Abstract
The c-Jun/Sp1 interaction is essential for growth factor- and phorbol 12-myristate 13-acetate (PMA)-induced genes expression, including human 12(S)-lipoxygenase, keratin 16, cytosolic phospholipase A2, p21WAF1/CIP1, and neuronal nicotinic acetylcholine receptor β4. Here, we examined the mechanism underlying the PMA-induced regulation on the interaction between c-Jun and Sp1. We found that treatment of cells with PMA induced a dephosphorylation at the C terminus of c-Jun at Ser-243 and a concomitant inhibition of PP2B by using PP2B small interfering RNA, resulting in reduction of PMA-induced gene expression as well as the c-Jun/Sp1 interaction. The c-Jun mutant TAM-67-3A, which contains three substitute alanines at Thr-231, Ser-243, and Ser-249 compared with TAM-67, binds more efficaciously with Sp1 and is about twice as efficacious as TAM-67 in inhibiting the PMA-induced activation of the 12(S)-lipoxygenase promoter. Importantly, PP2B not only dephosphorylates the c-Jun at Ser-243 but also interacts with c-Jun in PMA-treated cells. PMA stimulates the association of the PP2B/c-Jun/Sp1 complex with the promoter. These findings indicate the dephosphorylation of c-Jun C terminus is required for the c-Jun/Sp1 interaction and reveal that PP2B plays an important role in regulating c-Jun/Sp1 interaction in PMA-induced gene expression.
INTRODUCTION
The proto-oncogene c-Jun is one of the components of adaptor protein-1 (AP)1, a transcription factor complex thought to mediate cell proliferation, survival, and death (Angel and Karin, 1991; Shaulian and Karin, 2001). Its activity is regulated in a cell type-dependent manner by a variety of signals that are relayed through transcriptional and posttranscriptional mechanisms. The transcriptional activity of c-Jun is dependent on protein phosphorylation and dephosphorylation in which c-Jun is phosphorylated on Ser-63, Ser-73, Thr-91, and/or Thr-93 within the N-terminal domain of the molecule by extracellular signal-regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK) (Smeal et al., 1991; Adler et al., 1992; Treisman, 1996). Phosphorylation at these sites induces an increase in the DNA binding and transactivation potential of the protein. Dephosphorylation of c-Jun N terminus is due to the inhibition of JNK activity by the activation of PP2A (Shanley et al., 2001; Kins et al., 2003). In contrast, the C terminus of c-Jun is constitutively phosphorylated by casein kinase II (CKII) (Lin et al., 1992) and glycogen synthase kinase 3 (GSK3) (Boyle et al., 1991). The phosphorylation of c-Jun on three C-terminal residues close to the DNA binding domain has been reported to inhibit DNA binding (Hunter and Karin, 1992). The phosphorylation of N-terminal transactivation domain of c-Jun by JNK (Derijard et al., 1994) causes a conformational change in c-Jun that facilitates the dephosphorylation of the C-terminal residues, resulting in an increased DNA binding (Papavassiliou et al., 1995). In addition, many genes are regulated by c-Jun through the binding between the C-terminal domain of c-Jun and transcription factors Sp1, ATF, smad, nuclear factor of activated T cells (NFAT), Ets, and signal transducer and activator of transcription (Chinenov and Kerppola, 2001). Thus, the regulation of cellular function by C terminus of c-Jun is achieved not only by stabilizing the DNA binding but also by interacting with transcription factors that modify the regulatory specificities of AP1 proteins in a cell- or tissue-specific manner. In spite of this wealth of knowledge regarding the regulatory mechanisms underpinning c-Jun, the regulation of dephosphorylation of c-Jun C terminus and its interaction with transcription factors are not completely clarified. Similarly, Sp1, the first mammalian transcription factor cloned (Kadonaga et al., 1987), is also phosphorylated by multiple cellular kinases, such as CKII (Armstrong et al., 1997), DNA-dependent protein kinase (Jackson et al., 1990), and protein kinase A (Rohlff et al., 1997). CKII phosphorylates Sp1 on a threonine residue in the second zinc finger of Sp1, thereby prevents it from binding with DNA. This phosphorylation is correlated with the reduced expression of Sp1 site-dependent genes in differentiated liver cells (Armstrong et al., 1997).
The four major types of phosphatases that dephosphorylate serine and threonine residues are PP1, PP2A, PP2B (calcineurin), and PP2C (Hunter, 1995). PP2B is a heterodimer consisting of calcineurin A, a 58- to 64-kDa catalytic and calmodulin-binding entity, and calcineurin B, a regulatory 19-kDa Ca2+-binding protein (Klee et al., 1988). The functional role of PP2B has been thoroughly studied. PP2B is a ubiquitously expressed protein phosphatase that is activated upon the binding of Ca2+-calmodulin (Guerini, 1997). It plays an essential role in T-cell activation through the NFAT signaling pathway (Liu et al., 1991), and it regulates the gene expression of interleukin-2 (Emmel et al., 1989). FK506 and cyclosporin A, when bound to their respective binding proteins, FKBP12 and cyclophilin A, are specific inhibitors of PP2B (Klee et al., 1998). PP2B also regulates the cellular functions by binding to several proteins, including a kinase anchor protein family (Sim et al., 2003), PP2B inhibitor CAIN (Kim et al., 2002), Down Syndrome candidate region 1 (Kingsbury and Cunningham, 2000), and acute myeloid leukemia 1 (Liu et al., 2004). PP2B has relatively narrower substrate specificity including transcription factors, e.g., NFAT (Luo et al., 1996a), the transcription factor Elk1 (Tian and Karin, 1999), and the heat–shock protein hsp25 (Gaestel et al., 1992). These indicate that PP2B may play a role in the regulation of gene expression through the modification of transcription factors.
An increasing number of genes, e.g., 12(S)-lipoxygenase, keratin 16, human cytosolic phospholipase A2 (cPLA2), neuronal nicotinic acetylcholine receptor β4, p21WAF1/CIP1, human vimentin, and lamin A/C regulated by the c-Jun/Sp1 interaction are identified (Kardassis et al., 1999; Chen and Chang, 2000; Blaine et al., 2001; Melnikova and Gardner, 2001; Wang and Chang, 2003; Wu et al., 2003; Okumura et al., 2004). Although c-Jun/Sp1 complex plays a pivotal role in the regulation of gene expression, the mechanism involved in the regulation of c-Jun/Sp1 interaction has not been completely understood. Although PMA enhances the dephosphorylation of c-Jun C terminus (Boyle et al., 1991), resulting in an increased DNA binding, the functional role of the dephosphorylated c-Jun C terminus in regulating a protein–protein interaction is not clear. We reported previously that PMA induces the interaction between c-Jun and Sp1 in the same manner as that induced by epidermal growth factor (EGF), indicating that protein kinase C activation could also induce the interaction between c-Jun and Sp1 (Chen et al., 2002). Because EGF and PMA induce the interaction between c-Jun and Sp1 via the C-terminal domain of c-Jun (Chen and Chang, 2000; Chen et al., 2002), there exists a possibility that the dephosphorylation of the C-terminal cluster is essential for the interaction between c-Jun and Sp1. Here, we present several lines of evidence showing that PP2B dephosphorylates c-Jun C terminus that results in interactions among the three proteins c-Jun, Sp1, and PP2B in PMA-treated cells. The finding provides a new role of PP2B in the regulation of c-Jun/Sp1–dependent genes.
MATERIALS AND METHODS
Cell Culture
Human epidermoid carcinoma A431 and nonsmall-cell lung carcinoma (NSCLC) A549 cells were grown at 37°C under 5% CO2 in 10-cm plastic dishes containing 10 ml of DMEM (Invitrogen, Carlsbad, CA) and Nutrition mixture F12 Kaighn's modification medium (Biowest, Miami, FL), respectively and supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin. An A431 cell line stably expressing myc-TAM-67, was cloned by limiting dilution after Geneticin (G-418) selection and maintained in the presence of 1 mg/ml G-418 (Sigma-Aldrich, St. Louis, MO). In this series of experiments, cells were treated with 5 nM PMA (Sigma-Aldrich) in culture medium supplemented with 10% fetal bovine serum, unless stated otherwise.
Plasmid Construction
In vitro-directed mutagenesis was performed by the QuikChange site- directed mutagenesis kit (Stratagene, La Jolla, CA). The desired mutation constructs were extended by using Pfu turbo DNA polymerase. Synthetic primers were shown in the following: c-Jun-Ser231 mutant primer, 5′-GAGCCTCAGGCAGTGCCCGAG-3′; c-Jun-Ser-243 mutant primer, 5′-ACACCGCCCCTGGCACCCATCGACATG-3′; and c-Jun-Ser-249 mutant primer, 5′-ATCGACATGGAGGCACAGGAGCGGATC-3′. Mutated positions in the sequence of the primers were underlined. Mutagenesis of more than one site was done by subsequent mutation of the corresponding previously mutated construct. The vector sequence was confirmed by DNA sequencing. TAM-67 was also amplified by polymerase chain reaction (PCR) and inserted into the EcoRI and BamHI sites in pcDNA3.1/myc-His to generate myc-TAM-67. PP2BAα short interfering RNA (siRNA) oligonucleotides were purchased from Invitrogen. The sequence of PP2BAα siRNA oligonucleotides targeting the PP2BAα gene was AAA UGU UCC UGA GUC UUC UCA UUU C. To generate the autoinhibitory domain truncated-PP2B, PP2B-ΔAI (1-430 amino acid) expression vector, the fragment was amplified by PCR and inserted into pECFP-N1 vector. The forward and reverse primers were 5′-CCCAAGCTTGAGATGTCCGAGCCC-3′ and 5′-CGCGGATCCCAGCCAGTTGGGGT-3′, respectively. The nucleotide sequence of the mutant was confirmed by automatic DNA sequencing.
Transfection of Cells with Plasmids and Luciferase Assay
A luciferase reporter plasmid (pXLO-7-1) bearing a promoter region (−224 base pairs) of human 12(S)-lipoxygenase gene was used. Two Sp1 binding sites at −158 to −150 base pairs and −123 to −114 base pairs present in the promoter were essential for PMA response of 12(S)-lipoxygenase gene activation (Liaw et al., 1998). Transient transfection of cells with plasmids was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions but with slight modifications. A431 cells were replated 36 h before transfection at a density of 3 × 105 cells in 2 ml of fresh culture medium in a 3.5-cm plastic dish. For use in transfection, 2 μl of Lipofectamine 2000 was incubated with 0.5 μg of pXLO-7-1 plasmid, 0.2 μg of β-galactosidase plasmid, or plasmids where indicated like as TAM-67, TAM-67-3A, and PP2BAα siRNA, in 1 ml of Opti-MEM medium for 30 min at room temperature. Cells were transfected by changing the medium with 1 ml of Opti-MEM medium containing the plasmids and Lipofectamine 2000 and then incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 h. After the change of Opti-MEM medium to 2 ml of fresh culture medium, cells were incubated further for an additional 24 h, unless stated otherwise. The luciferase and β-galactosidase activities in cell lysate were determined as described previously (Liu et al., 1997).
Reverse Transcription-PCR
Total RNA was isolated using the TRIzol RNA extraction kit (Invitrogen), and 5 μg of RNA was subjected to reverse transcription-PCR with SuperScriptII (Invitrogen). The 12(S)-lipoxygenase-specific primers (sense, 5′-AGT TCC TCA ATG GTG CCA AC-3′; and antisense, 5′-CAC CTG TGC TCA CTG CCT TA-3′) and β-actin primers were used. The PCR products were separated by 1% agarose-gel electrophoresis and visualized with ethidium bromide staining.
DNA Affinity Precipitation Assay
This assay was performed according to the method of Zhu et al. (2002) with a slight modification. The binding assay was performed by mixing 200 μg of nuclear extract proteins, 2 μg of biotinylated-conjugated Sp1-binding element (from −170 to approximately −110 base pairs) of 12(S)-lipoxygenase promoter (Liu et al., 1997), and 20 μl of streptavidin-agarose beads (4%) with 50% slurry. The mixture was incubated at room temperature for 1 h with rotating. Beads were pelleted and washed three times with phosphate-buffered saline (PBS). The binding proteins were eluted by loading buffer and separated by SDS-PAGE, followed by Western blot analysis probed with specific antibodies.
Chromatin Immunoprecipitation (ChIP) Assay
Chromatin immunoprecipitation assay was done as reported previously (Saccani et al., 2001) with minor modification. Briefly, A431 or A549 cells were treated with 1% formaldehyde for 15 min. The cross-linked chromatin was sonicated to 400- to 500-base pair fragments. Lysates were precleared with protein A beads and incubated overnight at 4°C with antibodies specific to PP2B (BD Biosciences, San Jose, CA), c-Jun (Santa Cruz Biotechnology, Santa Cruz, CA), and Sp1 (Santa Cruz Biotechnology). Immune complex was precipitated with protein A beads preabsorbed with sonicated single-stranded DNA and bovine serum albumin. After reversal of cross-linking, levels of precipitated 12(S)-lipoxygenase, cPLA2α, and thrombomodulin promoter DNA were determined by PCR by using oligonucleotides spanning the Sp1 binding sites (sense, 5′-GGG AAG TGT TCT CAT CTA TG-3′ and antisense, 5′-GGC CAC TTC CAA CCT TTA AA-3′); sense, 5′-CTC GAG ACA GAA ATC CGC AAC AGC ACT C-3′ and antisense, 5′-AAG CTT GAT CCT TTT TCA GCT CCG GA-3′; and sense, 5′-GAG AAC CCA GCA ATC CCG AGT ATG-3′ and antisense, 5′-CGT GCA GGC GCC GGG GAA AG-3′, respectively. The PCR products were separated by 1% agarose-gel electrophoresis and visualized with ethidium bromide staining.
Western Blotting
The cytoplasmic fractions and nuclear extracts of cells were prepared for Western blot analysis according to the method described previously (Andrews and Faller, 1991). An analytical 10% SDS-PAGE was performed, and 30 μg of protein of each were analyzed, unless stated otherwise. For immunoblotting, proteins in the SDS gels were transferred to a polyvinylidene difluoride membrane by an electroblot apparatus. Antibodies against human Sp1 (Santa Cruz Biotechnology), c-Jun (Santa Cruz Biotechnology), PP2B (BD Biosciences), β-actin (Santa Cruz Biotechnology), phospho-serine (Zymed Laboratories, South San Francisco, CA) and phospho-c-Jun (Ser-63) (Cell Signaling Technology, Beverly, MA) were used as the primary antibodies. Mouse or rabbit IgG antibodies coupled to horseradish peroxidase were used as secondary antibodies. An enhanced chemiluminescence kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was used for detection. Phospho-specific antibodies that recognize c-Jun phosphorylated at Ser-243 were raised in rabbit and purified at Immuno-Biological Laboratories (Tokyo, Japan). Phospho-specific antibodies were raised against the following peptide: GETPPLS*PIDMES, residues 237-249 of human c-Jun. The asterisk denotes the phosphorylated residue.
Coimmunoprecipitation
Two hundred micrograms of protein of nuclear extracts was incubated under gentle shaking at 4°C overnight with anti-c-Jun or PP2B antibody-agarose conjugate (Santa Cruz Biotechnology) or a mixture of anti-c-Jun antibodies and protein A agarose in 300 μl of immunoprecipitation buffer (20 mM HEPES, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2 and 25% glycerol [vol/vol], 0.5 mM phenylmethylsulfonyl fluoride, 1 mM orthovanadate, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin). Beads were pelleted at 7500 × g for 2 min and washed three times with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.5, 1% IGEPAL CA-630 [vol/vol], 150 mM NaCl, and 0.5% sodium deoxycholate). Protein was removed from the beads by boiling in sample buffer (120 mM Tris-HCl, pH 6.8, 10% glycerol, 3% SDS, 20 mM dithiothreitol [DTT], and 0.4% bromphenol blue) for 5 min and subjected to SDS-PAGE on a 10% gel. Western blot analysis was carried out as described above.
In Vitro Phosphorylation and GST Protein Interaction Assay
One microgram of glutathione S-transferase (GST) fusion protein was incubated with CKII or GSK3β (Upstate Biotechnology, Lake Placid, NY) in kinase buffer (25 mM Tris-HCl, pH 7.4, 150 mM KCl, 10 mM MgCl2, and 50 μM ATP) with addition of 2.5 μl of [γ-32P]ATP (5000 Ci/mmol; PerkinElmer Life and Analytical Sciences, Boston, MA) in a 50 μl of reaction volume. The kinase reaction was carried out at 30°C for 30 min. The reaction mixture was incubated with 30 μl of glutathione-Sepharose 4B, and the mixture was rotated at 4°C for 1 h. After spinning down, beads were incubated with 300 μg of nuclear extract in 300 μl of immunoprecipitation buffer (20 mM HEPES, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 25% glycerol (vol/vol), 0.5 mM phenylmethylsulfonyl fluoride, 1 mM orthovanadate, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin) at 4°C for overnight. After washing with RIPA buffer twice, the samples were separated by SDS-PAGE and detected by Western blotting or autoradiography.
In Vitro Dephosphorylation Assay
GSK3β-catalyzed phospho-GST-TAM-67 was incubated with PP2B and calmodulin (Upstate Biotechnology) in phosphatase buffer (50 mM Tris-HCl, pH 7.5, 1 mM CaCl2, 100 mM NaCl, 1 mg/ml bovine serum albumin, 0.025% NP-40, and 1 mM DTT). The reactions were carried out at 37°C for 30 min and repurified on glutathione-Sepharose 4B. The phosphorylated or dephosphorylated GST-c-Jun were finally incubated with 500 μg of nuclear extract in 300 μl of reaction buffer (20 mM HEPES, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 25% glycerol (vol/vol), 0.5 mM phenylmethylsulfonyl fluoride, 1 mM orthovanadate, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin) at 37°C for 30 min. After washing with RIPA buffer twice, the proteins were separated by SDS-PAGE and visualized by autoradiography or detected by antibodies against PP2B, Sp1, and c-Jun.
RESULTS
PP2B Dephosphorrylates c-Jun C Terminus
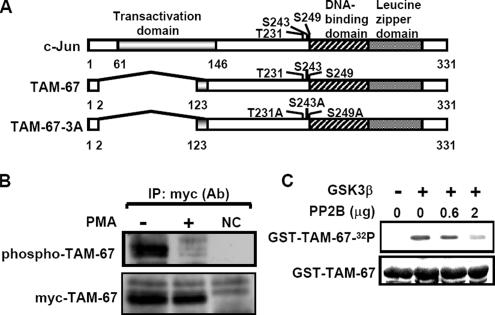
Based on the report that PMA enhances the dephosphorylation of c-Jun C terminus (Boyle et al., 1991), we studied the Ser/Thr phosphatase involved in regulating dephosphorylation of c-Jun C terminus. First, we measured the phosphorylation level of the C-terminal domain of c-Jun in PMA-treated cells. To diminish the N-terminal phosphorylation when probing the phosphorylation level of c-Jun C terminus, we used an N-terminally truncated mutant of c-Jun, TAM-67 (Figure 1A). Consistent with the results of the previous report (Boyle et al., 1991), PMA significantly attenuated the phosphorylation level of myc-TAM-67 (Figure 1B). Next, we identified whether the Ser/Thr phosphatase PP2B was involved in the regulation of c-Jun C-terminal dephosphorylation. The c-Jun C-terminal Thr-239, Ser-243, and Ser-249 sites were reported to be phosphorylated by GSK3, and Thr-231 and Ser-249 sites were reported to be constitutively phosphorylated by CKII (Lin et al., 1992). We examined whether c-Jun, like Elk1 (Tian and Karin, 1999), could be a substrate of and dephosphorylated by PP2B. The substrate used in this experiment was GST-TAM-67 fusion protein that was phosphorylated in vitro by GSK3 (Figure 1C). By using the same amount of GST-TAM-67 as a substrate, phospho-GST-TAM-67 was found to be dephosphorylated by PP2B in a dose-dependent manner (Figure 1C).
Figure 1.
PP2B directly dephosphorylates phospho-c-Jun. (A) Schematic diagram of c-Jun and TAM mutants. T, threonine; S, serine; and A, alanine. (B) Cells that constitutively expressed myc-TAM-67 were treated with 5 nM PMA for 90 min. Nuclear extracts were immunoprecipitated with protein A-agarose–conjugated antibodies against myc and analyzed by Western blotting with anti-phospho-serine and anti-myc antibodies. NC: negative control from vector only-transfected cell lines. (C) GST-TMA-67 fusion proteins were expressed in E. coli and purified on glutathione-Sepharose. GST fusion proteins (1 μg) were in vitro phosphorylated by recombinant GSK3 and repurified on glutathione-Sepharose. The 32P-labeled substrates were incubated with the indicated concentrations of PP2B and calmodulin purified from bovine brain in phosphatase buffer containing 1 mM Ca2+ for 30 min at 37°C. The proteins were separated by SDS-PAGE and visualized by autoradiography. Equal loading of GST-TAM-67 proteins was confirmed by Coomassie blue staining.
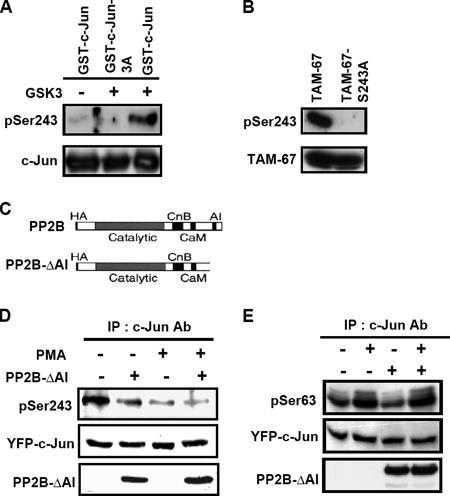
To study whether the dephosphorylation of c-Jun C terminus was mediated by PP2B in vivo, we detected the change of c-Jun phosphorylation by using the phospho-specific antibodies that recognize phospho-Ser-243 of c-Jun in cells transfected with constitutively activated-PP2B expression vector PP2B-ΔAI (Figure 2C) (Hashimoto et al., 1990; Shibasaki and McKeon, 1995). The specificity of the antibodies that recognize phosphorylated Ser-243 was verified by in vitro kinase assay. The GSK3-catalyzed phosphorylation of GST-c-Jun at Ser-243 but not GST-c-Jun-3A was detected (Figure 2A). The antibodies were also verified in vivo and only recognized phosphorylated TAM-67 but not TAM-67-S243A mutant (Figure 2B). The phospho-specific antibodies were then used to study the phosphorylation of c-Jun at Ser-243 in cells. Consistent with the result of Figure 1, both PMA and dominant-active PP2B enhanced the dephosphorylation of c-Jun at Ser-243 (Figure 2D). There was no effect of PP2B on the phosphorylation of c-Jun at Ser-63 in PMA- or nontreated cells (Figure 2E). The result indicates that PP2B specifically dephosphorylates the phospho-C terminus of c-Jun in cells.
Figure 2.
PMA and PP2B induce dephosphorylation of c-Jun at Ser-243 in cells. (A) GST-c-Jun and GST-c-Jun-3A fusion proteins were expressed in E. coli and purified on glutathione-Sepharose. Each of the GST fusion proteins (0.5 μg) was in vitro phosphorylated by recombinant GSK3β. The proteins were separated by SDS-PAGE and analyzed by Western blotting with antibodies against c-Jun and phospho-specific antibodies that recognize c-Jun phosphorylation at Ser-243. (B) Cells were transfected by lipofection with 2 μg of TAM-67 and TAM-67-S243A expression vectors and incubated further for 24 h. Cell lysates were prepared and analyzed by Western blotting with antibodies against c-Jun and phospho-c-Jun at Ser-243. (C) Schematic diagram of PP2B and constitutively activated-PP2B expression vector, PP2B-ΔAI (1-430 amino acids). (D and E) Cells were transfected with 2 μg of YFP-c-Jun and constitutively active CFP-PP2B-ΔAI expression vectors by lipofection, incubated further for 48 h, and treated with 5 nM PMA for 1 h. Cell lysates were immunoprecipitated with antibodies against c-Jun and analyzed by Western blotting with antibodies against c-Jun, PP2B and phospho-c-Jun at Ser-243 or at Ser63. Similar results were obtained in three or four independent experiments.
Phosphorylation of c-Jun C Terminus Destabilizes Its Interaction with Sp1
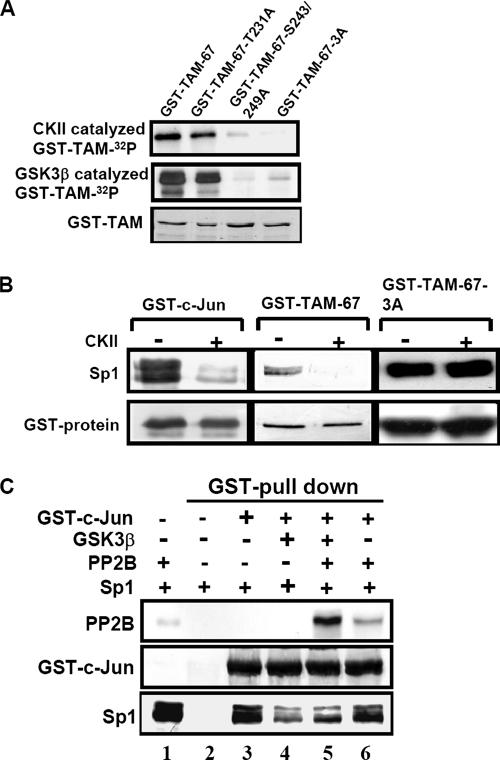
Based on the results that PMA enhances the dephosphorylation of c-Jun C terminus (Figure 1B and 2D) and the interaction between c-Jun and Sp1 via the C-terminal domain of c-Jun (Chen et al., 2002), we proposed that Sp1 might bind c-Jun resulted from decreased phosphorylation of c-Jun C terminus. We tested the effect of phosphorylation and dephosphorylation of the c-Jun C terminus on its interaction with Sp1 by using an expression vector TAM-67-3A containing three substitutive alanine residues at Thr-231, Ser-243, and Ser-249 (Figure 1A) in the absence or presence of CKII or GSK3. The phosphorylation intensity of GST-TAM-67-T231A, GST-TAM-67-S243/249A and GST-TAM-67-3A was weaker than that of GST-TAM-67 in an in vitro CKII and GSK3 kinase assay (Figure 3A). Furthermore, the effect of phosphorylatin of c-Jun on the interaction between c-Jun and Sp1 was studied. The interaction between GST-c-Jun and GST-TAM-67 with Sp1 was stronger than that between CKII-catalyzed phospho-GST-c-Jun and phospho-GST-TAM-67 with Sp1 (Figure 3B). The interaction between GST-TAM-67-3A and Sp1 was not affected by CKII (Figure 3B). These experiments indicate that the phosphorylation of c-Jun C terminus reduces the interaction between c-Jun and Sp1. Dephosphorylation of c-Jun C terminus at Thr-231, Ser-243, and Ser-249 sites is apparently critical for the c-Jun/Sp1 interaction. Consistent with that observed in Figure 3B, the binding affinity of GSK3-catalyzed phospho-GST-c-Jun with Sp1 was lower than that of GST-c-Jun with Sp1 (Figure 3C, lanes 3 and 4). Furthermore, PP2B reversed the decreased interaction between phospho-GST-c-Jun and Sp1 (Figure 3C, lanes 4 and 5) by decreasing the phosphorylation of c-Jun C terminus (Figures 1C and 2D). This result reveals that PP2B enhances the interaction between c-Jun and Sp1. To further examine whether PP2B bound phosphorylated or dephosphorylated form of c-Jun to regulate the binding between phospho-c-Jun and Sp1, nonphospho-GST-c-Jun and phospho-GST-c-Jun were used as the target candidates for PP2B in GST pull-down assay. As shown in Figure 3C, PP2B preferred to bind GSK3-catalyzed phospho-GST-c-Jun (lanes 5 and 6). Although PP2B had lower affinity with GST-c-Jun, it slightly interfered with the interaction between GST-c-Jun and Sp1 (Figure 3C, lanes 3 and 6). These findings suggest that GSK3- or CKII-mediated phoshporylation of c-Jun C terminus reduces the interaction between c-Jun and Sp1. PP2B binds and then dephosphorylates phospho-c-Jun to enhance the interaction between c-Jun and Sp1.
Figure 3.
Interaction between Sp1 and unphosphorylated C terminus of c-Jun in vitro. (A) GST-TAM-67, GST-TAM-67-T231A, GST-TAM-67-S243/249A and GST-TAM-67-3A fusion proteins were expressed in E. coli and purified on glutathione-Sepharose. Each of the GST fusion proteins (1 μg) was in vitro phosphorylated by recombinant CKII and GSK3β, and repurified on glutathione-Sepharose. The proteins were separated by SDS-PAGE and visualized by autoradiography. The GST fusion proteins were detected by Coomassie blue. (B) Nuclear extracts were incubated with CKII-phosphorylated GST-fusion proteins and repurified on glutathione-Sepharose. The proteins were separated by SDS-PAGE and detected by anti-Sp1 and anti-c-Jun antibodies. (C) The phosphorylated and dephosphorylated forms of GST-c-Jun were incubated with PP2B and calmodulin purified from bovine brain in phosphatase buffer containing 1 mM Ca2+ for 30 min at 37°C. Samples were repurified on GSH-Sepharose and then incubated with 500 μg of nuclear proteins for 30 min at 37°C. The proteins were purified on GSH-Sepharose again and separated by SDS-PAGE and detected by antibodies specific for PP2B, Sp1, and c-Jun. All panels show representative examples of three experiments with similar results.
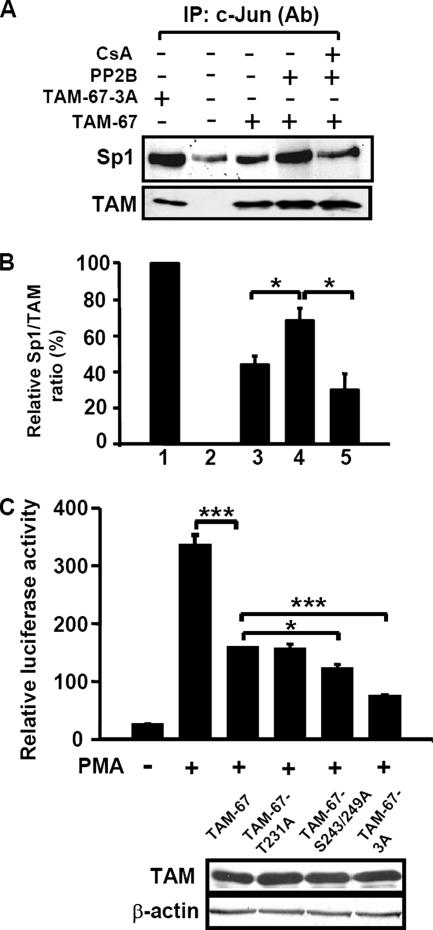
To examine whether dephosphorylated C terminus of c-Jun might physically associate with Sp1 in intact cells, we performed coimmunoprecipitation experiments on nuclear extracts obtained from transiently transfected cells. In the control cells transfected with pcDNA3.1 vector, no immunoreactive protein was detected (Figure 4A). As also shown in Figure 4A, although a small amount of Sp1 was detected to be associated with the endogenous c-Jun in cells transfected with the control vector, the interaction between TAM proteins and Sp1 was significantly enhanced in those cells transfected with expression vectors TAM-67 and TAM-67-3A. Because TAM-67 was phosphorylated in cells (Figures 1B and 2B), the relative ratio between Sp1 and TAM showed that TAM-67-3A exhibited a two-fold higher binding affinity with Sp1 than with TAM-67 (Figure 4B, lanes 1 and 3). These results indicate that the interaction between TAM-67-3A and Sp1 is stronger than that between TAM-67 and Sp1 in cells. Consistent with the results of Figure 3C, PP2B enhanced the TAM-67/Sp1 interaction by decreasing the phosphorylation of c-Jun C terminus (Figures 2D and 4B, lanes 3 and 4), and the enhancement was inhibited by treating the cells with inhibitor of PP2B, cyclosporin A (Figure 4B, lanes 4 and 5).
Figure 4.
Interactions between unphosphorylated C terminus of c-Jun and Sp1 in cells. (A) Cells were transfected by lipofection with 2 μg of TAM-67, TAM-67-3A, and PP2B expression vectors, respectively. pcDNA3.1 was used as a vector to adjust for the same amount of plasmids in each experiment. Cells were incubated further for 36 h in the presence of 15 μM cyclosporin A (CsA). Nuclear extracts were prepared, immunoprecipitated with antibodies against c-Jun, and analyzed by Western blotting with anti-c-Jun and anti-Sp1 antibodies. (B) Densitometry analysis of the relative Sp1/TAM ratio was calculated. Values represent means ± SEM from three experiments. Statistical significance (*p < 0.05) between Sp1/TAM-67 + PP2B and Sp1/TAM-67 or Sp1/TAM-67 + PP2B + CsA was analyzed by Student's t test. (C) Cells were transfected by lipofection with pXLO-7-1, pCMVβ-galactosidase, and 1 μg of TAM-67, or TAM-67 mutants. Cells were incubated with 5 nM PMA for 18 h. The luciferase and β-galactosidase activities were then determined. Values represent means ± SEM of three determinations. Expressions of TAM-67, TAM-67 mutants, and β-actin were analyzed by Western blot analysis by using anti-c-Jun antibodies. Statistical significance (*p < 0.05 and ***p < 0.001) between TAM-67 and TAM-67 mutants transfected or PMA-treated cells was analyzed by Student's t test.
Next, we examined whether the dephosphorylated form of c-Jun C terminus exerted more effect on PMA-induced gene expression perhaps by regulating the interaction between c-Jun and Sp1 in intact cells. Overexpression of the c-Jun dominant-negative mutant TAM-67 not only inhibited the coimmunoprecipitation of c-Jun and Sp1 by competing for the binding of Sp1 but also reduced the promoter activity of 12(S)-lipoxygenase gene in cells overexpressing c-Jun (Chen and Chang, 2000). We then tested whether the dephosphorylated form of TAM-67 was more efficient than TAM-67 in inhibiting PMA-induced promoter activity of the 12(S)-lipoxygenase gene in cells. TAM-67 inhibited the PMA-induced promoter activation of 12(S)-lipoxygenase gene (Figure 4C), indicating that the interaction between c-Jun and Sp1 played a critical role in PMA-induced expression of the 12(S)-lipoxygenase gene. Expression of TAM mutants in cells was observed after transient transfection with vectors containing TAM-67, TAM-67-T231A, TAM-67-S243/249A and TAM-67-3A (Figure 4C). When two proteins were expressed at a comparative level and the interaction between TAM-67-3A and Sp1 was stronger than that between TAM-67 and Sp1 (Figure 4B, lanes 1 and 3), the inhibition effects of TAM-67-3A and TAM-67 on PMA-induced promoter activity were ∼85 and 50%, respectively (Figure 4C).
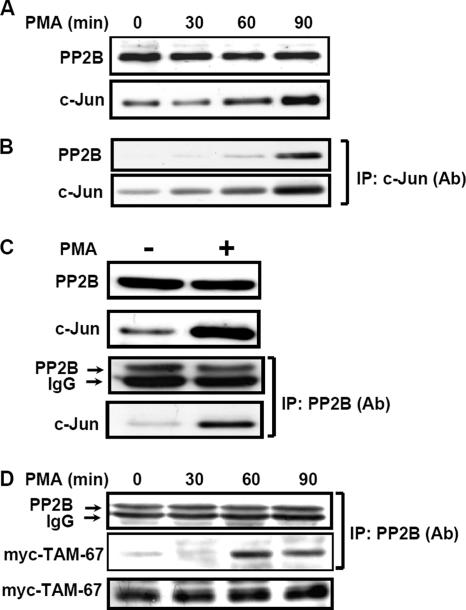
PMA Enhances the Interaction between c-Jun and PP2B
Because c-Jun was a substrate of PP2B, whether these two proteins could interact with each other was further clarified in vivo. Interestingly, coimmunoprecipitation assay by using c-Jun (Figure 5B) and PP2B antibodies (Figure 5C) indicated that PMA induced the interaction between c-Jun and PP2B. Because PMA-induced c-Jun biosynthesis and no change in PP2B expression were observed (Figure 5A), we speculated that the enhancement of c-Jun/PP2B interaction might be related to the c-Jun synthesis. In addition, c-Jun/PP2B interaction may be caused by protein modification in PMA-treated cells. We tested the possibility that whether PMA-induced c-Jun/PP2B interaction was through the protein modification in those cells transfected with expression vector myc-TAM-67. Indeed, in the equal amount of myc-TAM-67 expressed in cells, PMA enhanced myc-TAM-67/PP2B interaction in a time-dependent manner (Figure 5D). This result indicates that PMA induced c-Jun/PP2B interaction through protein modification, and c-Jun was a substrate of PP2B and, at least, dephosphorylated at Ser-243 by PP2B (Figure 2D).
Figure 5.
PMA induces interaction between c-Jun and PP2B. (A–C) Confluent cells were starved for 24 h in serum-free culture medium and then treated with 5 nM PMA for various times (as indicated) in the culture medium without serum. Nuclear extracts of cells were prepared and subjected to Western blot or immunoprecipitated with antibodies against c-Jun and PP2B bound to protein A-agarose. The proteins were subjected to SDS-PAGE and analyzed by Western blotting with antibodies against c-Jun and PP2B. (D) Cells that have been constitutively expressed myc-TAM-67 were treated with 5 nM PMA for various time durations. Nuclear extracts were immunoprecipitated with protein A-agarose conjugated antibodies against myc and analyzed by Western blotting with anti-PP2B and anti-myc antibodies. Similar results were obtained in three independent experiments.
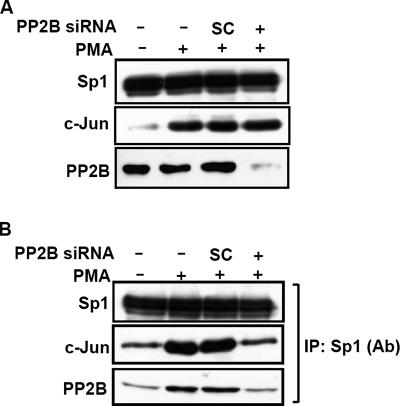
Inhibition of PP2B Reduces PMA-induced c-Jun/Sp1/PP2B Interaction
To confirm the functional role of endogenous PP2B in regulating the PMA-induced c-Jun/Sp1 interaction, PP2B-Aα siRNA oligonucleotides were transfected into A431 cells. As shown in Figure 6A, Sp1, PMA-induced c-Jun expressions and even the cellular distribution of c-Jun and Sp1 were not altered upon knockdown of endogenous PP2B. The PMA-induced c-Jun/Sp1/PP2B interaction was attenuated in those cells transfected with PP2B-Aα siRNA (Figure 6B). These results suggest that PP2B plays a functional role in the regulation of PMA-induced the complex formation of c-Jun/Sp1/PP2B.
Figure 6.
Effect of PP2B on PMA-induced interaction between c-Jun and Sp1 in cells. (A and B) Cells were transfected with 50 nM PP2B siRNA or scramble (SC) oligonucleotides by lipofection, incubated further for 36 h, and treated with 5 nM PMA for 3 h. Nuclear extracts were immunoprecipitated with antibodies against Sp1 bound to protein A-agarose. Nuclear extracts (A) or immunoprecipitates (B) were subjected to SDS-PAGE followed by Western blotting with antibodies against Sp1, c-Jun, and PP2B. Similar results were obtained in three independent experiments.
PP2B Is an Essential Component to Activate c-Jun/Sp1-regulated Genes
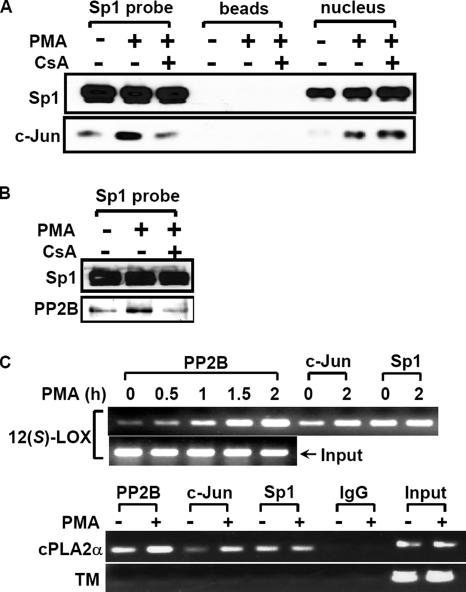
These findings described above suggest that PP2B is a key regulator of c-Jun/Sp1 interaction and the c-Jun/Sp1/PP2B complex may be essential for gene expression in PMA-treated cells. To directly examine the contribution of endogenous PP2B to activate the transcription of c-Jun/Sp1-regulated genes, we used DNA affinity precipitation assay (DAPA) and chromatin immunoprecipitation (ChIP) assay to examine whether PP2B could be bound to the target promoter in PMA-treated cells. The probes used in DAPA contained three Sp1 binding sites but no AP1 site in the promoter region of 12(S)-lipoxygenase (Liu et al., 1997). It has been found that c-Jun but not Sp1 binding to the promoter under the PMA stimulation was increased (Figure 7, A and C). In the cultures without PMA treatment, there was a slight association of PP2B and c-Jun with the promoter (Figure 7, B and C). In contrast, in PMA-treated cultures, there was a much greater association of PP2B with the promoter (Figure 7, B and C), although no change in PP2B expression was observed (Figure 5A). PMA-induced binding of c-Jun/PP2B to the promoter was inhibited in cells treated with cyclosporine A (Figure 7, A and B). In addition, we also found that the promoter region containing Sp1 binding sites but no AP1 binding site of cPLA2α was essential for c-Jun/Sp1-induced gene expression in PMA-treated A549 cells (our unpublished data). We then studied whether the binding of c-Jun and PP2B to the cPLA2α promoter was also enhanced under PMA treatment in cells. As shown in Figure 7C, the binding of PP2B and c-Jun but not Sp1 to the promoter of cPLA2α was also increased in PMA-treated cells as well as 12(S)-lipoxygenase promoter. To further study whether PP2B and c-Jun specifically bound to c-Jun/Sp1-regulated genes, we also analyzed the biding affinity between these factors and thrombomodulin promoter. The low expression of thrombomodulin was detected in A549 cells compared with other cell lines. The c-Jun/Sp1 complex had no effect on the expression of thrombomodulin in A549 cells (our unpublished data). As shown in Figure 7C, there was no significant binding of c-Jun, PP2B, and Sp1 to thrombomodulin promoter even in PMA-treated A549 cells. These results suggest that PMA stimulation facilitates access of PP2B to the promoter resulting in the formation of the PP2B/c-Jun/Sp1 complex (Figure 6B), and enhances the complex binding to the c-Jun/Sp1-regulated promoter (Figure 7).
Figure 7.
Binding of PP2B, c-Jun, and Sp1 to genes promoter. (A and B) Confluent cells were starved for 18 h in serum-free culture medium and then treated with 15 μM CsA for 30 min, followed by 5 nM PMA treatment for 2 h. Nuclear extracts from CsA- and PMA-treated cells were prepared and DNA affinity precipitation assay was performed as described under Materials and Methods. Binding of Sp1, c-Jun, and PP2B proteins to Sp1 probes were analyzed by Western blot. The streptavidin-agarose beads were used to serve as a nonspecific binding control. (C) Cross-linked chromatin derived from PMA-treated cells was immunoprecipitated with PP2B, c-Jun, and Sp1 antibodies and analyzed by PCR with specific primers for 12(S)-lipoxygenase promoter [12(S)-LOX], cPLA2α promoter, and thrombomodulin promoter (TM), which was used as a negative control. Input, nonimmunoprecipitated cross-linked chromatin. Similar results were obtained in two or three independent experiments.
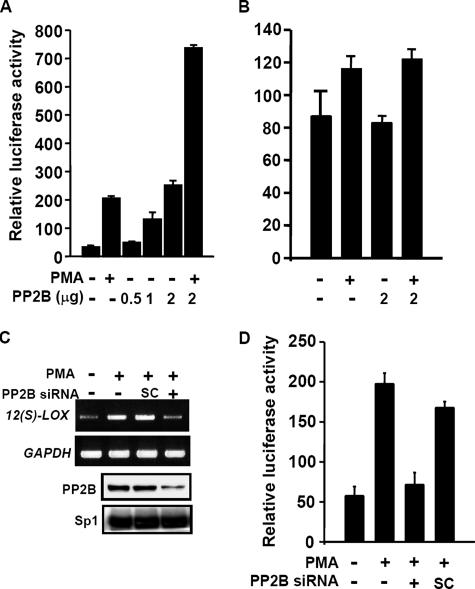
Next, we studied the contribution of the endogenous PP2B to activate the transcription of c-Jun/Sp1–regulated genes by reporter assay. Transient transfection of the catalytic subunit A of PP2B expression vector in cells induced the promoter activity of 12(S)-lipoxygenase in a dose-dependent manner (Figure 8A). The PMA treatment together with PP2B transfection produced a synergistic effect on the promoter activity (Figure 8A). To identify the potential role of Sp1 binding sites of the promoter in PP2B response, the luciferase reporter vector bearing the promoter with mutations at Sp1 binding sequences (SPM7) was used (Chen et al., 2000). Both PMA- and PP2B-induced promoter activities of 12(S)-lipoxygenase were nearly abolished in vector SPM7 (Figure 8B). The effect of PP2B on c-Jun/Sp1-regulated gene was further confirmed by using PP2B-Aα siRNA. Transient transfection of PP2B-Aα siRNA led to an efficient depletion of PP2B proteins and reduced the effect of PMA on the expression of 12(S)-lipoxygenase gene (Figure 8C). In addition, PMA-induced promoter activity of cPLA2α was also attenuated in those cells transfected with PP2B-Aα siRNA (Figure 8D). Together, these results demonstrate that PMA-induced the expression of 12(S)-lipoxygenase and cPLA2α genes is, at least in part, mediated by PP2B.
Figure 8.
Effect of PP2B on PMA-induced genes expression. (A and B) Cells were transfected with pXLO-7-1, SPM7, pCMVβ-galactosidase, or various amounts of PP2B expression vector by lipofection. Cells were treated with 5 nM PMA and further cultured in fresh medium up to 18 h. The luciferase and β-galactosidase activities were then determined. Values represent means ± SEM of three determinations. (C) Cells were transfected with 50 nM PP2B siRNA or SC oligonucleotides by lipofection. After PMA treatment for 18 h, total RNA was extracted for reverse transcription PCR with 12(S)-lipoxygenase and glyceraldehyde-3-phosphate dehydrogenase primers. Expressions of PP2B and Sp1 proteins were analyzed by Western blot analysis by using anti-PP2B and Sp1 antibodies, respectively. Similar results were obtained in two independent experiments. (D) A549 cells were transfected with pPLA 599, pCMVβ-galactosidase, 50 nM PP2B siRNA, or SC oligonucleotides by lipofection. After PMA treatment for 16 h, the luciferase and β-galactosidase activities were then determined. Values represent means ± SEM of three determinations.
DISCUSSION
A large group of transcription factors including the bZIP, NFAT, Ets, Smad, and basic helix loop helix families can activate or repress the transcription in conjunction with c-Jun C terminus by binding to regulatory elements adjacent to AP1 sites (Chinenov and Kerppola, 2001). However, it is not clear exactly how proteins interact with each other. In the present study, we found that dephosphorylation at the C terminus domain of c-Jun was required for its interaction with Sp1. Our results are in agreement with those from several other reports on cAMP response element-binding protein (CREB)/CREB binding protein (Sun et al., 1994; Parker et al., 1998) and RB/E2F (Mittnacht, 1998), showing that protein phosphorylation negatively regulates the protein interaction.
We also found that PP2B played a functional role in enhancing the interaction between c-Jun and Sp1 ensuing the transcriptional activation of 12(S)-lipoxygenase gene upon the PMA treatment. PP2B has been shown to mediate gene expression including nitric-oxide synthase (Marumo et al., 1995) and p21WAF1/CIP1 (Santini et al., 2001). The role of PP2B in regulating the transcription through dephosphorylation of downstream targets such as the PP2B-dependent cytoplasmic subunits of the NFATc transcription complex has been documented (Shibasaki et al., 1996; Rao et al., 1997). PP2B dephosphorylates the serines within the serine/proline (SP) repeats (SP1 to SP3) and the serine-rich regions of NFATc family members. When NFATc was phosphorylated, these residues seem to obscure the two nuclear localization sequences required for nuclear import (Beals et al., 1997). In this study, we found that PP2B siRNA inhibited c-Jun/Sp1 interaction but that it had no effect on the PMA-induced expression of c-Jun and nuclear distribution of c-Jun. Our results suggest that the regulation of PMA-induced gene expression mediated by PP2B was not due to an enhancement in the nuclear transport of c-Jun and Sp1 but that it might be due to the regulation of c-Jun/Sp1 interaction.
By using in vitro and in vivo assays, PP2B was found to bind and to dephosphorylate c-Jun at Ser-243, indicating that phospho-c-Jun could be a substrate of PP2B. Although PP2B reduced the phospho-c-Jun at Ser-243, we could not rule out the possibility that other phosphatases might also involve in regulating phosphorylated c-Jun. At least two possible underlying mechanisms could be suggested to explain the dephosphoryation of c-Jun under PMA treatment in cells. One mechanism is the increase of PP2B activity, and the other mechanism is the enhancement of interaction between c-Jun and PP2B. We have used a specific enzyme activity assay to determine the PP2B activity in cell lysates, and we found partial change (at most up to 30%) in cellular PP2B activity in the PMA-treated cells (our unpublished data). However, a significant enhancement in the interaction between c-Jun and PP2B upon PMA treatment was indeed observed. With the use of ChIP assay in this study, we demonstrated that PMA stimulated the association of both PP2B and c-Jun with the promoter in vivo. Because no AP1 binding site is present in the gene promoter of the 12(S)-lipoxygenase (Yoshimoto et al., 1992), the increase in the binding of nuclear PP2B/c-Jun to the promoter may be due to the formation of a PP2B/c-Jun/Sp1 complex. Indeed, Sp1 could function as an anchor protein to recruit c-Jun to the promoter (Chen and Chang, 2000). Because the samples used were from nuclear extracts in immunoprecipitation experiments and that PMA stimulated the binding of PP2B to the promoter in the ChIP assay, these results suggest that the PP2B-mediated dephosphorylation of c-Jun could occur in the cell nucleus, and the c-Jun/PP2B interaction seems to be another example of a targeting protein interaction with protein phosphatase. For example, PP2B directly binds to NFATc (Luo et al., 1996b) and itself is then directed to the inositol trisphosphate and ryanodine receptors via an interaction with FKBP12 (Cameron et al., 1995). Although PP2B is widely considered to be a cytoplasmic protein (Guerini, 1997) containing no nuclear translocation signal (Luo et al., 1996b), it has been reported that after PP2B binds to AML1 or NFAT4, it accompanies AML1 or NFAT4 to the nucleus by virtue of its tight association with transcription factors (Shibasaki et al., 1996; Liu et al., 2004). In our system, whether the nuclear import of PP2B is via its interaction with AML1, NFAT, or via other factors remains to be determined.
Because PMA treatment causes the dephosphorylation of the c-Jun DNA binding domain (residues 227-252) in human osteosarcoma MG63 cells (Boyle et al., 1991), and c-Jun has also been reported to be phosphorylated at Thr-231, Thr-239, Ser-243, and Ser-249 by GSK3 and CKII (Boyle et al., 1991; Lin et al., 1992), we speculate that the PP2B targeting sites of c-Jun are located at these phosphorylation sites. By using the in vitro assays, we found that TAM-67-3A was almost inert to GSK3- and CKII-catalyzed phosphorylation and PP2B did dephosphorylate the phospho-TAM-67 protein. Although both the phosphorylated and dephosphorylated forms of NFAT1 bind to PP2B (Wesselborg et al., 1996), we found that PP2B preferred to bind the phosphorylated form of c-Jun. It was previously reported that PMA stimulates the dephosphorylation of Thr-239 in fibroblasts, but the inhibition of GSK3 is not required for the dephosphorylation of Thr-239 in response to lipopolysaccharide (Morton et al., 2003). Therefore, the Thr-239 site of c-Jun may not be a functional target for PP2B. In addition, there is only one SP repeat of the dephosphorylation site of PP2B (Beals et al., 1997) located at Ser-243 of c-Jun. The mutation of Ser-243 prevents c-Jun from being earmarked for destruction by the Fbw7 ubiquitin ligase complex (Wei et al., 2005). Although we found that PP2B dephosphorylated the c-Jun at Ser-243 but not at Ser-63, we could not rule out the possibility that phosphorylation site of c-Jun at Thr-231 or Ser-249 might also be regulated by PP2B. Together, these results indicate that PP2B-regulated dephosphorylation of c-Jun C terminus may thus not only participate in the regulation of c-Jun/Sp1 interaction but also play a role in the stabilization of c-Jun.
In this study, we found that dephosphorylation of c-Jun C terminus regulated by PP2B was required for the c-Jun/Sp1 interaction in PMA-induced gene regulation. Overexpression of c-Jun transactivated the human p21WAF1/CIP1 gene by acting as a superactivator of Sp1 in HepG2 cells (Kardassis et al., 1999). Our present findings strongly support the notion that the up-regulation of p21WAF1/CIP1 gene expression by PMA may also be mediated through PP2B by regulating the interaction of c-Jun with Sp1. It is apparent that PP2B also participates in regulating other genes in addition to 12(S)-lipoxygenase through the c-Jun/Sp1 interaction. Indeed, we found that PP2B regulated the EGF-induced promoter activity of keratin 16 gene (our unpublished data) and PMA-induced cPLA2α expression. Because genes regulated by the c-Jun/Sp1 interaction play important functions in cells, further elucidation of the mechanisms underlying the c-Jun/Sp1 interaction could provide important clues to how genes are regulated in general.
ACKNOWLEDGMENTS
We are greatly indebted to Drs. Shozo Yamamoto, Gerald R. Crabtree, and Michael J. Birrier for providing the plasmids pXLO-7-1, PP2B, and TAM-67, respectively. We thank Drs. J. R. Chen, T. P. Su, and W. M. Kan for their critical review of the manuscript. This work was supported in part by grant NSC 93-2320-B-006-091 from the National Science Council of the Republic of China, and the Ministry of Education Program for Promoting Academic Excellent of University under Grant 91-B-FA09-1-4 of the Republic of China, and National Cheng Kung University Project of Promoting Academic Excellence and Developing World Class Research Centers.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0797) on January 10, 2007.
REFERENCES
- Adler V., Franklin C. C., Kraft A. S. Phorbol esters stimulate the phosphorylation of c-Jun but not v-Jun: regulation by the N-terminal delta domain. Proc. Natl. Acad. Sci. USA. 1992;89:5341–5345. doi: 10.1073/pnas.89.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;10:2–3. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Armstrong S. A., Barry D. A., Leggett R. W., Mueller C. R. Casein kinase II-mediated phosphorylation of the C terminus of Sp1 decreases its DNA binding activity. J. Biol. Chem. 1997;272:13489–13495. doi: 10.1074/jbc.272.21.13489. [DOI] [PubMed] [Google Scholar]
- Beals C. R., Clipstone N. A., Ho S. N., Crabtree G. R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- Blaine S. A., Wick M., Dessev C., Nemenoff R. A. Induction of cPLA2 in lung epithelial cells and non-small cell lung cancer is mediated by Sp1 and c-Jun. J. Biol. Chem. 2001;276:42737–42743. doi: 10.1074/jbc.M107773200. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Cameron A. M., Steiner J. P., Roskams A. J., Ali S. M., Ronnettt G. V., Snyder S. H. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Chen B. K., Chang W. C. Functional interaction between c-Jun and promoter factor Sp1 in epidermal growth factor-induced gene expression of human 12(S)-lipoxygenase. Proc. Natl. Acad. Sci. USA. 2000;97:10406–10411. doi: 10.1073/pnas.180321497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. K., Kung H. C., Tsai T. Y., Chang W. C. Essential role of mitogen-activated protein kinase pathway and c-Jun induction in epidermal growth factor-induced gene expression of human 12-lipoxygenase. Mol. Pharmacol. 2000;57:153–161. [PubMed] [Google Scholar]
- Chen B. K., Tsai T. Y., Huang H. S., Chen L. C., Chang W. C., Tsai S. B. Functional role of extracellular signal-regulated kinase activation and c-Jun induction in phorbol ester-induced promoter activation of human 12(S)-lipoxygenase gene. J. Biomed. Sci. 2002;9:156–165. doi: 10.1007/BF02256027. [DOI] [PubMed] [Google Scholar]
- Chinenov Y., Kerppola T. K. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001;20:2438–2452. doi: 10.1038/sj.onc.1204385. [DOI] [PubMed] [Google Scholar]
- Derijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK 1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Gaestel M., Benndorf R., Hayess K., Priemer E., Engel K. Dephosphorylation of the small heat shock protein hsp25 by calcium/calmodulin-dependent (type 2B) protein phosphatase. J. Biol. Chem. 1992;267:21607–21611. [PubMed] [Google Scholar]
- Guerini D. Calcineurin: not just a simple protein phosphatase. Biochem. Biophys. Res. Commun. 1997;235:271–275. doi: 10.1006/bbrc.1997.6802. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J. Biol. Chem. 1990;265:1924–1927. [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., MacDonald J. J., Lees-Miller S., Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990;63:155–165. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987;51:1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kardassis D., Papakosta P., Pardali K., Moustakas A. c-Jun transactivates the promoter of the human p21WAF1/Cip1 gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J. Biol. Chem. 1999;274:29572–29581. doi: 10.1074/jbc.274.41.29572. [DOI] [PubMed] [Google Scholar]
- Kim M. J., et al. Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc. Natl. Acad. Sci. USA. 2002;99:9870–9875. doi: 10.1073/pnas.152336999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury T. J., Cunningham K. W. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- Kins S., Kurosinski P., Nitsch R. M., Gotz J. Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am. J. Pathol. 2003;163:833–843. doi: 10.1016/S0002-9440(10)63444-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv. Enzymol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Ren H., Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Liaw Y. W., Liu Y. W., Chen B. K., Chang W. C. Induction of 12-lipoxygenase expression by phorbol 12-myristate 13-acetate in human epidermoid carcinoma A431 cells. Biochim. Biophys. Acta. 1998;5:23–33. doi: 10.1016/s0005-2760(97)00090-8. [DOI] [PubMed] [Google Scholar]
- Lin A., Frost J., Deng T., Smeal T., al-Alawi N., Kikkawa U., Hunter T., Brenner D., Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell. 1992;70:777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- Liu H., Holm M., Xie X.-Q., Wolf-Watz M., Grundstrom T. AML1/Runx1 recruits calcineurin to regulate granulocyte macrophage colony- stimulating factor by Ets1 activation. J. Biol. Chem. 2004;279:29398–29408. doi: 10.1074/jbc.M403173200. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr., Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu Y. W., Arakawa T., Yamamoto S., Chang W. C. Transcriptional activation of human 12-lipoxygenase gene promoter is mediated through Sp1 consensus sites in A431 cells. Biochem. J. 1997;324:133–140. doi: 10.1042/bj3240133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Burgeon E., Carew J., McCaffrey P., Badalian T., Lane W., Hogan P., Rao A. Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol. Cell. Biol. 1996a;16:3955–3966. doi: 10.1128/mcb.16.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Shaw K.T.-Y., Raghavan A., Aramburu J., Garcia-Cozar F., Perrino B. A., Hogan P. G., Rao A. Interaction of calcineurin with a domain of the transcription factor NFAT1 that controls nuclear import. Proc. Natl. Acad. Sci. USA. 1996b;93:8907–8912. doi: 10.1073/pnas.93.17.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo T., Nakaki T., Hishikawa K., Suzuki H., Kato R., Saruta T. Cyclosporin A. inhibits nitric oxide synthase induction in vascular smooth muscle cells. Hypertension. 1995;25:764–768. doi: 10.1161/01.hyp.25.4.764. [DOI] [PubMed] [Google Scholar]
- Melnikova I. N., Gardner P. D. The signal transduction pathway underlying ion channel gene regulation by Sp1-c-Jun interactions. J. Biol. Chem. 2001;276:19040–19045. doi: 10.1074/jbc.M010735200. [DOI] [PubMed] [Google Scholar]
- Mittnacht S. Control of pRB phosphorylation. Curr. Opin. Genet. Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- Morton S., Davis R. J., McLaren A., Cohen P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 2003;22:3876–3886. doi: 10.1093/emboj/cdg388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Hosoe Y., Nakajima N. c-Jun and Sp1 family are critical for retinoic acid induction of the lamin A/C. retinoic acid-responsive element. Biochem. Biophys. Res. Commun. 2004;320:487–492. doi: 10.1016/j.bbrc.2004.05.191. [DOI] [PubMed] [Google Scholar]
- Papavassiliou A. G., Treier M., Bohmann D. Intramolecular signal transduction in c-Jun. EMBO J. 1995;14:2014–2019. doi: 10.1002/j.1460-2075.1995.tb07193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D., Jhala U. S., Radhakrishnan I., Yaffe M. B., Reyes C., Shulman A. I., Cantley L. C., Wright P. E., Montminy M. Analysis of an activator:coactivator complex reveals an essential role for secondary structure in transcriptional activation. Mol. Cell. 1998;2:353–359. doi: 10.1016/s1097-2765(00)80279-8. [DOI] [PubMed] [Google Scholar]
- Rao A., Luo C., Hogan P. G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Rohlff C., Ahmad S., Borellini F., Lei J., Glazer R. I. Modulation of transcription factor Sp1 by cAMP-dependent protein kinase. J. Biol. Chem. 1997;272:21137–21141. doi: 10.1074/jbc.272.34.21137. [DOI] [PubMed] [Google Scholar]
- Saccani S., Pantano S., Natoli G. Two Waves of Nuclear Factor {kappa}B Recruitment to Target Promoters. J. Exp. Med. 2001;193:1351–1360. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini M. P., Talora C., Seki T., Bolgan L., Dotto G. P. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21WAF1/CIP1 expression in keratinocyte differentiation. Proc. Natl. Acad. Sci. USA. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley T. P., Vasi N., Denenberg A., Wong H. R. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J. Immunol. 2001;166:966–972. doi: 10.4049/jimmunol.166.2.966. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., McKeon F. Calcineurin functions in Ca(2+)-activated cell death in mammalian cells. J. Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F., Price E. R., Milan D., McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- Sim A. T., Baldwin M. L., Rostas J. A., Holst J., Ludowyke R. I. The role of serine/threonine protein phosphatases in exocytosis. Biochem. J. 2003;373:641–659. doi: 10.1042/BJ20030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Sun P., Enslen H., Myung P. S., Maurer R. A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Tian J., Karin M. Stimulation of Elk1 transcriptional activity by mitogen-activated protein kinases is negatively regulated by protein phosphatase 2B. (Calcineurin) J. Biol. Chem. 1999;274:15173–15180. doi: 10.1074/jbc.274.21.15173. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Wang Y. N., Chang W. C. Induction of disease-associated keratin 16 gene expression by epidermal growth factor is regulated through cooperation of transcription factors Sp1 and c-Jun. J. Biol. Chem. 2003;278:45848–45857. doi: 10.1074/jbc.M302630200. [DOI] [PubMed] [Google Scholar]
- Wei W., Jin J., Schlisio S., Harper J. W., Kaelin J., William G. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wesselborg S., Fruman D. A., Sagoo J. K., Bierer B. E., Burakoff S. J. Identification of a physical interaction between calcineurin and nuclear factor of activated T cells (NFATp) J. Biol. Chem. 1996;271:1274–1277. doi: 10.1074/jbc.271.3.1274. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang X., Zehner Z. E. c-Jun and the dominant-negative mutant, TAM67, induce vimentin gene expression by interacting with the activator Sp1. Oncogene. 2003;22:8891–8901. doi: 10.1038/sj.onc.1206898. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Arakawa T., Hada T., Yamamoto S., Takahashi E. Structure and chromosomal localization of human arachidonate 12-lipoxygenase gene. J. Biol. Chem. 1992;267:24805–24809. [PubMed] [Google Scholar]
- Zhu Y., Saunders M. A., Yeh H., Deng W.-G., Wu K. K. Dynamic regulation of cyclooxygenase-2 promoter activity by isoforms of CCAAT/enhancer-binding proteins. J. Biol. Chem. 2002;277:6923–6928. doi: 10.1074/jbc.M108075200. [DOI] [PubMed] [Google Scholar]