Abstract
The epidermal growth factor (EGF)-dependent trafficking of the intact EGF receptor to the nucleus and its requirement for growth factor induction of cyclin D and other genes has been reported. Unresolved is the mechanism by which this or other transmembrane proteins are excised from a lipid bilayer before nuclear translocalization. We report that, after the addition of EGF, the cell surface EGF receptor is trafficked to the endoplasmic reticulum (ER) where it associates with Sec61β, a component of the Sec61 translocon, and is retrotranslocated from the ER to the cytoplasm. Abrogation of Sec61β expression prevents EGF-dependent localization of EGF receptors to the nucleus and expression of cyclin D. This indicates that EGF receptors are trafficked from the ER to the nucleus by a novel pathway that involves the Sec61 translocon.
INTRODUCTION
Although intracellular trafficking destinations for activated epidermal growth factor (EGF) receptors, such as the lysosome or recycling to the cell surface, are relatively well understood (Sorkin and von Zastrow, 2002), little is known regarding the mechanism by which this or other cell surface receptors reach the nucleus. In addition to the EGF receptor (Lin et al., 2001), the list of nuclear-translocated hormone receptors includes several receptor tyrosine kinases (ErbB-2, Wang et al., 2004; ErbB-3, Offterdinger et al., 2002; ErbB-4, Ni et al., 2001; FGFR-1, Maher, 1996; Stachowiak et al., 1996; FGFR-2, Schmahl et al., 2004), as well as G protein–coupled receptors (angiotensin I, Lee et al., 2004; angiotensin II, Chen et al., 2000; endothelin, Boivin et al., 2003; bradykinin, Lee et al., 2004). In the case of ErbB-4 it is a secretase-produced intracellular domain fragment that is translocated to the nucleus (Ni et al., 2001). In the other instances, however, a full-length receptor is found in the nucleus in a nonmembranous environment, raising the question of how these transmembrane molecules are extracted from lipid layers.
Nuclear localization of the EGF receptor (Lo et al., 2006), ErbB-2 (Giri et al., 2005), and FGFR-1 (Reilly and Maher, 2001) require endocytosis and association of the receptor with importin-β. However, this does not suggest how a transmembrane receptor is processed to the nuclear nonmembrane-bound receptor. As cells do have protein complexes that translocate proteins into and out of lipid bilayers (Wickner and Schekman, 2005), we have explored the possibility that one of these, the Sec61 translocon, could mediate nuclear localization of the EGF receptor. This translocon is located exclusively in the endoplasmic reticulum (ER) and ER/Golgi transitional region (Greenfield and High, 1999) and functions to insert secretory and transmembrane proteins into the ER during protein synthesis (Tsai et al., 2002). The translocon is bidirectional and retrotranslocates misfolded proteins in the ER to the cytosol for degradation as part of the ER-associated degradation (ERAD) pathway. Although Sec61 has no known role in signal transduction, it does retrotranslocate certain toxins trafficked from the cell surface to the ER to the cytosol and is an essential part of the intoxication process (Sandvig and van Deurs, 2002).
In the instance of the EGF receptor it is reported that the nuclear translocation is EGF-dependent and that the nuclear receptor associates with promoters for cyclin D (Lin et al., 2001), iNOS (Lo et al., 2005a), and c-myb (Hanada et al., 2006). This suggests that nuclear localization of the EGF receptor is both a trafficking and a signal transduction pathway. Nuclear EGF receptors have been identified by a variety of biochemical and morphological techniques in both cell lines and tumor tissue specimens (Lin et al., 2001, 2005b; Psyrri et al., 2005). Also, nuclear EGF receptor expression portends a poorer prognosis for breast (Lo et al., 2005b) and oropharyngeal (Psyrri et al., 2005) cancer patients.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle's medium (DMEM) containing l-glutamine and high glucose and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA), human breast cancer cell line MDA-MB-468 and HeLa cells were from ATCC (Manassas, VA). EZ-link Sulfo-HS-LC-Biotin was from Pierce (Rockford, IL). Protease inhibitor cocktail tablet was from Roche (Indianapolis, IN). OptiPrep density gradient medium, Pseudomonas exotoxin A (Exo A), and antibody to Pseudomonas exotoxin A were from Sigma (St. Louis, MO). Recombinant human EGF was obtained from R&D Systems (Minneapolis, MN), and Endoglycosidase H (Endo H), and EGFR kinase inhibitor AG 1478 were from Calbiochem (San Diego, CA). [α-32P]dATP was purchased from New England Life Science Products (Boston, MA). Prime-It II random primer labeling kit was from Stratagene (La Jolla, CA), and Lipofectamine 2000 reagent was from Invitrogen (Carlsbad, CA). Antibodies to EGF receptor (06–847), phospho EGF receptor, and Sec61β were from Upstate (Lake Placid, NY), and antibodies to cyclin D1, HSP70, HSP70 agarose conjugate, c-Fos, HDAC1, EEA1, phospholipase C γ-1, Erk 1, 2, and dual phosphorylated Erk 1and 2 were from Santa Cruz Biotechnology (Santa Cruz, CA); and antibodies to Lamp-1 and calnexin were from BD Transduction Laboratories (Lexington, KY). Antibody to transferrin receptor was from Zymed (South San Francisco, CA). pDsRed2-ER construct [calreticulin red fluorescent protein (RFP)] was from Clontech (Palo Alto, CA). The EGFR-GFP construct was a gift from Dr. A. Sorkin (University of Colorado Health Science Center, Denver). Mouse cyclin D1 cDNA was a gift from Dr. B. Law (Vanderbilt University, Nashville, TN). Human Sec61 β cDNA was a gift from Dr. S. High (University of Manchester, United Kingdom). Cyclophilin cDNA was a gift from Dr. C. Hao (Vanderbilt University). Sec61 β siRNAs were synthesized by Dharmacon (Boulder, CO).
Cell Culture and Biotinylation
MDA-MB-468 and HeLa cells were cultured in DMEM containing 10% FBS. Forty to 60% confluent cells were incubated overnight in DMEM before stimulation by EGF (25 ng/ml). Cells were washed three times with PBS (pH 8.0) and then incubated with 0.5 mg/ml Sulfo-NHS-Biotin reagent at room temperature for 30 min. Cells were then washed three times with PBS plus 100 mM glycine to quench the reaction.
Purification of ER
The basic procedure was described previously (Higashi et al., 2002; see also OptiPrep Application S16. Axis-Shield POC, AS. http://www.axis-shield.com/optiprep/S14.pdf). Briefly, cells cultured in 10 15-cm dishes were treated with EGF for 3 h as indicated. Subsequently, the cells were harvested, washed twice in ice-cold PBS, and resuspended in 6 ml of homogenization buffer (10 mM Tris-HCl, pH 7.5, 250 mM sucrose, and protease inhibitor cocktail tablet, 1 tablet/10 ml). Cells were homogenized (20 strokes) in the same buffer and centrifuged (12,000 × g, 20 min) at 4°C. The resulting supernatant was centrifuged (100,000 × g, 45 min) at 4°C to obtain a microsomal pellet, which was resuspended in 3 ml homogenizing buffer and 6.67 vol of microsome suspension was mixed with 3.33 vol of Optiprep (final iodixanol concentration 20%; p = 1.127 g/ml). The mixture was transferred to tubes (1 ml/tube) and centrifuged (200,000 × g, overnight). One-drop ER fractions were collected by tube puncture.
ER Retrotranslocation Assay In Vitro
OptiPrep ER fractions (7–14) were combined and mixed with an equal volume of homogenization buffer. ER was recovered by centrifugation (100,000 × g, 20 min) and resuspended briefly in ice-cold 10× translocation buffer (20 mM HEPES, pH 7.2, 40 mM Mg acetate, 10 mM DTT, and 1 mM PMSF) before dilution into retrotranslocation assays. Cytosol was obtained from the 100,000 × g supernatant in ER purification. The following reaction conditions were used: Control reaction (cytosol 180 μl + 20 μl 10× translocation buffer); ER-containing reactions (20 μl ER suspension was mixed with either 180 μl cytosol or homogenization buffer). The mixtures were then incubated for 60 min at 37°C, before centrifugation (100,000 × g, 10 min) to yield a pellet (P) and soluble (S) fractions. For inhibitor experiments 20 μl of the ER suspension was preincubated with or without 1 μl Sec61β antibody or Exo A (1 μg) for 30 min at room temperature.
Preparation of Nuclear Extracts and SDS Lysates
The basic nuclear fraction protocol was described previously (Lin et al., 2001). Briefly, cells in a 10-cm dish were rinsed twice with ice-cold PBS and removed with a rubber cell scraper in 1 ml buffer A (10 mM HEPES, pH 7.5, 10 mM KCl, 2 mM MgCl2, protease inhibitor tablet with EDTA at 1 tablet/10 ml) containing 1% NP-40. Cells were disrupted by 10 passes through a 21-gauge needle and the extent of nuclear isolation was monitored microscopically. Nuclei were centrifuged (500 × g, 5 min) and washed once with buffer A. The resulting supernatant was designated as the nonnuclear fraction. The nuclear pellet was resuspended in 50 μl buffer A supplemented with 500 mM NaCl and 25% glycerol and kept on ice for 30 min. Samples were centrifuged (12,000 × g, 5 min), and the supernatant (nuclear extracts) was aliquoted and frozen at −80°C. The pellet (SDS lysate) was solubilized in 1× SDS-PAGE sample loading buffer.
Coprecipitation and Western Blotting
Cells were lysed in cold buffer A containing 1% NP-40 and incubated for 30 min in ice. After centrifugation (12,000 × g, 5 min), anti-Sec613β antibody and protein A beads were added to the supernatant and incubated overnight. The precipitate was then washed three times with buffer A. After SDS-PAGE and transfer to nitrocellulose membranes, the samples were probed with the indicated antibody. For Western blots, cell lysates were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and probed with the indicated antibody. Bound antibody was detected by enhanced chemiluminescence (ECL).
Northern Blotting
Total RNA was isolated using a TRIzol Reagent according to the manufacturer's instructions (Invitrogen). An aliquot (5 μg) of total RNA was electrophoresed, transferred to Duralon-UV membranes (Stratagene), and probed with a labeled cDNA fragment of cyclin D1 according to standard procedures. A probe for cyclophilin was used as an internal control. The probe was labeled with [α32P]dATP using Prime-It II random primer labeling kit.
siRNA Knockdown of Sec61 β
siRNAs for human Sec61 β cDNA were selected using an advanced version of siRNA Sequence Selector (Clontech). Only those sequences with more than three mismatches against unrelated genes were selected. Four different siRNAs were inserted into pSuper vector (Oligoengine, Seattle, WA) and transfected transiently into MDA-MB-468 cells to monitor expression of Sec61β protein. The most effective Sec61β siRNA was chosen for experiment. The RNA sequence is 5′-GCAAGUACACUCGUUCGUA-3′ from site 347-366 of human Sec61β mRNA (NM_006808). The mismatch siRNA has a two-base pair change in the middle of the antisense and the sequence is 5′-GCAAGUAGAGUCGUUCGUA-3′. The siRNA duplexes were synthesized in-house as 21-mers with UU overhangs using a modified method of 2′-acid labile orthoester chemistry (Scaringe, 2000), and the anti-sense strand was chemically phosphorylated to ensure maximized activity (Martinez et al., 2002). The siRNA duplex was resuspended in 1× siRNA Universal buffer (Dharmacon) to 2 μM before transfection. Cells in six-well plate (50–60% confluent) were transfected with 100 μl of 2 μM siRNA duplex and 4 μl of transfection reagent (Dharmacon) in 100 μl DMEM. Cells were subcultured (1:1 split) 24 h after transfection and placed into normal culture medium for 2 d before experiments, which were performed 3 d after the initial transfection. The final concentration of siRNA was 0.1 μM and the data in Supplementary Figure S1 show, in titration experiments, that this was the minimal effective concentration for maximal depletion of Sec61β protein.
Confocal Microscopy and EGFR-mGFP
EGFR-GFP construct (Carter and Sorkin, 1998) was used to make a point mutation of A206K in the GFP sequence by QuikChange (Stratagene) to prevent GFP dimerization as described elsewhere (Zacharias et al., 2002). MDA-MB-468 cells were cotransfected with pEGFR-mGFP and pDsRed2-ER DNA (Clontech) using Lipofectamine 2000 according to the manufacturer's instruction. The cells were subcultured (1:1 split) 24 h after transfection and placed into normal culture medium for 24 h. Cells were serum-starved overnight and incubated with or without EGF (25 ng/ml) for the indicated time. Cells were imaged with a Zeiss LSM510 confocal scanning microscope Thornwood, NY) and a Plan-Neofluar 40× 1.3 NA oil immersion lens was used for imaging all the samples with a 1.0–1.5-μm optical slice. Green fluorescent protein (GFP) was excited with an argon laser with excitation at 488 nm, and RFP was excited at a 543 nm. The emission was detected with filter sets (505–550 bandpass for GFP and 560 longpass for RFP). Image analysis was performed using Metamorph software (Universal Imaging, West Chester, PA). A 20-μm-width line intensity scan was used to show colocalization of GFP and RFP.
RESULTS
Translocation of Cell Surface EGF Receptor to the ER
To assess possible trafficking of EGF receptors from the cell surface to the ER, both biochemical and morphological approaches have been used. In the former experiments, ER was OptiPrep gradient purified to obtain a fraction with minimal contamination by other organelles, particularly those expected to contain mature EGF receptor (plasma membrane, endosomes). As shown in Supplementary Figure S2, markers of late endosomes/lysosomes (LAMP-1), cytoplasm (PLC-γ1), nuclei, (HDAC1), early endosomes (EEA1), and plasma membrane (biotinylated cell surface proteins) were not present at detectable levels in the purified ER preparation, whereas the ER marker calnexin was enriched.
To test EGF-dependent trafficking of cell surface receptors to the ER, MDA-MB-468 cells were cell surface biotinylated and treated with or without EGF for 3 h before ER isolation. As a control, the cells were also biotinylated after the incubation with EGF. Analysis of the purified ER fraction from these cells (Figure 1A) shows that biotinylated EGF receptor is recovered in the ER fraction from cells treated with EGF after, but not before, biotinylation. A low amount of receptor signal is detectable in the absence of exogenous EGF and likely results from autocrine production of transforming growth factor α by these tumor cells (Bjorge et al., 1989) that overexpress the EGF receptor (Filmus et al., 1985). Analysis of the Optiprep fractions with an antibody to pY1173 EGF receptor (Figure 1B) shows that tyrosine-phosphorylated EGF receptor is present in the ER from cells incubated with EGF at 37°C, but not at 4°C. The data in Figure 1C show that EGF treatment of cells at either temperature results in comparable levels of activated EGF receptor. Together these results indicate that after the addition of EGF activated cell surface EGF receptors are trafficked to the ER in a manner that requires cellular metabolism.
Figure 1.
Activated EGFR trafficking to the ER. (A) MDA-MB-468 cells were treated without or with EGF (25 ng/ml) for 3 h and were subjected to cell surface biotinylation before (pre) or after (post) EGF treatment. Subsequently, ER was purified by OptiPrep gradient techniques. Top panel, the ER was lysed, and immobilized NeutrAvidin beads were added. Subsequently, the precipitates were blotted with anti-EGFR. Middle panel, equal aliquots of ER lysate were blotted for the ER marker calnexin. Bottom panel, cell lysates were precipitated by NeutrAvidinTM beads and blotted with anti-EGFR. (B) MDA-MB-468 cells were treated with EGF for 3 h at 37 or 4°C. ER was isolated and ER fractions were subjected to Western blotting with anti-phosphoEGFR. The blot was stripped and then blotted with anti-calnexin. (C) MDA-MB-468 cells were untreated or treated with EGF for 10 min at 37 or 4°C. The cell lysates were blotted with anti-phospho EGFR and reblotted with anti-EGFR.
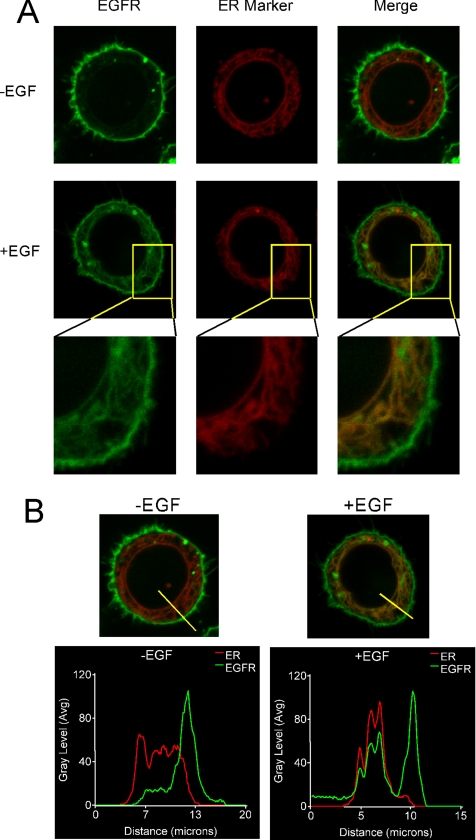
To independently assess receptor trafficking to the ER, a noninvasive morphological technique was used. Cells were cotransfected with EGFR-mGFP and the ER marker calreticulin-RFP for 48 h. The cells were then incubated with EGF for 6 h, and examined by confocal microscopy (Figure 2). In the absence of EGF, the receptor is predominantly distributed at the plasma membrane and in organelles that are most likely Golgi. Only a very low level of EGFR-mGFP signal is detectable in the ER. After incubation with EGF, however, there is a large increase in EGFR-mGFP signal that overlaps with the calreticulin-RFP signal. This occurs throughout the lattice-like ER network and includes the nuclear membrane, the outer portion of which is contiguous with ER. Images of additional control and EGF-treated cells are presented in Supplementary Figure S3. In Figure 2B, the overlap of marker profiles along a line from the nucleus to the plasma membrane is presented. This Metamorph software analysis shows increased nuclear EGF receptor (x-axis, 0–5 μm) in the EGF-treated cells.
Figure 2.
EGF-induced EGFR-mGFP translocation to ER. (A) MDA-MB-468 cells were transiently cotransfected with cDNAs encoding the ER protein calreticulin and EGFR fused, respectively, with RFP or mGFP. The cells were then treated with EGF for 0 or 6 h. Live-cell images were taken by confocal microscopy with a 1–1.5-μm optical slice. (B) The merged images from A are repeated with line intensity scan using Metamorph software to assess of colocalization of these two markers. A 20-μm-wide yellow line was drawn as indicated in control and EGF-treated cells. Intensity profiles corresponding to the yellow lines were determined using Metamorph software. Y- and X-axes represent the level of fluorescence and scanning position, respectively.
These results establish a novel destination for the intracellular trafficking of EGF receptors. Exposure of cells to the selective EGF receptor kinase inhibitor AG1478 prevents trafficking of receptor to the ER (data not shown) consistent with reports that kinase activity is required for EGF receptor internalization (Sorkina et al., 2002). Although nuclear EGFR-mGFP is difficult to discern in Figure 2, when the confocal plane is focused on the nucleus this becomes evident, as shown in Supplementary Figure S4. Interestingly, most but not all cells demonstrate a diffuse nuclear signal consistent with the reported nonmembranous mature nuclear EGF receptor (Lin et al., 2001).
Interaction of EGF Receptor and Sec61
The Sec61 translocon, which contains three transmembrane proteins (α, β, γ), is known to mediate the retrotranslocation of ER proteins to the cytosol (Tsai et al., 2002). To assess whether ER-localized EGF receptor could associate with Sec61, cells were incubated with or without EGF. Cell lysates were precipitated with antibody to Sec61β, and the precipitates were probed with anti-EGF receptor. As shown in Figure 3A, EGF increased the amount of receptor present in the Sec61β precipitates in a manner that increased over a period of 3 h and was blocked by the presence of the tyrosine kinase inhibitor AG1478. Control data in Figure 3B show that this association, which is unusually sensitive to salt and, therefore, probably weak, requires EGF addition at 37°C and does not occur when the cells are treated with EGF at 4°C, decreasing the possibility of post-lysis association. It is likely that Sec61β precipitates the Sec61 complex of α, β, and γ components, and the association data could reflect receptor interaction with any of these subunits or other translocon-associated proteins.
Figure 3.
EGF-dependent association of EGFR and Sec61β. (A) MDA-MB-468 cells were preincubated with 2 μM AG1478 for 30 min before the addition of EGF, as indicated. The cells were then lysed, anti-Sec 61β was added, and the precipitate was blotted with anti-EGFR or anti-Sec61β. (B) The cells were treated with EGF for 3 h at 37 and 4°C. The cells were then lysed, anti-Sec 61β and protein A beads were added, and the precipitate subsequently was blotted with anti-EGFR or anti-Sec61β. Lane 1 is a control representing no addition of anti-Sec61β. The lysates (lanes 2–4) were immunoprecipitated with anti-Sec61β and protein A beads. A duplicate blot was probed with anti-Sec61β. (C) The cells were treated with EGF for 3 h, and the lysates were precipitated with anti-Sec61β. The precipitates (lanes 1 and 2) were then incubated overnight with or without Endo H and blotted with anti-EGFR. Parallel cultures, not treated with EGF, were preincubated without or with swainsonine (SW; 1 μg/ml) for 2 d before precipitation with anti-EGFR, digestion with Endo H, and blotting with anti-EGFR. (D) Cells were preincubated with 10 μM actinomycin D (act) or 10 μg/ml cycloheximide (CH) for 30 min before the addition of EGF for 3 h. Top panel, the cell lysates were precipitated with anti-Sec61β and blotted with anti-EGFR. Arrows mark the 170-kDa mature EGF receptor and 150-kDa fragment. Bottom panel, the nuclear extracts were blotted with anti-EGFR. Arrows mark the 170-kDa mature EGF receptor and the 150- and 130-kDa fragments.
In this and other experiments the mature 170-kDa EGF receptor and a lower Mr fragment(s) of 150 kDa or less are routinely detected. In many experiments the amount of the fragment is significantly greater that the level of intact native receptor. Based on experiments with an N-terminal Flag construct, these fragments are produced by the loss of N-terminal sequences (data not shown). Others have reported similar EGF receptor fragments in cells treated with EGF for 2 h or more (Carter and Sorkin, 1998).
Although it might be expected that misfolded immature EGF receptor present in the ER would interact with Sec61 as part of the ERAD pathway, this would not be dependent on exogenous EGF or tyrosine kinase activity. Nevertheless, we have used endoglycosidase H (EndoH) digestion to test whether the Sec61-associated receptor is, in fact, mature receptor (Figure 3C). The EGF receptor has 10 N-linked oligosaccharide chains (Cummings et al., 1985) and when all are immature, high-mannose chains, EndoH removes all and the Mr decreases from 170 to 130 kDa (Soderquist and Carpenter, 1984), as demonstrated in lanes 5 and 6. In contrast, EndoH produces a small decrease, from 170 to ∼165 kDa, in Mr of the mature receptor, which has seven complex and three high-mannose chains (Cummings et al., 1985; Zhen et al., 2003), as shown in lanes 3 and 4. The lower band in lane 4 arises from a small fraction of immature receptor that is always present in the intracellular receptor pool. Importantly, lanes 1 and 2 show the relative insensitivity of the Sec61-associated EGF receptor to EndoH. The predominant EGF receptor species present before EndoH treatment is the 150-kDa fragment (lane 1) and EndoH treatment reduces this to a slightly lower Mr of ∼145 kDa, consistent with the removal of the few high-mannose oligosaccharides present in the mature receptor. Therefore, under these conditions the majority of EGF receptor associated with Sec61 is mature and not immature receptor.
Because EGF does provoke an increase in EGF receptor synthesis in these cells (Kudlow et al., 1986), RNA or protein synthesis was blocked before the addition of EGF and subsequent immunoprecipitation of Sec61. The results (Figure 3D) show that EGF induces receptor association with Sec61 in the absence of ongoing protein or mRNA synthesis. This result also indicates that it is mature and not immature EGF receptor associated with Sec61. Also, in Figure 3D data show that neither cycloheximide nor actinomycin D prevents the nuclear localization of the EGF receptor in EGF-treated cells.
Receptor Retrotranslocation by Sec 61
That EGF induces trafficking of its receptor to the ER and association with Sec61 suggests that, as a consequence, the receptor could be retrotranslocated to the cytosol. To test this, OptiPrep-purified ER from EGF-treated cells was prepared and examined for receptor retrotranslocation. The data in Figure 4A show that when this purified ER fraction is incubated in the presence of cytosol, but not in its absence, EGF receptor initially present in the ER is recovered in the cytosol. In contrast, the ER marker Sec61β is not lost from the ER indicating that incubation with cytosol does not promote loss of ER integrity.
Figure 4.
Retrotranslocation of EGFR from the ER to cytosol. (A) Cells were incubated with EGF for 3 h and the ER was OptiPrep was purified. Cytosol prepared from control cells was added as indicated to equal aliquots of ER. After a 60-min incubation at 37°3 C, the reaction mixture was centrifuged to yield pellet (P) and soluble (S) fractions. These fractions were then blotted for EGFR or Sec61β as indicated. (B) ER was incubated with control or HSP70 immunodepleted cytosol and then blotted with antibodies as indicated. (C and D) ER was preincubated with anti-Sec61β (C) or exotoxin A (D) for 30 min at room temperature before addition of cytosol. (E) Cells were subjected to cell surface biotinylation and incubated with EGF for 3 h. The ER fraction was isolated and incubated for 60 min at 37°C without or with cytosol prepared from untreated cells. The reaction mixture was centrifuged to yield pellet (P) and soluble (S) fractions. Detergent was then added to solubilize the ER fraction, and anti-EGFR was added to each fraction and the precipitates were blotted with HRP-streptavidin. The film was exposed for two different times to more clear show receptor bands in the P and S fractions. Equal aliquots of P and S fractions were also blotted for Sec61β. (F) ER fraction was incubated for 60 min at 37°C with cytosol. The soluble fraction was precipitated with anti-EGFR, and the precipitate was incubated with Endo H. The mixtures were then subjected to blotting for EGFR (lanes 1 and 2). EGFR immunoprecipitates from cell lysates from control (lanes 3 and 4) or swainsonine-treated (SW) cells (lanes 5 and 6) were digested by Endo H and blotted for EGFR. Arrows mark the 170-kDa mature receptor and a 150-kDa receptor fragment.
In subsequent experiments the retrotranslocation of EGF receptors in this system is shown to be dependent on cytosolic HSP-70 (Figure 4B) and blocked by preincubation with anti-Sec61β (Figure 4C) or exotoxin A (Figure 4D), which targets Sec61 (Koopmann et al., 2000) in vitro. These results establish that movement of EGF receptor from the ER to the cytosol in these assays is Sec61-dependent. That the assay is measuring retrotranslocation of mature EGF receptors derived from the cell surface is evidenced by the data in Figure 4E in which cell surface proteins were biotinylated before EGF treatment and ER isolation. Also, EndoH digestion (Figure 4F) shows that the retrotranslocated receptor is slightly sensitive to this glycosidase, typical of the mature receptor. Lanes 1 and 2 show that two EGF receptor species are recovered in the cytosol after retrotranslocation and the Mr of each is slightly decreased by exposure to EndoH. This is in marked contrast to the large Mr decrease provoked by EndoH digestion of immature receptor accumulated in swainsonine-treated cells.
In these retrotranslocation assays the rate of receptor movement is slow compared with other substrates in other retrotranslocation assay systems, which usually employ crude microsomes. Also, in our system the addition of ATP is inhibitory, whereas in other systems it is stimulatory. The exotoxin A data (Figure 4D) indicate that the OptiPrep purified ER is not tightly sealed in contrast to microsomal systems in which transient detergent permeabilization is necessary for exotoxin A inhibition of Sec61 (Koopmann et al., 2000). In our assays detergent has not been used and yet the exotoxin A blocks and associates with Sec 61β retrotranslocation (Figure 4D). If the OptiPrep-purified ER is not tightly sealed, this may account for the slow rate of retrotranslocation and the inhibitory effect of ATP, which can inhibit early lumenal steps in the retrotranslocation process (Lyman and Schekman, 1997). That ATP inhibits receptor translocation and yet HSP70 is required indicates that the chaperone function of HSP70 is not necessary only its capacity to bind hydrophobic substrate regions, in this case probably the transmembrane domain of the EGF receptor.
If EGF receptor is retrotranslocated to the cytoplasm in intact cells, then it might be detectable in the cytosol of the EGF-treated cells unless it is degraded or rapidly translocated elsewhere. However, attempts to detect cytosolic EGF receptor in vivo have not been successful. While it is theoretically conceivable that retrotranslocation might occur directly into the nucleoplasm from the inner nuclear membrane, this would require gated movement of Sec61 and EGF receptor from the outer nuclear membrane and is unlikely.
Role of Sec61β in Nuclear Localization and Cyclin D Expression
The preceding data suggest that Sec61-dependent processing of the EGF receptor could function as a precursor to remove the receptor from the lipid bilayer, present it to the cytoplasm, and thereby mediate nuclear translocation. To test this, siRNA depletion of Sec61β was used. The high-resolution structures of bacterial Sec61 orthologues suggest that the α and γ component are essential channel components, whereas the more peripheral β protein has a less clear functional role (van den Berg et al., 2003). This observation may suggest that knockdown of Sec61β may be more tolerable than other Sec61 subunits, particularly regarding interference with EGF receptor biosynthesis.
The data in Figure 5A show that transient knockdown of Sec61β substantially depletes the intracellular pool of Sec61β protein and mRNA, but does not attenuate the level of EGF receptor protein nor cyclophilin mRNA. Also, this figure shows that after depletion of Sec61β the addition of EGF readily provokes activation of the EGF receptor equivalent to that of control cells.
Figure 5.
Influence of Sec61β knockdown on the EGFR translocation to the nucleus. (A) MDA-MB-468 cells were transiently transfected with Sec61β siRNA duplex for 3 d. Cell lysates were then blotted with EGFR and Sec61β antibodies, and total RNA was blotted with human Sec61β or cyclophilin cDNA as indicated. Also, cells were stimulated with or without EGF for 5 min, and equal lysate aliquots were blotted with anti-phosphoEGFR or EGFR antibodies. (B) Cells were treated with EGF (25 ng/ml) for the indicated times, high-salt nuclear extracts were isolated, and blotted with a C-terminal antibody to EGFR. The same blot was stripped and reblotted with HDAC1. Arrows mark the 170-kDa mature EGF receptor and the 150- and 130-kDa fragments. (C) Cells were incubated without or with EGF for 3 h, and the nuclear fraction was prepared as described in Materials and Methods. The nuclear high-salt extracts and SDS-solubilized residual nuclear material were blotted with anti-EGFR, stripped, and reblotted with the nuclear marker, HDAC1.
To determine whether Sec61β knockdown effects EGF receptor nuclear localization, we first assessed the time course of this process using a nuclear fraction prepared as described elsewhere (Lin et al., 2001) and analyzed for various markers, as shown in Supplementary Figure S5A. Only a small level of the ER marker calnexin could be detected. As shown in Figure 5B, during the first 30–90 min after the addition of EGF there is a rapid increase in the level of high-salt extractable mature 175-kDa EGF receptor from the nucleus, as noted by others (Lin et al., 2001). That the receptor is salt extractable indicates it is not membrane-bound. However, we note that the level of nuclear receptor continues to increase during the first 3 h, and during this time 150- and 130-kDa fragments, observed in our previous experiments, are also detected.
When the EGF-dependent nuclear translocation of EGF receptor is measured in control and Sec61β-depleted cells (Figure 5C), it is clear that the level of high-salt extractable receptor is significantly reduced in the knockdown cells. This indicates that Sec 61β is necessary for the nonmembranous nuclear localization of the EGF receptor. Interestingly, if the residual high-salt extracted nuclear fraction is subsequently treated with SDS, EGF receptor is recovered from the small interfering RNA (siRNA)-treated cells. This suggests that the receptor in this nuclear fraction remains in a membrane environment, either in the nucleus or more probably in peripheral ER present in the nuclear fraction. The ER marker calnexin can be recovered from this nuclear fraction with SDS, but is not high-salt extractable (Supplementary Figure S5B).
Others have identified the cyclin D promoter as a target of the nuclear EGF receptor (Lin et al., 2001) and have provided evidence that nuclear localization of the receptor is necessary for cyclin D expression in EGF-treated cells (Lo et al., 2005a). Therefore, we have used Sec61β-depleted cells to test whether the loss of this translocon component interferes with EGF induction of cyclin D. This assay also allows the assessment of Sec61 function in cells that do not overexpress the EGF receptor, such as HeLa cells that express 20-fold fewer receptors than MDA-MB-468 cells (Berkers et al., 1991). As a control, the expression of c-Fos has been measured. The data show that when measured at the protein (Figure 6A) or mRNA level (Figure 6B), cyclin D and c-Fos are induced by EGF in both cell types. However, the induction of cyclin D, but not c-Fos, is significantly diminished in either cell type exposed to Sec61β siRNA. That the EGF-induction of c-Fos is not impaired by Sec61 β siRNA indicates that the knockdown does not perturb EGF signaling to the nucleus in general. Also, a two-base change mutant Sec61 β siRNA did not abrogate EGF-induced cyclin D1 expression (Supplementary Figure S6). These results indicate that Sec61 is required not only for nuclear localization of the EGF receptor, but also for the receptor's capacity to act as a cotranscriptional activator.
Figure 6.
Influence of Sec61 mRNA knockdown on cyclin D1 expression. MDA-MB-468 cells or HeLa cells were transiently transfected with Sec61β siRNA and 72 h later EGF was added for the indicated times. (A) The cells were lysed and the expression of cyclin D and Sec61β was estimated by blotting, whereas nuclear extracts were blotted for c-Fos. Erk-1 or phospholipase C-γ1 were used as loading controls, as indicated. (B) Total RNA was isolated from control and EGF-treated and Northern blotted with a cyclin D1 probe. Cyclophilin3 served as a loading control. Also, control blots for Sec61β or Erk-1 are shown for both cell types. Control blots show equivalent level of EGFR in both cells types with or without siRNA knockdown by Sec61β (data not shown).
DISCUSSION
The data presented herein, as depicted in Figure 7, describe a new route of intracellular trafficking not only for the activated EGF receptor, but for any hormone receptor. However, this pathway from the cell surface to the ER is known for certain toxins (Sandvig and van Deurs, 2002) and for the SV40 virus (Pelkmans and Helenius, 2003). In the former case toxins, such as cholera toxin, are internalized primarily, but not exclusively, from caveolae and trafficked first to the Golgi and then retrogradely transported to ER. Transport to the cytosolic site of toxin targets is mediated by the Sec61 translocon. SV40 is internalized from caveolae at the cell surface and via intracellular vesicles, termed caveosomes, trafficked to the ER. It is not know how virus particles exit the ER, but interruption of the pathways blocks virus replication, which requires nuclear localization. Preliminarily, it has been reported that translocons in the ERAD pathway mediate virus penetration to the cytosol (Marsh and Helenius, 2006). These systems show that, in addition to their role in protein quality control, translocons in the ER facilitate the mechanism of action of biological agents.
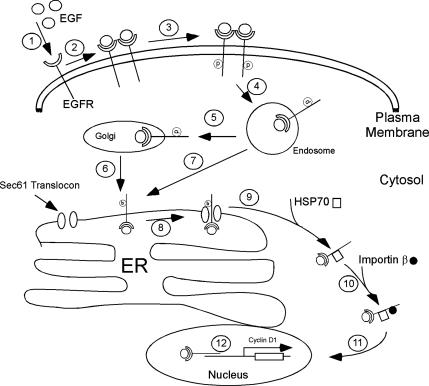
Figure 7.
Schematic diagram of Sec61-dependent trafficking of EGF receptor from the cell surface to the nucleus. Steps 1–4 represent the well described binding of EGF to its receptor, dimerization activation, translocation to cell surface coated pits, and internalization. Endosomal sorting directly to the ER or indirectly via the Golgi is presented in steps 5–7. ER association of the receptor with Sec61 (step 8) leads to retrotranslocation to the cytosol (step 9) and association with HSP70 (step 10). Interaction with importin β (Lo et al., 2006) precedes nuclear localization (step 11) and interaction with target promoters, such as cyclin D (step 12). Because Lin et al. (2001) have reported the presence of EGF in the nucleus, the intact ligand receptor:complex is depicted.
Intracellular trafficking of EGF receptors to the ER has not been previously reported (Sorkin and von Zastrow, 2002) and may have been missed for several reasons. We have attempted to quantitate the level of EGF receptor trafficked to the ER using cell surface biotinylation and OptiPrep purification of ER (as in Figure 1A, with calnexin as an ER marker) and by Metamorph software analysis of EGFR-mGFP (as in Figure 2B). At 3 h after the addition of EGF, these analyses indicate that 6 or 12%, respectively, of the total receptor is present in the ER. At 6 h, Metamorph analysis indicates that ∼25% of the total EGFR-mGFP is present in the ER of EGF-treated cells. Because neither of these methodologies is precise for quantitation, the values are only approximate. Therefore, EGF receptor trafficking from the cell surface to the ER is relatively slow and involves, in the first hour, a small pool of internalized receptor. Because most published trafficking studies of the EGF receptor have focused on events within 1 h after growth factor addition, it seems likely that the ER pool was too small to be considered significant in previous investigations.
Interestingly, trafficking of SV40 and cholera toxin from the cell surface to the ER is also on the order of 2–3 h (Pelkmans et al., 2001; Fujinaga et al., 2003). In terms of a mechanism of trafficking of the EGF receptor to the ER, little is known, including whether the Golgi is an intermediate (Figure 7). Others (Lo et al., 2006) have reported that the receptor-mediated endocytosis and importin β are required for nuclear localization of the EGF receptor and this is consistent with trafficking to the ER after coated-pit internalization (Figure 7).
A major obstacle in understanding trafficking to the nucleus is reconciling a known mechanism with the fact that the nuclear EGF receptor is a transmembrane domain-containing molecule in a nonmembranous environment (Lin et al., 2001). Sec61 provides a mechanism to extract the receptor from its lipid bilayer. As part of the ERAD pathway, Sec61 retrotranslocates malfolded transmembrane proteins to the cytosol for proteosomal degradation. This often requires a cytoplasmic chaperone, such as HSP70 (Römisch, 2005), which we have observed in in vitro assays. HSP70 may simply function to prevent receptor aggregation in the cytosol (Figure 7).
Efficient proteosomal degradation of retrotranslocated glycoproteins requires the prior removal of N-linked oligosaccharides by peptide-N-glycanase (Hirsch et al., 2003). Glycoprotein substrates in the ERAD system contain only high-mannose chains and peptide-N-glycanase exhibits a strong preference for high-mannose oligosaccharide-containing substrates. Therefore, the mature EGF receptor should be a poor substrate for proteosomal degradation, and this may indirectly promote receptor translocation to the nucleus. Recently, EGF receptor has been reported to be associated with mitochondria (Boerner et al., 2004), which could be another trafficking site for retrotranslocated receptor.
That depletion of Sec61β abrogates not only nuclear localization of the EGF receptor, but also EGF-dependent cyclin D expression indicates that the Sec61 translocon participates in a growth factor signal transduction pathway. Such a role for Sec61 has not been reported previously for any receptor signaling pathway. However, there is an increasing level of evidence that EGF receptor trafficking and signaling are functionally interrelated (Miaczynska et al., 2004a). In particular, endocytosis of EGF receptors is required for nuclear translocation of STAT (Bild et al., 2002) and APPL-1 (Miaczynska et al., 2004b). Whether the Sec61 pathway also facilitates the delivery of receptor-associated signaling molecules remains unresolved.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Carpenter for manuscript preparation. Experiments and data analysis were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by National Institutes of Health Grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126) and with the assistance of S. Wells and J. S. Goodwin. Support for the authors from the Department of Defense (BC045152) and NIH (P30 CA98131, P50 CA68485) is acknowledged.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0802) on January 10, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org)
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org)
REFERENCES
- Berkers J.A.M., van Bergen en Henegouwen P.M.P., Boonstra J. Three classes of epidermal growth factor receptors on HeLa cells. J. Biol. Chem. 1991;266:922–927. [PubMed] [Google Scholar]
- Bild A. H., Turkson J., Jove R. Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO J. 2002;21:3255–3263. doi: 10.1093/emboj/cdf351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorge J. D., Paterson A. J., Kudlow J. E. Phorbol ester or epidermal growth factor (EGF) stimulates the concurrent accumulation of mRNA for the EGF receptor and its ligand transforming growth factor-α in a breast cancer cell line. J. Biol. Chem. 1989;264:4021–4027. [PubMed] [Google Scholar]
- Boerner J. L., Demory M. L., Silva C., Parsons S. J. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell. Biol. 2004;24:7059–7071. doi: 10.1128/MCB.24.16.7059-7071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B., Chevalier D., Villeneuve L. R., Rousseau É, Allen B. G. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J. Biol. Chem. 2003;278:29153–29163. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- Carter R. E., Sorkin A. Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 1998;273:35000–35007. doi: 10.1074/jbc.273.52.35000. [DOI] [PubMed] [Google Scholar]
- Chen R., Mukhin Y. V., Garnovskaya M. N., Thielen T. E., IIjima Y., Huang C., Raymond J. R., Ullian M. E., Paul R. V. A functional angiotensin II receptor-GFT fusion protein: evidence for agonist-dependent nuclear translocation. Am. J. Physiol. Renal Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Soderquist A. M., Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J. Biol. Chem. 1985;260:11944–11952. [PubMed] [Google Scholar]
- Filmus J., Pollak M. N., Cailleau R., Buick R. N. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem. Biophys. Res. Commun. 1985;128:898–905. doi: 10.1016/0006-291x(85)90131-7. [DOI] [PubMed] [Google Scholar]
- Fujinaga Y., Wolf A. A., Rodighiero C., Wheeler H., Tsai B., Allen L., Jobling M. G., Rapoport T., Holmes R. K., Lencer W. I. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to the endoplasmic reticulum. Mol. Cell. Biol. 2003;14:4783–4793. doi: 10.1091/mbc.E03-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri D. K., Ali-Seyed M., Li L.-Y., Lee D.-F., Ling P., Bartholomeusz G., Wang S.-C., Hung M.-C. Endosomal transport of ErbB-2, mechanism for nuclear entry of the cell surface receptor. Mol. Cell. Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield J.J.A., High S. The Sec61 complex is located in both the ER and the ER-Golgi intermediate compartment. J. Cell Sci. 1999;112:1477–1486. doi: 10.1242/jcs.112.10.1477. [DOI] [PubMed] [Google Scholar]
- Hanada N., Lo H.-W., Day C.-P., Pan Y., Nakajima Y., Hung M.-C. Co-regulation of b-Myb expression by E2F1 and EGF receptor. Mol. Carcinogen. 2006;45:10–17. doi: 10.1002/mc.20147. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Itabe H., Fukase H., Mori M., Fujimoto Y., Sato R., Imanaka T., Takano T. Distribution of microsomal triglyceride transfer protein within sub-endoplasmic reticulum regions in human hematoma cells. Biochim. Biophys. Acta. 2002;1581:127–136. doi: 10.1016/s1388-1981(02)00157-9. [DOI] [PubMed] [Google Scholar]
- Hirsch C., Blom D., Ploegh H. L. A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J. 2003;22:1036–1046. doi: 10.1093/emboj/cdg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann J.-O., Albring J., Hüter E., Bulbuc N., Spee P., Neefjes J., Hämmerling G. J., Momburg F. Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity. 2000;13:117–127. doi: 10.1016/s1074-7613(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Kudlow J. E., Cheung C.-Y.M., Bjorge J. D. Epidermal growth factor stimulates the synthesis of its own receptor in a human breast cancer cell line. J. Biol. Chem. 1986;261:4134–4138. [PubMed] [Google Scholar]
- Lee D. K., Lança A. J., Cheng R., Nguyen T., Ji X. D., Gobeil F., Jr., Chemtob S., George S. R., O'Dowd B. F. Agonist-independent nuclear localization of the apelin, angiotensin AT1, and bradykinin B2 receptors. J. Biol. Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Lin S.-Y., Makino K., Xia W., Matin A., Wen Y., Kwong K. Y., Bourguignon L., Hung M.-C. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- Lo H.-W., Hsu S.-C., Ali-Seyed M., Gunduz M., Xia W., Wei Y., Bartholomeusz G., Shih J. Y., Hung M.-C. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS-NO pathway. Cancer Cell. 2005a;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lo H.-W., Xia W., Wei U., Ali-Seyed M., Huang S.-F., Hung M.-C. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005b;65:338–348. [PubMed] [Google Scholar]
- Lo H.-W., Ali-Seyed M., Wu Y., Bartholomeusz G., Hsu S.-C., Hung M.-C. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin, β1 and CRM1. J. Cell. Biochem. 2006;98:1570–1583. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- Lyman S. K., Schekman R. Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell. 1997;88:85–96. doi: 10.1016/s0092-8674(00)81861-9. [DOI] [PubMed] [Google Scholar]
- Maher P. A. Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J. Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Patkaniowska A., Urlaub H., Lürmann R., Tuschi T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Miaczynska M., Pelkmans L., Zerial M. Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 2004a;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004b;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Ni C.-Y., Murphy M. P., Golde T. E., Carpenter G. Ã-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Offterdinger M., Schöfer C., Weipoltshammer K., Grunt T. W. C-erbB-3, a nuclear protein in mammary epithelial cells. J. Cell Biol. 2002;157:929–939. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L., Kartenbeck J., Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Helenius A. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 2003;15:414–422. doi: 10.1016/s0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- Psyrri A., Yu Z., Weinberger P. M., Sasaki C., Haffty B., Camp R., Rimm D., Burtness B. A. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin. Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- Reilly J. F., Maher P. A. Importin β-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J. Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römisch K. Endoplasmic reticulum-associated degradation. Annu. Rev. Cell Dev. Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250. [DOI] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002;529:49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A. Advanced 5′-silyl-2′-orthoester approach to RNA oligonucleotide synthesis. Methods Enzymol. 2000;317:3–18. doi: 10.1016/s0076-6879(00)17003-x. [DOI] [PubMed] [Google Scholar]
- Schmahl J., Kim Y., Colvin J. S., Ornitz D. M., Capel B. FGF9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development. 2004;131:3627–3636. doi: 10.1242/dev.01239. [DOI] [PubMed] [Google Scholar]
- Soderquist A.M., Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J. Biol. Chem. 1984;259:12586–12594. [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Huang F., Beguinot L., Sorkin A. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J. Biol. Chem. 2002;277:27433–27441. doi: 10.1074/jbc.M201595200. [DOI] [PubMed] [Google Scholar]
- Stachowiak M. K., Maher P. A., Joy A., Mordechai E., Stachowiak E. K. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol. Biol. Cell. 1996;7:1299–1317. doi: 10.1091/mbc.7.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B., Ye Y., Rapoport T. A. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 2002;3:246–255. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- van den Berg B., Clemons W. M., Jr., Collinson I., Modis Y., Hartmann E., Harrison S. C., Rapoport T. A. X-ray structure of a protein-conducting channel. Nature. 2003;42:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- Wang S.-C., et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wickner W., Schekman R. Protein translocation across biological membranes. Science. 2005;310:1452–141456. doi: 10.1126/science.1113752. [DOI] [PubMed] [Google Scholar]
- Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- Zhen Y., Caprioli R. M., Staros J. V. Characterization of glycosylation sites of the epidermal growth factor receptor. Biochemistry. 2003;42:5478–5492. doi: 10.1021/bi027101p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.