and an unrelated control antibody (Figure 3C). These data strongly suggest that xPAK1 function is required downstream of activated EphA4 in the loss-of-adhesion pathway.
xPAK1 Induces Loss of Blastomere Adhesion Independently of Its Kinase Activity
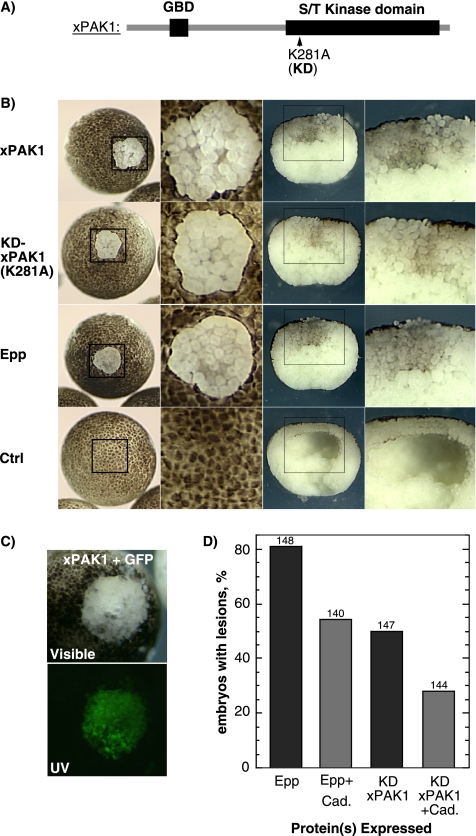
Because xPAK1 was a potential target of EphA4 signaling, we investigated its effects on loss-of-adhesion. As shown in Figures 1 and 2, activated EphA4 (Epp) causes a disruption of superficial and deep animal pole cell layers (the animal cap) and a characteristic absence of the blastocoel (Winning et al., 1996). Ectopic expression of xPAK1 phenocopied this disruption of the animal cap (Figure 4B). Coinjection of GFP showed that the effect was cell autonomous, being limited to the xPAK1 expressing blastomeres (Figure 4C). Surprisingly, kinase dead KD-xPAK1 induced loss-of-adhesion as effectively as the wild type (Figure 4B). A range of other proteins, including the Ncks, catalytically inactive Epp (Epp-K) (Winning et al., 1996), dominant-negative (kinase domain-deleted) EphA4 (DN-Eph) (Smith et al., 1997), xMLK2 (MAP3K10) (a xPAK1 target) (Poitras et al., 2003a, b), the EGFR ligand transforming growth factor-α (Winning et al., 1996), and unrelated proteins such as GFP were all unable to induce loss-of-adhesion (data not shown).
Figure 4.
xPAK1 induces the loss-of-adhesion phenotype that can be suppressed by C-cadherin. (A) xPAK1 domain structure indicating the ATP binding site mutation used to inactivate the kinase domain. (B) Left panel shows examples of control embryos and embryos displaying loss of blastomere adhesion induced by xPAK1 or the KD mutant in comparison with the activated EphA4 (Epp) phenotype. The corresponding boxed regions are shown at higher magnification in the middle-left panels. The middle-right panels show manually cross-sectioned embryos, and the corresponding boxed regions are shown at higher magnification in the right panels. (C) Coexpression of xPAK1 with GFP shows that the effect of xPAK1 is restricted to the expressing cells. Visible and UV refer to incident and fluorescent light images, respectively. (D) Epp and KD-xPAK1 RNAs were coinjected with or without C-cadherin RNA, and loss-of-adhesion lesions was scored. The ratio of Epp or xPAK1 to cadherin RNA was maintained at 1-4. Scoring was on the basis of visible lesions; no correction for lesion size was made. The numbers above the histogram columns refer to the total number of embryos scored.
Ectopic expression of activated EphA4 may weaken blastomere adhesion by affecting the strength of adherens junctions, because Epp loss-of-adhesion can be counteracted by coexpression of C-cadherin. Furthermore, it is rescued within minutes when embryos are transferred to low salt medium (Sargent, personal communication; our unpublished data). These characteristics also held true for xPAK1. C-cadherin was found to suppress the KD-xPAK1–induced loss-of-adhesion (Figure 4D), and disrupted embryos were rapidly rescued on transfer to low salt medium (0.1× MMR; data not shown). Thus, as judged by developmental and biochemical criteria, both wild-type and kinase dead xPAK1 were able to fully phenocopy the EphA4 loss-of-adhesion phenotype.
Membrane Recruitment of xPAK1 Enhances Loss-of-Adhesion
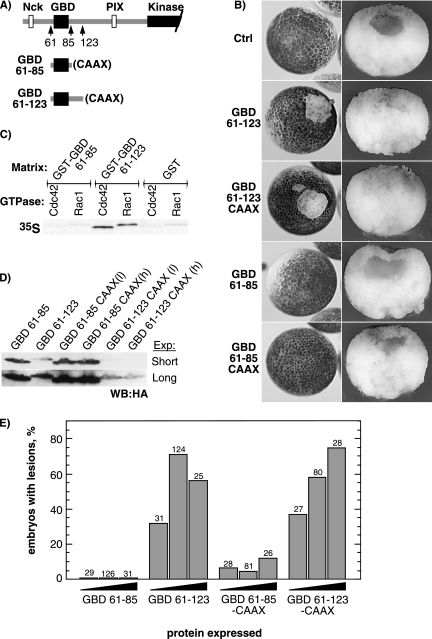
Deletion of the N-terminal control region of xPAK1 (ΔN159) completely abrogated the loss-of-adhesion phenotype (Figure 5, A and B). To further determine which protein domains were required, they were each separately inactivated. It should be noted that due to the inhibitory function of the N-terminal control region, its mutation generally leads to xPAK1 activation, resulting in blastomere fragmentation and necrosis (Bisson et al., 2003). Hence, all mutants were created in the KD mutant background.
Mutation of the Nck interaction site of xPAK1 (P13/14A) (Galisteo et al., 1996) significantly reduced the loss-of-adhesion phenotype KD-Nck− (Figure 5, B and C), and this effect was evident over a range of RNA injections (Figure 5C). It was yet further evident when the relative sizes of loss-of-adhesion lesions were scored, the KD-Nck− mutation clearly causing a reduction in lesion size compared with the control KD-xPAK1 (Figure 5D). To determine whether constitutive membrane recruitment would rescue the full activity of the KD-Nck− mutant, it was targeted to the plasma membrane by using the CAAX motif (CCIF) from hCdc42 (G25K). As can be seen in Figure 5D, both the number of embryos exhibiting loss-of-adhesion lesions and lesion size was increased by addition of the C-terminal CAAX motif. Indeed, the average lesion size induced by KD-Nck−-CAAX mutant surpassed that of the KD-xPAK1 control, consistent with constitutive membrane targeting rather than targeting being limited to the availability of activated receptors.
Loss of Cell Adhesion Induced by xPAK1 Requires Its GTPase Binding Domain
PAK1 is generally considered to be a downstream effector of Cdc42 or of Rac (see Introduction). However, our data showed that the catalytic activity of xPAK1 was not required to phenocopy EphA4 activation, excluding a role for kinase-dependent downstream signaling. Under certain circumstances, PAK1 seems to function upstream rather than downstream of Cdc42/Rac (Obermeier et al., 1998) and can mediate the activation of Cdc42/Rac by recruiting the GEF PIX/COOL (Bagrodia et al., 1998; Manser et al., 1998). PAK1 also represses the GTPase activity of Cdc42 and Rac, significantly extending the half-life of the GTP-bound form (Manser et al., 1994). Thus, overexpression and membrane targeting of KD-xPAK1 could enhance Cdc42 and Rac activity. Alternatively, it might sequester or mask the active GTPase and hence compete with other interactors.
The L98F mutation in the GBD (also known as the CRIB domain) of xPAK1 renders its activation independent of GTPase activity (Brown et al., 1996; Bisson et al., 2003). However, it also strongly augments the affinity of xPAK1 for Cdc42 (Figure 5E). In the context of KD-xPAK1, this mutation did not alleviate, and indeed significantly exacerbated, loss-of-adhesion (Figure 5, B and C). Mutation of the putative binding site for PIX/COOL family of GEFs (PR180/181GA) (Bagrodia et al., 1998; Manser et al., 1998) had no effect on loss-of-adhesion, either alone or when combined with the L98F mutation (see PIX− and L98F-PIX−; Figure 5B). In contrast, point mutation (H74,77L) or deletion of the minimal GBD of xPAK1 (ΔGBD) very strongly suppressed loss-of-adhesion (Figure 5, B and C). Together, these data suggested that the loss-of-adhesion phenotype did not depend on the activity of the GEF PIX and hence did not rely on GTPase activation, but it did depend on the ability of xPAK1 to interact with Cdc42.
The GBD of xPAK1 Is Sufficient to Phenocopy EphA4 Activation
To identify the minimal region of xPAK1 required to induce loss of cell adhesion, two polypeptides, one polypeptide containing the minimal the GBD (Thompson et al., 1998), amino acids 61-85 (GBD 61-85), and the other polypeptide a slightly larger region, amino acids 61-123 (GBD 61-123), were expressed in embryo (Figure 6). Only the longer GBD construct (GBD 61-123) induced loss-of-adhesion (Figure 6, B and E), despite the fact that it was expressed at a significantly lower level than GBD 61-85 (Figure 6D). The phenotype induced by GBD 61-123 was indistinguishable from that of xPAK1 or activated EphA4, and it displayed the characteristic lack of a blastocoel (Figure 6B). When the relative affinities of the two GBD constructs for Rac and Cdc42 were analyzed by “pull-down” (Figure 6C), GBD 61-123 bound both GTP-loaded Cdc42 and -Rac1, whereas GBD 61-85 did not detectably bind either GTPase. This is fully consistent with the mapping of the GBD in human PAK and with the binary structure of Cdc42 complexed with PAK1, which reveals significant contacts at least as far C-terminally as a.a. 107 (a.a. 98 of xPAK1) (Morreale et al., 2000). Thus, the region of xPAK1 required for loss-of-adhesion corresponded closely with its functional GBD. CAAX membrane targeting of the GBD construct did not at first seem to enhance penetrance of the phenotype, although the shorter GBD construct (GBD 61-85) did begin to show a mild internal disorganization of animal cap cells and a small number of lesions (Figure 6, B and E). However, the expression levels of GBD 61-123 CAAX were found to be very significantly below those of the original GBD 61-123 (Figure 6D). Despite the large difference in expression levels, the dose–response curves for these two forms were very similar (Figure 6E). Thus, membrane targeting very significantly enhanced the specific activity of GBD 61-123.
Figure 6.
The functional GBD of xPAK1 is sufficient to induce the loss-of-adhesion phenotype, but its function is enhanced by membrane targeting. (A) The structure of the GBD constructs (xPAK1 a.a. 61-85 and a.a. 61-123). (B) Phenotype of the GBD-induced lesions, left panels show external animal pole views, and right panels show sections through the lesions. (C) Binding of in vitro-translated GTP-charged wild type Cdc42 and Rac1 GTPases to the GST-immobilized xPAK1 GBDs 61-85 and 61-123. (D) Western analysis of expression levels of the GBD constructs using an antibody to the common HA epitope-tag (h embryos in 1× MMR and l embryos in 0.1× MMR). Two exposures of the same analysis are shown to increase the visible dynamic range. (E) Statistical analysis of lesion penetrance. Embryos were injected in parallel with 70, 200, or 400 pg of GBD 61-123 and GBD 61-123-CAAX RNAs. Embryos displaying obvious lesions, irrespective of size, were scored. Total number of embryos analyzed is shown above each column. In B and D, each embryo was injected with 200 pg of mutant RNA.
Activated Cdc42, Rac, and Rho All Rescue Loss-of-Adhesion
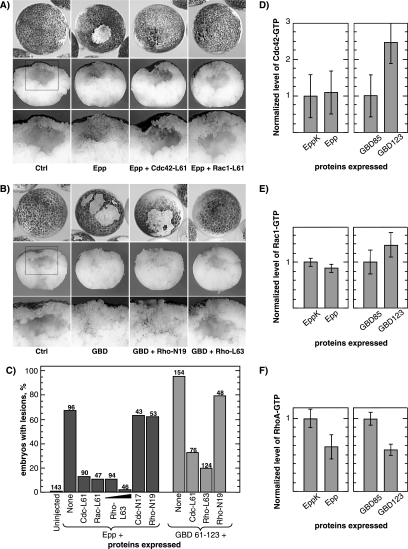
It was reported previously that activated RhoA (V14) could rescue loss-of-adhesion induced by the activated EphA4 receptor Epp (Winning et al., 2002) (we have also shown that this is the case for the activated RhoA L63 mutant; see Supplemental Figure S1). Cdc42, Rac, and Rho are known to be linked in a signaling cascade (Mackay and Hall, 1998). Thus, although RhoA does not interact directly with xPAK1 (Faure et al., 1997; Islam, unpublished data), its activity could be regulated by changes in the levels of GTP-bound Cdc42 or Rac. Consistent with this, we found that both activated Cdc42 (L61) and Rac1 (L61) were also able to rescue Epp-induced loss-of-adhesion, the blastocoel being fully reestablished (Figure 7, A and C). We further found that activated RhoA (L63) was able to rescue the loss-of-adhesion phenotype induced by the xPAK1-GBD, again with the blastocoel being restored (Figure 7, B and C). Ephexin1, a Dbl family GEF for Rho, Cdc42, and Rac (Shamah et al., 2001; Sahin et al., 2005) that was previously shown to rescue the Epp phenotype (Winning et al., 2002), also rescued the xPAK-GBD–induced phenotype (Supplemental Figure S2). Thus, loss-of adhesion induced by Epp was again at this level exactly phenocopied by the xPAK1-GBD and seemed to be due to a down-regulation of GTPase activity.
Figure 7.
Activated Cdc42, Rac1, and RhoA rescue EphA4 induced loss-of-adhesion. (A) Epp RNA was coinjected with activated Cdc42 or Rac1 GTPases RNA (ratio 1:3) at the two-cell stage. Lower panels show corresponding sections of embryos in top panels, and corresponding boxed regions are shown at higher magnification in the bottom panels. (B) As in A, but the xPAK1-GBD 61-123 was coinjected either alone or with dominant-negative (N19) or activated RhoA (L63) (ratio 1:2). Bottom panels show sections of the corresponding embryos, and corresponding boxed regions are shown at higher magnification in the bottom panels. Typical examples of resulting embryos are shown. See Supplemental Figure 1 for sample images of dominant-negative GTPase coinjection. (C) Quantitation of Epp and GBD rescue with active and dominant-negative forms of Cdc42, Rac1, and RhoA. The numbers above the histogram columns refer the total number of embryos scored. (D) Levels of active (GTP-bound) Cdc42 in embryos expressing Epp or EppK (kinase dead Epp that does not give the loss-of-adhesion phenotype) and xPAK1 GBD constructs. Active Cdc42 levels were measured by pull-down with the xPAK1 GBD. The average of three experiments is shown. (E) Levels of active (GTP-bound) Rac1, measured as in D. The average of two experiments is shown. (F) Levels of active (GTP-bound) RhoA in embryos expressing Epp or EppK or xPAK1 GBD constructs. Active RhoA levels were measured by pull-down with the rhotekin GBD. The average of two experiments is shown.
Active RhoA but Not Cdc42 and Rac Levels Are Suppressed by EphA4 Activation and by the xPAK1 GBD
In contrast to the ability of active Cdc42 and Rac to rescue loss-of-adhesion, neither Epp nor the xPAK1-GBD induced a reduction in the levels of GTP-bound Cdc42 or Rac (Figure 7, D and E). Moreover, consistent with the ability of PAK1 to suppress the GTPase activity of its targets (Manser et al., 1994), embryos undergoing xPAK1-GBD–induced loss-of-adhesion actually displayed enhanced levels of both GTP-Cdc42 and Rac1. However, the levels of GTP-RhoA were significantly reduced in embryos undergoing Epp-induced loss-of-adhesion, whereas the inactive receptor (EppK) neither induced lesions nor reduced GTP-RhoA levels, (Figure 7F). Similarly, embryos undergoing xPAK1-GBD-induced loss-of-adhesion displayed a closely comparable reduction in RhoA-GTP levels, whereas the inactive GBD (61-85) had no effect (Figure 7F). Because the GBD of xPAK1 interacts only with Cdc42 and Rac and active mutants of these GTPases rescued the loss-of-adhesion phenotype, down-regulation of Rho was very probably the result of an effective deficit in active Cdc42 and/or Rac levels. This suggested that xPAK1 recruitment to the EphA4 receptor sequestered or masked the active forms of these GTPases, and it was this masking that led to down-regulation in RhoA and loss-of-adhesion.
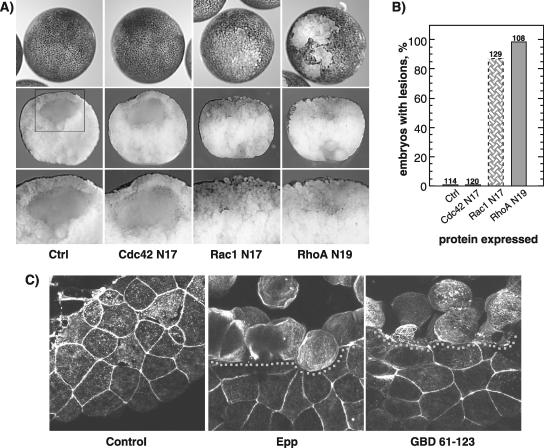
DN-RhoA and DN-Rac1 Also Induce Loss-of-Adhesion
Because activated Cdc42, Rac, and Rho all rescued activated EphA4 loss-of-cell adhesion, it seemed likely that the activated EphA4 and PAK-GBD induced phenotype resulted from a deficit in Cdc42 activity and that this in turn led to down-regulation of Rac and Rho (Mackay and Hall, 1998). Consistent with this, expression of a dominant-negative (inactive) RhoA (N19) alone fully reproduced the loss-of-adhesion phenotype, inducing a break-down of the outer embryonic cell layer, internalization of pigment granules, and a complete loss of the blastocoel (Figure 8, A and B). This yet again reinforces the notion that this phenotype is the result of a reduction in RhoA activity. Consistent with its role as an upstream activator of RhoA, dominant-negative Rac1 (N17) was able to induce a loss of the blastocoel. However, DN-Rac1 induced only a partial disruption of the outer embryonic cell layer and an incomplete internalization of pigment (Figure 8A). However, dominant-negative Cdc42 (N17) displayed no detectable effect on either blastocoel formation, pigment disposition, or integrity of the outer embryonic cell layer (Figure 8A). Although this contrasts with the ability of the active form of Cdc42 to rescue loss-of-adhesion, DN-Cdc42 would by definition be unable to interact with many of the Cdc42 targets that are used to relay its downstream effects. Thus, the gradation of effects we observe for the DN-GTPases, from Cdc42 through Rac to Rho, may well be indicative of their hierarchy in the signaling chain.
Figure 8.
DN-RhoA and DN-Rac1 also induce loss-of-adhesion. (A) Phenotypes of the DN-Cdc42 (N17), DN-Rac1 (N17), and DN-RhoA (N19) expression in embryo. Top panels show external animal pole views, middle panels show sections through the lesions, and corresponding boxed regions are shown at higher magnification in the bottom panels. Typical examples of resulting embryos are shown. (B) Histogram of the percentage of embryos displaying loss-of-adhesion lesions. Scoring was on the basis of visible lesions; no correction for lesion size was made. The same amount of GTPases was injected for all mutants. The numbers above the histogram columns refer to the total number of embryos scored over two independent experiments. For DN-Rac1, the shaded bar represents the penetrance of the distinct phenotype shown in A. (C) Confocal imaging of the actin cytoskeleton in a phalloidin-stained control embryo and in embryos undergoing Epp and xPAK1-GBD (61-123) loss-of-adhesion. The boundary of the loss-of-adhesion lesion is indicated by a dotted line.
The Actin Cytoskeleton Is a Downstream Target in Loss-of-Adhesion
Given that Epp induces loss-of-adhesion via an effect of xPAK1 on the cascade of Rho GTPases, it is very likely that some of its action is on the actin cytoskeleton. That this is probably the case can be deduced from the loss of surface pigment from the outer blastomeres during loss-of-adhesion, because the pigment granules are embedded within the cortical actin (Bisson et al., 2003). F-actin forms a well-defined cortical layer in the blastomeres of the normal animal cap epithelium. However, after Epp or xPAK-GBD–induced loss-of-adhesion, this cortical actin layer disappeared, and cytoplasmic actin filaments became visible (Figure 8C). This disruption of cortical actin is consistent with the observed loss of cell–cell contact adhesion and the role of the Rho-GTPase cascade.
DISCUSSION
Several Eph receptors and their ligands, including EphA4, -A2, -B2, and -B3 and their ligands ephrinB1 and -B2, are present in the early Xenopus embryo (Winning and Sargent, 1994; Jones et al., 1995; Scales et al., 1995). Their ability to regulate blastomere adhesion is probably of key importance in regulating the cell intercalation and convergent extension movements that occur during gastrulation. Just a few hours later, these same receptors and ligands are also important for determining boundaries within the brain and for the migration of the neural crest. However, the mechanisms by which these receptors and ligands mediate their effects on cell adhesion and cell repulsion have been hampered by the lack of a relatively simple model system in which Eph receptor activity can be modulated and the physiological effects precisely defined. Activation of EphA4 in early Xenopus embryos induces a loss of blastomere adhesion (loss-of-adhesion), which provides a reliable physiological readout of receptor function.
Using this readout, our data delineate a novel signaling cascade running from the EphA4 receptor through Nckβ and xPAK1 to Cdc42, Rac, and Rho. We have shown that disruption of the normally tight adhesion between blastomeres induced by constitutive activation of the EphA4 receptor is rescued by the ectopic expression of Nckβ (Grb4) but not Nckα. This rescue requires the SH2 domain of Nckβ, and it is mediated by interaction with phosphotyrosines at positions 595 and 601 on the receptor. xPAK1 is recruited to the EphA4 receptor via Nck and is required for loss-of-adhesion. Ectopic expression of xPAK1 was found to phenocopy EphA4 activation, reproducing cell autonomous loss of blastomere adhesion, disruption of the blastocoel and blastoceol roof, and disassembly of cortical actin, all characteristic of EphA4 activation. Furthermore, both the xPAK1- and EphA4-induced phenotypes could be rescued by enhanced C-cadherin expression, transfer to low salt medium, and the constitutively activated GTPases Cdc42, Rac, and Rho. However, loss-of-adhesion did not require the catalytic activity of xPAK1 nor the binding site for the PIX/COOL family of Cdc42/Rac GEFs (Bagrodia et al., 1998; Manser et al., 1998). Indeed, we found that the functional GBD of xPAK1 was sufficient to phenocopy EphA4 activation, whereas membrane targeting of this GBD very significantly enhanced phenotypic penetrance.
Together these data suggested that loss-of-adhesion was the result of the recruitment of xPAK1 to the EphA4 receptor and that this led to a reduction in the effective levels of active Cdc42, Rac, and Rho, these GTPases being linked in a cascade (Nobes and Hall, 1995; Mackay and Hall, 1998). This was confirmed when RhoA-GTP levels were found to be significantly reduced and dominant-negative RhoA was found to recapitulate loss-of-adhesion. In contrast, neither activated EphA4 nor the xPAK1-GBD reduced active Cdc42 or Rac1 levels. This left an apparent incongruity; whereas enhanced levels of active Cdc42 and Rac rescue loss-of-adhesion, induction of this phenotype does not reduce the activity of either GTPase. However, the data also show that an increase in the affinity of xPAK1 or the xPAK-GBD for Cdc42 significantly enhances penetrance of loss-of-adhesion phenotype (Figures 5, C and E, and 6, C and E). Because p21 GTPase effectors, and indeed GTPase-activating proteins, GEFs, and guanine dissociation inhibitors, all bind to overlapping sites on the GTPases (Dvorsky and Ahmadian, 2004), such an increased affinity would also more effectively sequester active Cdc42 from the cell machinery. This would lead to a reduction in the active Cdc42 available to the Rho cascade and hence to a down-regulation of Rho. This is a novel masking or sequestration role for PAK and one that places it upstream of Cdc42, as a GTPase regulator.
How does the EphA4–xPAK1 axis then modulate cell adhesion? During early Xenopus cleavage divisions, the adherens junctions form the major means of maintaining blastomere–blastomere contacts. Because loss of this contact after EphA4 activation or ectopic xPAK1 expression is suppressed by C-cadherin and by low ionic conditions, both EphA4 and xPAK1 seem to have their effect by down-regulating cadherin-dependent cell adhesion. It is well established that activation of the Rho family GTPases is required to maintain cadherin-mediated cell adhesion (Braga, 2000; Fukata and Kaibuchi, 2001). Thus, sequestration of active Cdc42 would be expected to down-regulate cadherin adhesion, weakening the tight contacts between blastomeres and resulting in the observed disruption of the blastocoel roof. Of the other proteins identified as potential mediators of Eph signaling in Xenopus embryos, none are able to induce the EphA4 loss-of-adhesion phenotype. For example, members of the Src family and the Abl and Arg TKs (Kullander and Klein, 2002; Murai and Pasquale, 2003) have been the subject of extensive studies in the early Xenopus embryos, (Adler et al., 2000; Weinstein and Hemmati-Brivanlou, 2001) and do not lead to loss-of-adhesion.
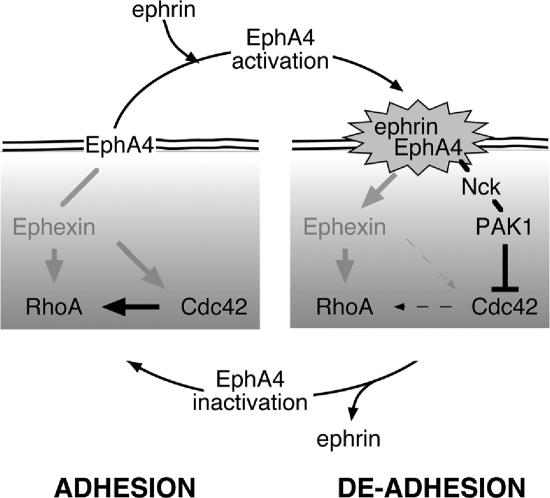
Targeted migration, such as is directed by Eph signaling, requires regulation of both adhesion and deadhesion (Tepass et al., 2002). To dissect the EphA4 pathway, we have used a cell autonomous means of activating this receptor. However, EphA4 activation would normally depend on an ephrin B1/2 signal from adjacent cells. Our data suggest that an EphA4 activation–deactivation cycle may occur at points of cell contact such as to promote migration. This cycle would consist of 1) cadherin-dependent adhesion; 2) ephrin signaling and receptor activation; 3) local recruitment of xPAK1, Cdc42 sequestration, and Rho down-regulation; 4) a concomitant down-regulation of cadherin and hence loss of adhesion; 5) loss of local ephrin signaling; and 6) reestablishment of new cadherin adhesions and the potential for a new round of ephrin signaling (Figure 9). In this way, cells could migrate across each other, continuously testing-out their surroundings for cell surfaces and cell partners until they find partners that do not signal repulsion.
Figure 9.
Schematic diagram describing the probable mechanisms of induction of the loss-of-adhesion lesions by EphA4. Pointed arrowed lines show activating and blunt ended lines inhibitory signaling and line density indicates signaling strength.
Constitutive recruitment of GEFs of the Ephexin (Dbl) family to EphA4 occurs in several cell types, although its status in Xenopus has still to be determined. In the absence of receptor activation, Ephexin1 enhances the activities of all three small GTPases—Cdc42, Rac, and Rho. In our model, this would equate with a stabilization of adhesion. Receptor activation suppresses the activity of Ephexin1 for Cdc42 and Rac, probably leading to their local down-regulation, but maintains its activity toward Rho (Sahin et al., 2005). In the model, signaling through Nck-xPAK1 sequesters active Cdc42 and probably Rac, but not Rho, effectively inducing a similar imbalance in available GTPase levels. Thus, Ephexin1 and Nck-xPAK1 could represent redundant signaling pathways, or, if present in the same cell type, they might synergize to accentuate the GTPase activity imbalance.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Sargent, R. Winning, and J. Scales for the EphA4 and Ephexin constructs and for helpful discussions; D. Wilkinson (National Institute for Medical Research, Mill Hill, London, UK) for DN-EphA4; B. Gumbiner (Department of Cell Biology, University of Virginia, Charlottesville, VA) for C-cadherin; J. Schlessinger (Department of Pharmacology, Yale University, New Haven, CT) and A. Pawson (Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada) for Nckα; W. Li (Department of Medicine, University of Southern California, Los Angeles, CA) for Nckβ and point mutants; and N. Lamarche-Vane (Department of Anatomy and Cell Biology, McGill University, Montreal, Quebec, Canada) for GTPase constructs. We also thank J. Lavoie and N. Lamarche-Vane for critical reading of an earlier version of this article, and C. St-Pierre and the Cancer Centre Imaging Unit for help with confocal microscopy. This work was supported by an operating grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society, a Fonds pour la Recherche en Santé du Québec (FRSQ) scholarship to L.P., a Natural Sciences and Engineering Research Council and more recently a National Cancer Institute of Canada studentship with funds from the Terry Fox Foundation to N.B., and a Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) scholarship to A.M.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0294) on January 10, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Adler C. E., Miyoshi-Akiyama T., Aleman L. M., Tanaka M., Smith J. M., Mayer B. J. Abl family kinases and Cbl cooperate with the Nck adaptor to modulate Xenopus development. J. Biol. Chem. 2000;275:36472–36478. doi: 10.1074/jbc.M005424200. [DOI] [PubMed] [Google Scholar]
- Bagrodia S., Taylor S. J., Jordon K. A., Van Aelst L., Cerione R. A. A novel regulator of p21-activated kinases. J. Biol. Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Becker E., Huynh-Do U., Holland S., Pawson T., Daniel T. O., Skolnik E. Y. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol. Cell. Biol. 2000;20:1537–1545. doi: 10.1128/mcb.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard V., Bohl B. P., Bokoch G. M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bisson N., Islam N., Poitras L., Jean S., Bresnick A. R., Moss T. The catalytic domain of xPAK1 is sufficient to induce myosin II dependent in vivo cell fragmentation independently of other apoptotic events. Dev. Biol. 2003;263:264–281. doi: 10.1016/j.ydbio.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Wang Y., Bohl B. P., Sells M. A., Quilliam L. A., Knaus U. G. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J. Biol. Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Braga V. Epithelial cell shape: cadherins and small GTPases. Exp. Cell Res. 2000;261:83–90. doi: 10.1006/excr.2000.5050. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Stowers L., Baer M., Trejo J., Coughlin S., Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr. Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- Chen M., She H. Y., Kim A., Woodley D. T., Li W. Nckbeta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor bb. Mol. Cell. Biol. 2000;20:7867–7880. doi: 10.1128/mcb.20.21.7867-7880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. E., Kemp H. A., Moens C. B. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 2005;15:536–542. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Cowan C. A., Henkemeyer M. Ephrins in reverse, park and drive. Trends Cell Biol. 2002;12:339–346. doi: 10.1016/s0962-8924(02)02317-6. [DOI] [PubMed] [Google Scholar]
- Dvorsky R., Ahmadian M. R. Always look on the bright site of Rho: structural implications for a conserved intermolecular interface. EMBO Rep. 2004;5:1130–1136. doi: 10.1038/sj.embor.7400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden S., Rohatgi R., Podtelejnikov A. V., Mann M., Kirschner M. W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Faure S., Vigneron S., Dorée M., Morin N. A member of the Ste20/PAK family of protein kinases is involved in both arrest of Xenopus oocytes at G2/prophase of the first meiotic cell cycle and in prevention of apoptosis. EMBO J. 1997;16:5550–5561. doi: 10.1093/emboj/16.18.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- Galisteo M. L., Chernoff J., Su Y. C., Skolnik E. Y., Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase pak1. J. Biol. Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Gupta R. W., Mayer B. J. Dominant-negative mutants of the SH2/SH3 adapters Nck and Grb2 inhibit MAP kinase activation and mesoderm-specific gene induction by eFGF in Xenopus. Oncogene. 1998;17:2155–2165. doi: 10.1038/sj.onc.1202158. [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid I. B., He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Holland S. J., Gale N. W., Gish G. D., Roth R. A., Zhou S. Y., Cantley L. C., Henkemeyer M., Yancopoulos G. D., Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot J. Ephrin signaling in axon guidance. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:813–818. doi: 10.1016/j.pnpbp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Irie F., Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat. Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Islam N., Poitras L., Moss T. The cytoskeletal effector xPAK1 is expressed during both ear and lateral line development in Xenopus. Int. J. Dev. Biol. 2000;44:245–248. [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jones T. L., Karavanova I., Maéno M., Ong R. C., Kung H. F., Daar I. O. Expression of an amphibian homolog of the Eph family of receptor tyrosine kinases is developmentally regulated. Oncogene. 1995;10:1111–1117. [PubMed] [Google Scholar]
- Kullander K., Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Bong Y. S., Moore K. B., Soria K., Moody S. A., Daar I. O. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat. Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lu W. G., Katz S., Gupta R., Mayer B. J. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Mackay D.J.G., Hall A. Rho GTPases. J. Biol. Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Zhen Z.-S., Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E., Loo T. H., Koh C. G., Zhao Z. S., Chen X. Q., Tan L., Tan I., Leung T., Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Maruta H., Nheu T. V., He H., Hirokawa Y. Rho family-associated kinases PAK1 and rock. Prog. Cell Cycle Res. 2003;5:203–210. [PubMed] [Google Scholar]
- Mellitzer G., Xu Q., Wilkinson D. G. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- Morreale A., Venkatesan M., Mott H. R., Owen D., Nietlispach D., Lowe P. N., Laue E. D. Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat. Struct. Biol. 2000;7:384–388. doi: 10.1038/75158. [DOI] [PubMed] [Google Scholar]
- Murai K. K., Pasquale E. B. ‘Eph’ective signaling: forward, reverse and crosstalk. J. Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Noren N. K., Pasquale E. B. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Obermeier A., Ahmed S., Manser E., Yen S. C., Hall C., Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E. B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Penzes P., Beeser A., Chernoff J., Schiller M. R., Eipper B. A., Mains R. E., Huganir R. L. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Poitras L., Bisson N., Islam N., Moss T. A tissue restricted role for the Xenopus Jun N-terminal kinase kinase kinase MLK2 in cement gland and pronephric tubule differentiation. Dev. Biol. 2003a;254:200–214. doi: 10.1016/s0012-1606(02)00040-4. [DOI] [PubMed] [Google Scholar]
- Poitras L., Jean S., Islam N., Moss T. PAK interacts with NCK and MLK2 to regulate the activation of jun N-terminal kinase. FEBS Lett. 2003b;543:129–135. doi: 10.1016/s0014-5793(03)00424-1. [DOI] [PubMed] [Google Scholar]
- Poliakov A., Cotrina M., Wilkinson D. G. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rao Y., Zipursky S. L. Domain requirements for the Dock adapter protein in growth-cone signaling. Proc. Natl. Acad. Sci. USA. 1998;95:2077–2082. doi: 10.1073/pnas.95.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera G. M., Briceno C. A., Takeshima F., Snapper S. B., Mayer B. J. Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr. Biol. 2004;14:11–22. doi: 10.1016/j.cub.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Sahin M., et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Scales J. B., Winning R. S., Renaud C. S., Shea L. J., Sargent T. D. Novel members of the eph receptor tyrosine kinase subfamily expressed during Xenopus development. Oncogene. 1995;11:1745–1752. [PubMed] [Google Scholar]
- Shamah S. M., et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Smith A., Robinson V., Patel K., Wilkinson D. G. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- Stein E., Huynh-Do U., Lane A. A., Cerretti D. P., Daniel T. O. Nck recruitment to Eph receptor, EphB1/ELK, couples ligand activation to c-Jun kinase. J. Biol. Chem. 1998;273:1303–1308. doi: 10.1074/jbc.273.3.1303. [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. Adhesion in development: an historical overview. Dev. Biol. 1996;180:377–388. doi: 10.1006/dbio.1996.0312. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamo T., Ota S., Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 2003;22:847–858. doi: 10.1093/emboj/cdg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Lu W., Gupta R., Mayer B. J. Expression of mutated Nck SH2/SH3 adaptor respecifies mesodermal cell fate in Xenopus laevis development. Proc. Natl. Acad. Sci. USA. 1997;94:4493–4498. doi: 10.1073/pnas.94.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Godt D., Winklbauer R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr. Opin. Genet. Dev. 2002;12:572. doi: 10.1016/s0959-437x(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Thompson G., Owen D., Chalk P. A., Lowe P. N. Delineation of the Cdc42/Rac-binding domain of p21-activated kinase. Biochemistry. 1998;37:7885–7891. doi: 10.1021/bi980140+. [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. K., Li F., Adam L., Nguyen D., Ohta Y., Stossel T. P., Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21- activated kinase 1. Nat. Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- Weinstein D. C., Hemmati-Brivanlou A. A. Src family kinase function during early Xenopus development. Dev. Dyn. 2001;220:163–168. doi: 10.1002/1097-0177(2000)9999:9999<1::AID-DVDY1098>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Winning R. S., Sargent T. D. Pagliaccio, a member of the Eph family of receptor tyrosine kinase genes, has localized expression in a subset of neural crest and neural tissues in Xenopus laevis embryos. Mech. Dev. 1994;46:219–229. doi: 10.1016/0925-4773(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Winning R. S., Scales J. B., Sargent T. D. Disruption of cell adhesion in Xenopus embryos by Pagliaccio, an Eph-class receptor tyrosine kinase. Developmental Biology. 1996;179:309–319. doi: 10.1006/dbio.1996.0262. [DOI] [PubMed] [Google Scholar]
- Winning R. S., Ward E. K., Scales J. B., Walker G. K. EphA4 catalytic activity causes inhibition of RhoA GTPase in Xenopus laevis embryos. Differentiation. 2002;70:46–55. doi: 10.1046/j.1432-0436.2002.700105.x. [DOI] [PubMed] [Google Scholar]
- Winning R. S., Wyman T. L., Walker G. K. EphA4 activity causes cell shape change and a loss of cell polarity in Xenopus laevis embryos. Differentiation. 2001;68:126–132. doi: 10.1046/j.1432-0436.2001.680206.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.