Abstract
The spindle assembly checkpoint is essential to maintain genomic stability during cell division. We analyzed the role of the putative Drosophila Mad2 homologue in the spindle assembly checkpoint and mitotic progression. Depletion of Mad2 by RNAi from S2 cells shows that it is essential to prevent mitotic exit after spindle damage, demonstrating its conserved role. Mad2-depleted cells also show accelerated transit through prometaphase and premature sister chromatid separation, fail to form metaphases, and exit mitosis soon after nuclear envelope breakdown with extensive chromatin bridges that result in severe aneuploidy. Interestingly, preventing Mad2-depleted cells from exiting mitosis by a checkpoint-independent arrest allows congression of normally condensed chromosomes. More importantly, a transient mitotic arrest is sufficient for Mad2-depleted cells to exit mitosis with normal patterns of chromosome segregation, suggesting that all the associated phenotypes result from a highly accelerated exit from mitosis. Surprisingly, if Mad2-depleted cells are blocked transiently in mitosis and then released into a media containing a microtubule poison, they arrest with high levels of kinetochore-associated BubR1, properly localized cohesin complex and fail to exit mitosis revealing normal spindle assembly checkpoint activity. This behavior is specific for Mad2 because BubR1-depleted cells fail to arrest in mitosis under these experimental conditions. Taken together our results strongly suggest that Mad2 is exclusively required to delay progression through early stages of prometaphase so that cells have time to fully engage the spindle assembly checkpoint, allowing a controlled metaphase–anaphase transition and normal patterns of chromosome segregation.
INTRODUCTION
The spindle assembly checkpoint (SAC; Minshull et al., 1994) is a carefully orchestrated quality control mechanism required to ensure accurate chromosome segregation during cell division. The SAC is responsible for preventing anaphase onset in cells whose chromosomes have not yet reached a stable bipolar attachment. SAC activation/maintenance is thought to be mediated by a signal continuously generated at unattached or improperly attached kinetochores during prometaphase (Rieder et al., 1995). Studies in primary spermatocytes demonstrated that not only microtubule occupancy but also tension across kinetochore pairs is required in order to satisfy the SAC (Nicklas et al., 1995; see also Pinsky and Biggins, 2005). The delayed metaphase–anaphase transition imposed by the SAC is ultimately controlled by the anaphase-promoting complex/cyclosome (APC/C), a multisubunit E3 ubiquitin ligase that targets several mitotic substrates (including mitotic cyclins and securin) for destruction by the 26S proteasome to allow sister chromatid separation and mitotic exit (reviewed by Peters, 2002).
The main components of the SAC molecular machinery were originally identified by genetic screens in budding yeast. MAD1-3 (Li and Murray, 1991) and BUB1 and BUB3 (Hoyt et al., 1991) were shown to be required for a mitotic arrest in the presence of spindle damage. These genes have been found to be conserved from yeast to man with the exception of Mad3, which in higher eukaryotes is called Bub1-related kinase (BubR1) because it is highly similar to Bub1 and unlike Mad3 contains a protein kinase domain within the C-terminal half (Jablonski et al., 1998; Taylor et al., 1998).
Significant progress has been made in unraveling the molecular mechanism by which SAC proteins like Mad2 impose the mitotic arrest in response to inappropriately attached kinetochores. Mad2 was shown to be required for the establishment of a checkpoint-mediated arrest in response to spindle damage in Xenopus egg extracts (Chen et al., 1996) and in mammalian cells in culture (Gorbsky et al., 1998). Studies in Xenopus and human cells have also shown that Mad2 could block mitotic exit by sequestering Cdc20, an APC/C activator (Musacchio and Hardwick, 2002; Bharadwaj and Yu, 2004). Mad2 localizes to kinetochores early in mitosis after binding Mad1 (Chung and Chen, 2003) where it undergoes rapid turnover (Howell et al., 2004). This rapid turn over at kinetochores is thought to underlay the formation of Mad2–Cdc20 inhibitory complexes that signal abnormal microtubule-kinetochore attachment (Sironi et al., 2001). Extensive work using fixed cells in a variety of organisms has supported this model by showing that indeed Mad2 accumulates strongly at kinetochores in the absence of microtubules (Chen et al., 1998, 1999; Logarinho et al., 2004). However, recent studies in Drosophila using a green fluorescent protein (GFP)-Mad2 transgene and time-lapse microscopy have suggested an alternative view. The data indicates that even after microtubule kinetochore attachment takes place, a low level of Mad2 continues to enter the kinetochore and is removed mostly along spindle microtubules in a poleward direction (Howell et al., 2004; Buffin et al., 2005). These results suggest that perhaps the inhibitory signal provided by unattached kinetochores results not from the absence of kinetochore microtubule attachment per se but from the inability of Mad2 (and maybe other checkpoint proteins) to exit the kinetochore through microtubules, causing the accumulation of Mad2 at the kinetochore and the consequent formation of complexes that can now freely diffuse throughout the cytoplasm and inhibit the APC/C (see also Buffin et al., 2005).
Although the role of kinetochores in the generation of a soluble inhibitory signal that delays metaphase–anaphase transition is consistent with most published data, recent experiments have suggested that cytoplasmic Mad2 is also required for the proper timing of early prometaphase independently of kinetochores (Meraldi et al., 2004). Studies in human tissue culture cells show that when Mad2 is depleted in cells with disrupted kinetochores, sister chromatid separation follows very shortly after nuclear envelope breakdown (NEBD). However, if kinetochore-deficient cells now contain cytosolic Mad2, prometaphase is extended significantly, even though these cells still show a defective SAC response (Meraldi et al., 2004). These results suggest that Mad2 has a kinetochore-associated function in maintaining SAC activity and a kinetochore-independent function in timing mitotic progression (for discussion see Kops et al., 2005). Mad2 might therefore perform additional, SAC-unrelated functions during progression through mitosis. Interestingly, recent studies have also shown that besides their role in maintaining SAC activity, other checkpoint proteins also perform additional roles during mitosis progression. Bub3 has been shown to be required for the accumulation of cyclins during G2 and early mitosis (Lopes et al., 2005). Furthermore, BubR1 (Lampson and Kapoor, 2005) and Bub1 (Meraldi and Sorger, 2005) were shown to be required for maintaining proper microtubule kinetochore interaction and chromosome congression.
Therefore, to gain insight into the primary role of Mad2 during mitosis, Drosophila S2 tissue culture cells were treated with double-stranded RNA against Mad2, and mitotic progression was analyzed in detail. Consistent with previous studies in other organisms, we find that depletion of Mad2 causes loss of the SAC response in Drosophila S2 cells. Moreover, Mad2-depleted cells fail to reach metaphase, exit mitosis very soon after NEBD, and show highly abnormal chromosome segregation that is characterized by the formation of extensive chromatin bridges and severe aneuploidy. However, our results indicate that Mad2 is unlikely to have any specific role in either chromosome condensation or microtubule kinetochore interaction because a checkpoint-independent arrest in mitosis allows normal chromosome condensation and congression. Also, release from the mitotic arrest allows cells to exit mitosis without chromatin bridges and with chromatid segregation profiles that are indistinguishable from controls. More significantly, if Mad2-depleted cells are released from the mitotic arrest into media containing the microtubule-depolymerizing agent colchicine, cells arrest in mitosis with intact sister chromatid cohesion and strong kinetochore accumulation of SAC proteins, suggesting an active SAC response. Taken together our results suggest that Mad2 has a major role in delaying mitotic progression during early stages of prometaphase so that the SAC can be activated and chromosome segregation can be properly conducted.
MATERIALS AND METHODS
Double-stranded RNA Interference in Drosophila S2 Cells
To deplete Mad2, S2 cells were transfected with double-stranded RNA (dsRNA) corresponding to a fragment of Mad2 defined by the primers (Forward) TAATACGACTCACTATAGGGAATAGCGGCAATTTAGC and (Reverse) TAATACGACTCACTATAGGGAGAAGCGCAGCTGGA. The PCR product was purified and used as a template for the synthesis of the dsRNA using the MEGAscript T7 kit (Ambion, Austin, TX) and following the manufacturer's instructions. RNA interference (RNAi) experiments were performed as previously described (Maiato et al., 2003) by adding 15 μg of dsRNA to 106 cells. Every 24 h after the addition of the dsRNA, cells were collected and processed for immunofluorescence, Western blot, and fluorescence-activated cell sorter (FACS) analysis. For BubR1 depletion, a fragment of BubR1 cDNA was cloned into pSPT18 and pSPT19 expression vectors, and the RNA was synthesized as done for Mad2 (Maia and Sunkel, unpublished results). Quantification of Mad2 and BubR1 protein levels by both Western blot analysis and immunofluorescence were performed using Image J software. For Western blot analysis, mean pixel intensity was quantified and normalized to tubulin levels. For immunofluorescence quantification, original images captured with equal settings and stacks were projected using Axiovision software (Carl Zeiss MicroImaging, Germany). The intensity of Mad2 and BubR1 at kinetochores was measured with a specific predefined region of interest (ROI) into which all kinetochores could fit. Selected ROI area was defined in the other channel (CID) to reveal kinetochore location.
Immunofluorescence in S2 Cells
Cells were centrifuged onto slides (5 min at 1000 rpm) and processed for simultaneous fixation and extraction in 3.7% methanol-free formaldehyde, 0.5% Triton X-100 in 1× PBS for 10 min, followed by three washes in 1× PBS, 0.05% Tween 20. For separate fixation/extraction protocol (used to reveal spindle morphology) the fixing solution was prepared using 3.7% methanol-free formaldehyde in 1× PBS for 10 min, and then extraction was performed two times for 5 min using 1× PBS, 0.5% Triton X-100. For Mad2 antibody detection, slides were fixed using 4% paraformaldehyde in 1× PBS for 12 min and further extracted for 8 min in 1× PBS, 0.1% Triton X-100. Blocking was performed in 1× PBS, 10% fetal bovine serum, 0.05% Tween 20 (PBS-TF) for 30 min. Primary antibody incubations were prepared in blocking solution for at least 1 h at room temperature or overnight at 4°C, followed by three 10-min washes in 1× PBS, 0.05% Tween. Secondary antibody incubations were performed as described for the primary antibodies, including the three 10-min washes at the end. Slides were then mounted using Vectashield mounting medium for fluorescence with DAPI (Vector Laboratories, Burlingame, CA). Calcium treatment was performed as previously described (Kapoor et al., 2000). Z-series optical sections were collected using the Zeiss Axiovert 200M microscope (Carl Zeiss, Oberkochen, Germany) using an AxioCam. Data stacks were deconvolved using either the Axiovision AxioVs40 V 4.2.0.0 (Carl Zeiss) or the Huygens Essential version 3.0.2p1 (Scientific Volume Imaging, Hilversrum, The Netherlands). Images were treated using Adobe Photoshop CS (Adobe Microsystems, CA).
Antibodies
The primary antibodies were anti-Mad2 (Rb 1223) rabbit, anti-BubR1 (Rb 666) rabbit, anti-Bub1 (Rb 1112) rabbit, and anti-Bub3 (Rb 730) rabbit (Logarinho et al., 2004). Anti-phospho Histone H3 rabbit (Polyclonal, Upstate Biotechnology, Lake Placid, NY), anti-α-tubulin clone B-5-1-2 mouse (Monoclonal, Sigma, St. Louis, MO), anti-Barren rabbit (Bhat et al., 1996), anti-Topo II (P2G3) mouse (Swedlow et al., 1993), anti-Polo (MABMA294) mouse monoclonal (Llamazares et al., 1991), DRAD21 (Rb 735) rabbit (Warren et al., 2000), anti-CID chicken (Blower and Karpen, 2001), and anti-CID rabbit (Henikoff et al., 2000).
In Vivo Time-Lapse Fluorescence Imaging
In vivo timing of mitotic progression was performed using S2 cells stably expressing GFP-tubulin (Rodgers, 2002). Control and Mad2-depleted cells (72 h) were plated onto glass coverslips previously treated with 100 mg/ml concanavalin A (Sigma). Images were obtained at 30-s intervals. Image treatment was performed using AxioVision 4.3 Software (Carl Zeiss).
FACS Analysis
Control and Mad2 RNAi-treated cells were centrifuged at 2000 rpm for 5 min, resuspended in 200 ml PBS, and fixed using 2 ml 70% ice-cold ethanol in PBS, added drop by drop while vortexing. Samples were kept on ice for 30 min before centrifugation at 1000 rpm for 5 min. The pellet was resuspended in 200 ml PBS with 100 mg/ml RNAse and 100 mg/ml propidium iodide. Samples were incubated for a further 30 min at 37°C. For DNA content analysis, we used a FACSCalibur (Becton Dickinson, Mountain View, CA) flow cytometer and data from 10,000 cells was obtained. Results were analyzed using Cell Quest data acquisition software (BD Biosciences, San Jose, CA).
Transient Mitotic Arrest with MG132
Cells were collected every at various time points and fixed onto slides for analysis. Time 0 min corresponds to 72 h after the addition of the dsRNA. At this time point cells were incubated with a low dose of MG132 (2 μM) to induce a mitotic arrest. After a 120-min incubation, the cell culture was diluted threefold either with fresh media or media containing the microtubule poison colchicine. Slides were fixed and stained accordingly for the various antibody incubations performed.
RESULTS
Depletion of Mad2 by RNAi in S2 Drosophila Cells
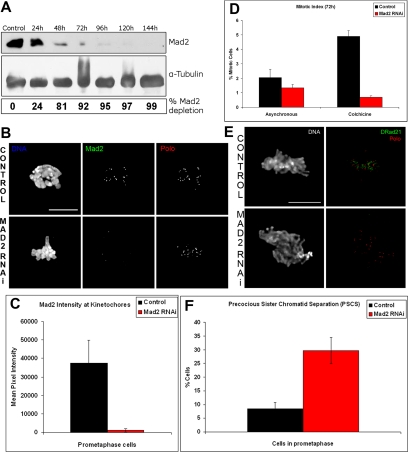
To study the role of the Drosophila Mad2 putative homologue, the protein was depleted from S2 cells by dsRNAi. Addition of dsRNA against Mad2 caused protein levels to drop by more than 90% by 72 h and was virtually absent by 96 h (Figure 1A). Immunolocalization studies with anti-Mad2 antibodies (Figure 1B) and quantification of Mad2 at kinetochores (Figure 1C) confirms these observations. Given that Mad2 is significantly depleted after 72 h of treatment, all our studies were carried out using this period of dsRNA incubation. In total we conducted five separate RNAi studies and quantification of Mad2 protein levels by Western blot analysis after 72 h of RNAi treatment indicated a significantly consistent (93 ± 3%) level of depletion. S2 Drosophila tissue culture cells show a functional SAC response (Logarinho et al., 2004). Therefore, in order to determine whether the SAC is functional in the absence of the putative Mad2 homologue, dsRNA-treated cells were incubated for 2 h with colchicine, and the mitotic index was determined. Spindle damage causes control cells to arrest at a prometaphase-like state, whereas Mad2-depleted cells fail to arrest in the presence of colchicine (Figure 1D). Consistent with these results, analysis of asynchronous prometaphase figures indicate that Mad2-depleted cells show a significant proportion of premature sister chromatid separation (PSCS) as determined by the loss of the cohesin subunit DRAD21 (Figure 1, E and F). These results indicate that in Drosophila S2 cells, Mad2 has a conserved role that is essential to maintain normal SAC activity.
Figure 1.
Depletion of Mad2 by RNAi in S2 cells, (A) Western blot analysis shows Mad2 depletion at different times after addition of Mad2 dsRNA. Below α-tubulin was used as a loading control. At 72 h Mad2 depletion is more than 90%. (B) Immunolocalization of Mad2 RNAi in control and dsRNAi-treated cells at 72 h shows DNA (blue), Mad2 (green), and Polo (red). After 72 h RNAi treated cells do not contain Mad2 at their kinetochores, whereas in control cells Mad2 staining is clearly visible. (C) Quantification of Mad2 levels in control and RNAi-treated prometaphase cells. At least 15 cells were analyzed in each case. (D) Control and RNAi-treated cells were incubated with 30 μM colchicine, and the mitotic index was recorded. Cells lacking Mad2 fail to accumulate in mitosis. (E) These cells were also immunostained to reveal the cohesin subunit DRAD21 to determine whether they exit mitosis prematurely. Note that there is a significant increase in the frequency of DRAD21 negative cells after colchicine incubation (mitotic population only). (F) Immunolocalization of the cohesin subunit DRAD21 in control and Mad2 RNAi-treated cells at 72 h followed by colchicine incubation to depolymerize microtubules revealed a threefold increase in PSCS. DNA (blue), DRAD21 (green), and Polo (red) are shown. Note that after 72 h RNAi treatment most prometaphase cells do not contain centromere-associated DRAD21, whereas in control cells the staining is clearly visible. Bar, (B and E) 5 μm.
Previous studies have suggested that checkpoint proteins assemble at kinetochores in a well-defined order in which localization of Bub1 was essential for Mad2 binding (Johnson et al., 2004). However, this study analyzed cells that were actively progressing through mitosis without a functional SAC and was therefore confined to look only at early prometaphase. To determine whether Mad2 has indeed any role in the kinetochore localization of other checkpoint proteins, cells have to be prevented from exiting mitosis in a checkpoint-independent manner and microtubules depolymerized. Accordingly, control and Mad2-depleted cells were prevented from exiting mitosis by incubation with the proteasome inhibitor MG132 (Genschik et al., 1998; Oliveira et al., 2005) and then treated with colchicine to prevent microtubule repolymerization and hence generate unattached kinetochores. Subsequently, control and Mad2-depleted cells were immunostained with antibodies against BubR1, Bub1, or Bub3 (Supplementary Figure 1). The results show that Mad2 is not required for the kinetochore localization of any of the checkpoint proteins tested.
Mitotic Progression after Depletion of Mad2
Although Mad2 has been extensively studied in a number of organisms, previous reports have not yet provided a comprehensive description of mitotic progression in the absence of the protein. Accordingly, we depleted Mad2 from S2 cells and carried out a full phenotypic analysis. Mitotic cells were identified as being anti-phospho histone H3 (PH3) positive, and immunolocalization of Polo was used to define successive mitotic stages (Llamazares et al., 1991). Overall quantification indicates that Mad2-depleted cells progress through early stages of mitosis normally, and as expected there is a decrease in the number of prometaphases (data not shown). More significantly, we find that Mad2-depleted cells show a strong decrease in the frequency of metaphases (Figure 2A). Also, these cells exit mitosis with highly abnormal anaphases/telophases containing extensive PH3-positive chromatin bridges that remain until very late stages of cell division (Figure 2B). Quantitative analysis shows that most Mad2-depleted cells contain chromatin bridges during anaphase, and ∼50% of the cells are unable to resolve these bridges because they are still present during telophase (Figure 2C). Analysis of DNA content by FACS shows that depletion of Mad2 also results in severe aneuploidy (Figure 2, D and E). DNA content profiles indicate that although control cultures show clearly defined 2N and 4N peaks throughout the experiment, from 72 h onward, cultures treated with dsRNA against Mad2 show a highly abnormal FACS profile, and a clear separation between the 2N and 4N peaks is no longer observed, suggesting an increase in aneuploidy along time. To confirm this observation, we quantified the number of kinetochores in control and Mad2-depleted prometaphase cells at different times during the course of the experiment (Figure 2F). Our results show that at the start of the experiment most cells contain 20–26 kinetochores, consistent with the expected average number of chromosomes in these cells (12 chromosomes). However, as the RNAi treatment progresses, we find a significant proportion of Mad2-depleted cells containing either more or less than 20–26 kinetochores.
Figure 2.
Mitotic progression after depletion of Mad2. (A–C) Cells were treated with dsRNAi for 72 h before fixation and immunostaining to reveal DNA (blue), phospho-histone H3 (green), and Polo (red). (A) Quantification of mitotic progression revealed that cells lacking Mad2 show a significant reduction in the frequency of metaphases among mitotic cells. (B) During anaphase and telophase, cells show extensive PH3-positive chromatin bridges. Bar, 5 μm. (C) Quantification shows that after depletion of Mad2 most cells show chromatin bridges during anaphase or telophase. Analysis of DNA content in control or (D) Mad2-depleted (E) cells at different times. Note that after 72 h Mad2-depleted cells fail to show a well-defined 4N peak, which becomes very broad, indicating extensive aneuploidy. (F) Quantification of kinetochore numbers in control and Mad2-depleted cells at prometaphase. Cells containing between 20 and 26 kinetochores were considered to be normal. C, control; M, Mad2 RNAi; n = 30 cells at each time point. Note the progressive shift in ploidy along time.
Taken together, these results suggest that cells lacking Mad2 that progress through mitosis are unable to organize proper metaphase plates, undergo PSCS and segregate their chromatids with extensive chromatin bridges that persist up to telophase, resulting in severe aneuploidy. One possible hypothesis to explain these observations is that Mad2-depleted cells simply transit through prometaphase rapidly and exit mitosis prematurely. Previous results on the role of Mad2 in human tissue culture cells have shown that in its absence cells show a highly accelerated transit through mitosis characterized by a severe reduction in the time between NEBD and anaphase onset (Meraldi et al., 2004). Therefore, we determined whether loss of Mad2 alters the timing of mitotic progression in S2 cells (Supplementary Figure 2 and Supplementary Movies 1 and 2). To address this directly, we analyzed mitotic progression in Mad2-depleted cells by in vivo time-lapse microscopy using a S2 cell line stably expressing GFP-tubulin (Rodgers, 2002) as previously described (Lopes et al., 2005). The results show very clearly that in the absence of Mad2 the time from NEBD to anaphase onset is significantly shortened (11 ± 2 min) when compared with control cells (33 ± 8 min). These results indicate, first, that in S2 cells mitotic exit can only take place 11 min after NEBD independently of checkpoint activity, and second, that during normal progression through mitosis Mad2 allows S2 cells to extend the length of prometaphase/metaphase up to threefold.
Chromosome Congression in Mad2-depleted Cells Prevented from Exiting Mitosis
Quantitative analysis of mitotic progression of Mad2-depleted cells showed a strong reduction in the number of metaphases. To investigate whether failure of these cells to reach proper chromosome congression is only due to an accelerated anaphase onset, Mad2-depleted cells were prevented from exiting mitosis by incubation with MG132 as described above. Cells were then fixed and stained to detect chromosomes, kinetochores, and spindle microtubules (Figure 3, A and B). The results show that if Mad2-depleted cells are prevented from exiting mitosis prematurely, proper chromosome congression is achieved. Interestingly, abnormal chromosome congression has also been associated with improper microtubule-kinetochore attachment after depletion of other checkpoint proteins like Bub1 (Meraldi and Sorger, 2005) or BubR1 (Lampson and Kapoor, 2005). Therefore, to ascertain whether Mad2 has any specific role in this process, Mad2-depleted cells were prevented from exiting mitosis by incubation in MG132, and kinetochore-microtubule interaction was analyzed. Fixed cells were stained for microtubules and kinetochores and imaged by fluorescence microscopy where each kinetochore pair was carefully followed through the stack of optical sections (see Supplementary Figure 3). The results show that most chromosomes are able to establish correct amphitelic attachment (Figure 3, C, D, and F), suggesting that Mad2 does not have a specific role in establishing and/or maintaining microtubule kinetochore interactions. To determine whether microtubule kinetochore interactions are functional in the absence of Mad2, we quantified the interkinetochore distance in untreated late prometaphase cells and in metaphase cells treated with MG132 as an indication that tension was exerted (Figure 3E). As expected we find that the interkinetochore distance in prometaphase cells is on average half of that after chromosomes have fully congressed, suggesting that proper tension is exerted upon kinetochore pairs even after Mad2 depletion. This is in full concordance with the amphitelic attachments observed in the absence of Mad2. Taken together, our results suggest that chromosome congression fails after Mad2-depletion only because cells exit mitosis prematurely.
Figure 3.
Chromosome congression after checkpoint-independent mitotic arrest. (A and C) Cells were incubated with the proteasome inhibitor MG132 for 90 min before fixation and immunostained to reveal DNA (blue), kinetochores (green), and tubulin (red). (B) Quantification shows that when Mad2-depleted cells are incubated with MG132, the frequency of metaphases is similar to controls. (C) To determine the frequency of proper attachment of kinetochore pairs, control and Mad2-depleted cells were treated with Ca2+ to reveal only kinetochore bundles, fixed, and immunostained as before. Kinetochore pairing was determined by following individual chromosomes through different optical layers. (D) Quantification of different kinetochore attachments in control and Mad2-depleted cells after incubation with MG132 to prevent exit from mitosis. Note that in the absence of Mad2 most kinetochore pairs appear to attach properly, and only ∼5% of kinetochore pairs could not be assigned to any particular type of attachment (n = 300 kinetochore pairs). (E) Interkinetochore distance measured in metaphase cells arrested with 20 μM MG132 and in asynchronous cells in culture. When treated with MG132, both control and Mad2-depleted cells are under tension, and the interkinetochore distance is increased. (F) Types of attachment used for quantification shown in D; CID (green) and tubulin (red). Bar, 5 μm.
Analysis of Chromosome Condensation in Mad2-depleted Cells
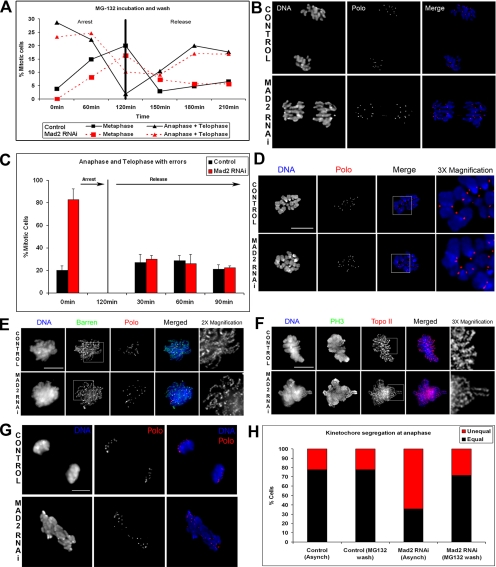
The analysis of mitotic progression after Mad2-depletion indicates that cells progress very rapidly during prometaphase and exit mitosis with PSCS, resulting in severe aneuploidy. Furthermore, during anaphase and telophase these cells show extensive chromatin bridges that are highly reminiscent of phenotypes previously reported to be associated with abnormal chromosome condensation (Coelho et al., 2003; Oliveira et al., 2005). To determine whether Mad2 has any specific role in chromosome condensation and chromosome segregation or whether these phenotypes result exclusively from an accelerated transit through mitosis, we devised a protocol that allowed a reversible checkpoint-independent transient mitotic arrest. Cells were incubated with a low dose of MG132 for up to 2 h and then the drug was washed by extensive dilution in fresh media. Samples where then taken every 30 min and mitotic progression was analyzed by immunostaining (Figure 4). Quantitative analysis shows that MG132 incubation causes a strong mitotic arrest and cells accumulate in metaphase, whereas the frequency of anaphases and telophases is severely reduced. Accordingly, after the MG132 wash, cells rapidly exit mitosis and the number of anaphases and telophases increases, whereas the frequency of metaphases is reduced (Figure 4A). The results clearly demonstrate that MG132 activity causes a transient accumulation of cells at metaphase, which can be reverted so that cells can then proceed through mitosis normally (Figure 4B). Additionally, our results show that the additional time in mitosis provided by the reversible MG132 arrest allows these cells to fully condense their chromosomes and exit mitosis without chromatin bridges at the same frequency as control cells (Figure 4C). Furthermore, if Mad2-depleted cells are arrested in mitosis and microtubules depolymerized to allow better visualization, chromosomes are able to condense and display a morphology that is indistinguishable from control cells (Figure 4D). To further confirm these observations, the localization of key components of the mitotic chromosome organization machinery was also analyzed in Mad2-depleted cells (Figure 4, E and F). We find that in early prometaphase Mad2-depleted cells that were not arrested in mitosis, the condensin subunit Barren, essential for the structural integrity of chromosomes during mitosis (Bhat et al., 1996) and Topoisomerase II (Topo II), a protein responsible for modifying DNA topology (Swedlow et al., 1993), localize properly along a well-organized chromosomal axis. These results demonstrate that Mad2 is unlikely to have any direct role in chromosome structure and suggest that the chromatin bridges observed in anaphase/telophase are exclusively due to a premature exit from mitosis.
Figure 4.
Reversion of the phenotypes caused by depletion of Mad2. To determine whether the Mad2-associated phenotypes could be reverted by providing additional time in prometaphase/metaphase, S2 cells previously treated with dsRNA against Mad2 for 72 h were incubated for 120 min with a low dose of MG132 (2 μM) and then released from the block by performing a threefold dilution on the cell culture media with fresh media. Samples were then collected every 30 min for immunofluorescence analysis. (A) Quantitative analysis of mitotic progression in control and Mad2-depleted cells before and after reversion. From 0 to 120 min both control and Mad2-depleted cells show a strong decrease in anaphases and telophases and a marked increase in the number of metaphases. After washing MG132 (120 min), the number of metaphases begins to decrease, and the number of anaphase and telophase figures increases. (B) Immunofluorescence shows that 180 min after washing the drug, most anaphases in Mad2-depleted cells do not show chromatin bridges. (C) Quantitative analysis of the anaphase and telophase figures before and after the MG132 treatment shows a complete reversal of the phenotype. (D) Normal chromosomes can be obtained in Mad2-depleted cells if cells are incubated with MG132 to prevent exit from mitosis. Before fixation cells were also treated with colchicine to depolymerize microtubules and induce a better chromosome spread. DNA is shown in blue and the kinetochore marker Polo in red. (E and F) Immunolocalization of essential components for the organization and structure of mitotic chromosomes. Barren and Topoisomerase II in control and Mad2-depleted prometaphases are properly localized to a well-defined sister chromatid axis, in asynchronous cell culture. (G) Analysis of kinetochore segregation in control and Mad2-depleted cells after 72 h of RNAi incubation was carried out on anaphase cells after immunostaining for DNA with anti-Polo antibody. (H) Quantification of chromosome segregation at anaphase shows that in control cells almost 80% of cells segregate kinetochores equally. After depletion of Mad2 nearly 70% of cells also show unequal kinetochore segregation. Providing extra time in prometaphase/metaphase to control cells by incubation in MG132 and then washing out the drug does not alter the frequency of unequal kinetochore segregation. However, a similar treatment in Mad2-depleted cells reduces to almost control levels the frequency of unequal kinetochore segregation. Bar, (D–G) 5 μm. A total of 40 cells were analyzed.
Our phenotypic analysis of Mad2-depleted cells (see above Figure 2, D–F) also showed that over time, cells become severely aneuploid. Because all mitotic abnormalities caused by loss of Mad2 could be reverted either by a permanent or a transient mitotic arrest (see above), we quantified kinetochore segregation in control and Mad2-depleted cells with and without a transient MG132-induced mitotic arrest (Figure 4, G and H). In asynchronous cultures not treated with the proteasome inhibitor, the majority of control cells show regular 1:1 kinetochore segregation. However, in cells depleted of Mad2, sister chromatids segregate unequally at a frequency almost threefold higher than in control cells. After a transient checkpoint-independent mitotic arrest Mad2-depleted cells are able to segregate sister chromatids similarly to control cells (Figure 4H), suggesting that the unequal segregation seen in the absence of Mad2 is caused only by the accelerated mitotic exit.
Analysis of the SAC in Cells Depleted of Mad2 or BubR1 after a Transient Mitotic Arrest
In the previous section we showed that S2 cells can be arrested transiently using MG132 and then after washing, the drug cells exit mitosis normally. Surprisingly, we find that Mad2-depleted cells that are also arrested transiently in mitosis by the MG132 reversible treatment can also undergo a mostly normal progression through mitosis. This suggests that Mad2 has an essential role in providing time during early stages of prometaphase so that cells can complete chromosome condensation and microtubule-kinetochore attachment before anaphase onset. So, given that these cells now show normal patterns of segregation it is important to determine if Mad2-depleted cells that are transiently arrested in prometaphase are able to respond to spindle damage. To study this further, we performed a similar experimental procedure as described in the previous section, with the exception that Mad2-depleted cells were released from the MG132 block into a medium containing the microtubule depolymerizing drug colchicine (Figure 5). Incubation in MG132 causes both control and Mad2-depleted cells to arrest, and when they are released into a normal medium, the frequency of prometaphase and metaphases is reduced and the frequency of sister chromatid separation increases (Figure 5, A and B). However, if either control or Mad2-depleted cells are released from the block into media containing colchicine to depolymerize spindle microtubules, they arrest in prometaphase at a high frequency (>90% of the mitotic cells) and the frequency of sister chromatid separation (determined by DRad21 immunostaining) is reduced significantly (Figure 5, A and B). Interestingly, 30 min after the release from MG132 arrest into media containing colchicine there is a slight increase in the cells in prometaphase. Given that the percentage of cells in prometaphase does not increase thereafter, we believe that the initial slight increase is due to some variability in the response to dilution of the drug from cells entering prometaphase. To determine whether the recovery of SAC activity after the transient mitotic arrest is specific for Mad2-depleted cells or a general behavior of cells depleted of any SAC protein, we repeated the experiment in cells depleted of BubR1. Further details on depletion of BubR1 will be published elsewhere (Maia, Lopes, and Sunkel, unpublished results). Western blotting shows that most BubR1 (>70%) can be effectively depleted by 72 h (Supplementary Figure 4) and could not be detected by immunofluorescence (data not shown). If BubR1-depleted cells are arrested with MG132, we observe an increase in accumulation at prometaphase and metaphase and a small reduction in the frequency of sister chromatid separation (Figure 5, A and B). When released from the MG132, we observed a reduction in the frequency of prometaphase and metaphases and a corresponding increase in the frequency of sister chromatid separation. Therefore in this experiment, although BubR1-depleted cells appear to respond less efficiently to the MG132 treatment, they do behave similarly to control and Mad2-depleted cells. However, if BubR1-depleted cells are released from MG132 into colchicine, the behavior is radically different from either control or Mad2-depleted cells because the frequency of prometaphases and metaphases is significantly reduced and the frequency of sister chromatid separation significantly increased (Figure 5, A and B). Thus, although a transient mitotic arrest causes Mad2-depleted cells to have a normal SAC response, BubR1-depleted cells fail to arrest after spindle damage, suggesting that the recovery of SAC activity after these treatments is Mad2 specific. However, it is still possible that the behavior of Mad2-depleted cells could be the result of low levels of Mad2 present after RNAi treatment, which during the MG132 incubation and colchicine treatment have time to accumulate at kinetochores and provide SAC function. To test this hypothesis, we took samples of cells treated with colchicine or MG132 or after the MG132-reversible protocol (180 min), fixed the cells, and immunostained for Mad2. Subsequently the levels of immunofluorescence were quantified (Figure 5C). The results indicate that unlike control cells, Mad2 is undetectable at the kinetochores of Mad2-depleted cells treated with colchicine and barely detectable after incubation in MG132 or when released into media containing colchicine. Accordingly, given that Mad2 is undetectable after Mad2-depletion, it is unlikely that the SAC activity we observed after the transient mitotic arrest is due to low Mad2 levels still present in the RNAi-treated cells. The results show that after a transient mitotic arrest, Mad2-depleted cells behave just like wild-type controls, displaying a normal SAC response.
Figure 5.
Maintenance of SAC activity in Mad2-depleted cells but not in cells lacking BubR1. To determine the status of the SAC Mad2-depleted cells were subjected to a transient mitotic arrest by incubation in MG132 for 2 h and then released either into normal media or media containing colchicine and the frequency of prometaphase and metaphases (A) or sister chromatid separation (B) determined by DRad21 immunostaining (mitotic population only). Note that Mad2-depleted cells when released into normal media rapidly exit mitosis, however, if released into media containing colchicine, accumulate in a prometaphase-like state similarly to control cells. In contrast, BubR1-depleted cells fail to accumulate at prometaphase when released into colchicine, suggesting an inactive SAC. (C) Quantification of Mad2 kinetochore signal by mean pixel intensity in cells treated with colchicine or MG132 and in cells released from the MG132 arrest into colchicine (180 min). Although almost no Mad2 was detected in any sample treated with Mad2 RNAi, in control cells there is a strong Mad2 accumulation in both cells treated with colchicine and those that were transiently arrested in mitosis with MG132 and then released into colchicine.
Sister Chromatid Cohesion after a Transient Mitotic Arrest in the Absence of Mad2 or BubR1
To explore further whether Mad2-depleted cells are able to fully activate the SAC if transiently arrested in mitosis, we set out to determine whether the APC/C was still being inhibited properly in these cells. For this, we chose to stain the cells from all the experimental protocols against one subunit of the cohesin complex. Accordingly, control, Mad2-, or BubR1-depleted cells in prometaphase from asynchronous cultures, after colchicine treatment or after the MG132-reversible treatment, were fixed and immunostained against Polo to label kinetochores and the cohesin complex subunit DRad21 (Figure 6). We find that in asynchronous control cultures all prometaphase cells display kinetochore pairs that are positive for DRad21, whereas most prometaphase cells depleted for either Mad2 or BubR1 do not show any DRad21 staining (Figure 6A). Loss of SAC activity was confirmed by immunostaining for DRad21 in cells incubated in colchicine, where we observed normal localization of cohesin in control cells, whereas either Mad2- or BubR1-depleted mitotic cells show no cohesin staining (Figure 6B). However, analysis of cells subjected to the MG132 reversible protocol followed by release in colchicine show that both control and Mad2-depleted mitotic cells contain chromosomes with proper localization of DRad21, whereas the cohesin subunit is undetectable in BubR1-depleted cells (Figure 6C). These results fully support the hypothesis that if Mad2-depleted cells are transiently arrested in mitosis, SAC activity can be specifically detected that prevents premature activation of the APC/C in the presence of spindle damage.
Figure 6.
Maintenance of centromere-associated DRad21 in Mad2-depleted cells. All cells were collected, fixed, and stained to reveal DNA (blue), Polo (green), and the cohesin subunit DRad21 (red). Cells from control and cells treated with dsRNA against Mad2 or BubR1 were analyzed without colchicine (A), after colchicine treatment (B), and in cells transiently arrested with MG132 (C) and then released into media containing colchicine (180 min). Note that Mad2-depleted cells released into this media are unable to degrade cohesion, whereas BubR1-depleted cells fail to maintain sister chromatid cohesion in the presence of colchicine, suggesting an inactive SAC response (see also higher magnification inserts). Bar, 5 μm.
Analysis of BubR1 Kinetochore Accumulation in Mad2-depleted Cells after a Transient Mitotic Arrest
In the previous sections we have shown that in contrast to a widely held view, cells that have been depleted of Mad2 can still activate the SAC and progress through a normal mitosis if they are prevented from premature mitotic exit by a transient arrest with the proteasome inhibitor MG132. Furthermore, we showed that this effect is unlikely to result from residual Mad2 levels after RNAi treatment and that it results in proper APC/C inhibition. To investigate whether the SAC activity detected in our Mad2-depleted cells presents other characteristics of the normal process like high accumulation of other SAC proteins, we set out to analyze the levels of BubR1 in Mad2-depleted cells before and after the transient mitotic arrest (Figure 7). We find that control or Mad2-depleted cells arrested in mitosis by MG132 incubation accumulate relatively low levels of BubR1 at kinetochores (Figure 7, A and C). This is fully consistent with previous observations describing that after normal microtubule kinetochore attachment and tension, BubR1 levels at kinetochores although still present is relatively low (Logarinho et al., 2004). However, if Mad2-depleted cells are then released into media containing colchicine to disrupt microtubules, kinetochore accumulation of BubR1 is significantly increased to levels that are comparable to that of control cells after spindle damage (Figure 7, B and C). These results are fully in accordance with all our observations and further suggest that the transient arrest of Mad2-depleted cells allows a mostly normal SAC response after spindle damage.
Figure 7.
Immunolocalization of BubR1 in cells lacking Mad2. Control and Mad2-depleted cells where either (A) incubated in MG132 for 2 h or (B) released from the arrest into media containing colchicine (180 min). These cells were fixed and stained to reveal DNA (blue), BubR1 (green), and CID (red). (C) Quantification of the BubR1 kinetochore signal in control and Mad2-depleted cells shows an identical twofold increase in cells released into the colchicine. Note that cells lacking Mad2, similarly to control cells, accumulate BubR1 at kinetochores strongly when released into colchicine. Bar, 5 μm.
DISCUSSION
Recent studies on SAC components have provided significant understanding on the molecular mechanisms in which Mad2 is involved. However, it is surprising that little or no data has been published characterizing the mitotic progression of cells that lack this essential SAC component. Here we provide the first detailed analysis of Mad2-depleted cells as they progress and eventually exit mitosis. Surprisingly, we find that all Mad2-associated phenotypes can be reverted and the checkpoint effectively activated if cells are subjected to a transient mitotic arrest. Thus, contrary to current models which view Mad2 at the center of the inhibition of the APC/C by the SAC, we hypothesize that Mad2 is only required for proper timing of mitotic progression during prometaphase, allowing cells to fully engage the SAC through kinetochore accumulation of other checkpoint proteins so that complete chromosome condensation and congression can be achieved before the controlled metaphase-to-anaphase transition takes place.
Mad2 Has a Conserved Role in Drosophila
Checkpoint proteins have been shown to be essential for the fidelity of mitosis because they are responsible for sensing errors in microtubule kinetochore interaction (Gorbsky et al., 1998; Canman et al., 2002; Mikhailov et al., 2002). Here we show that loss of the Mad2 homologue causes inactivation of the SAC in Drosophila S2 cells. To find out whether Mad2 is required for the kinetochore localization of other checkpoint components, we performed immunolocalization studies against other checkpoint proteins. We find that all three proteins tested (Bub1, Bub3, and BubR1) show strong accumulation at kinetochores, demonstrating that they do not require Mad2 for their localization and also that proper kinetochore localization of these checkpoint components does not per se prevent premature mitotic exit. Previous studies in Xenopus and HeLa cells were performed in the presence of microtubule poisons, and therefore it was unclear whether the absence of protein localization reflected a hierarchical relationship or the inability to analyze a large number of mitotic cells because of fast mitotic exit (Chen, 2002; Johnson et al., 2004). Because individual depletion of Bub3 in Drosophila (Lopes et al., 2005) and analysis of the hypomorphic allele of BubR1 (Basu et al., 1999) resulted in a nonfunctional SAC response, it is very likely that these proteins work through parallel signaling pathways that are mutually required at some stage to sustain checkpoint activity. Consistent with previous work, it seems probable that removing Mad2 may abrogate the spindle checkpoint not only because a sensor is being removed, but because the MCC (a far more potent APC/C inhibitor; Sudakin et al., 2001) cannot form in its absence.
Mad2-depleted Cells Show Abnormal Progression through Mitosis and Aneuploidy
Previous results have shown that inactivation of Mad2 by antibody microinjection during either prophase or prometaphase induced abnormal sister chromatid segregation in PtK1 cells (Gorbsky et al., 1998). Our phenotypic analysis revealed that Drosophila S2 cells lacking Mad2 also display severe abnormalities during mitotic progression. Mad2-depleted cells fail to reach metaphase and exit mitosis with extensive chromatin bridges. The anaphase bridges formed by Mad2-depleted cells appear to be exclusively due to a premature exit from mitosis because extending the time spent in mitosis is enough to revert this phenotype. This is fully consistent with recent data suggesting that proper chromosome condensation and sister chromatid resolution is only fully completed during early prometaphase (Maeshima and Laemmli, 2003). Furthermore, our results suggest that in S2 cells, full chromosome condensation is only achieved late in prometaphase, after the minimal time cells spend in prometaphase when the SAC is inactivated. This is in full agreement with our previous analysis of mitotic progression in Bub3-depleted cells (Lopes et al., 2005), where it was shown that in the absence of Bub3 the SAC is inactive but that cells do not exit mitosis with inappropriately condensed chromosome because of an extended period in prophase.
Several studies have shown that loss of SAC proteins causes PSCS and significant aneuploidy (Kops et al., 2005). We find that loss of Mad2 causes premature degradation of cohesins during prometaphase resulting in high levels of PSCS. In addition, quantification of kinetochore segregation at anaphase shows that loss of Mad2 results in a high frequency of cells showing unequal kinetochore segregation. Furthermore, FACS analysis shows that the DNA content of the mitotic population changes significantly over time in the absence of Mad2. These results suggest that unlike in yeast where MAD2 is not essential for chromosome segregation (Cohen-Fix and Koshland, 1997), Drosophila Mad2 is required to maintain the long viability of cells.
Mad2 Role in Timing of Prometaphase Is Essential for SAC Activation
Early studies on the role of Mad2 in the SAC response using cultured animal cells revealed premature anaphase onset (Gorbsky et al., 1998). More recently, it was found that the mitotic clock of unsynchronized rat basophilic leukemia cells has a marked precision in which ∼80% of cells complete mitosis in 32 ± 6 min and that Mad2 inactivation in these cells consistently shortened mitosis (Jones et al., 2004). Furthermore, depletion of Mad2 by RNAi showed that HeLa cells exit mitosis prematurely (Meraldi et al., 2004). Our results are fully consistent with this data because depletion of Mad2 in S2 cells causes a threefold reduction in the time from NEBD to anaphase onset. Interestingly, these same studies proposed a role of Mad2 in timing mitotic progression that is more complicated that previously expected. It was shown in HeLa cells that inactivation of kinetochore-bound Mad2 disrupts the SAC without significantly affecting the timing of mitotic progression. However, when the cytosolic pool of Mad2 present in these cells is depleted, then both the SAC response is abnormal and the timing of NEBD to anaphase onset is severely reduced, suggesting that Mad2 is also required to time mitotic progression in a kinetochore-independent manner. This contrasts with current models which propose that Mad2 plays an essential role in SAC activation and maintenance by providing a kinetochore-based signal that inhibits the APC/C (Musacchio and Hardwick, 2002). Our observations suggest a much more subtle role for Mad2 in ensuring a SAC response. Surprisingly, we find that after a transient mitotic arrest, Mad2-depleted cells were able to respond to spindle damage and arrest in mitosis with cohesin still located at the centromere of chromosomes and high kinetochore levels of BubR1, suggesting that the SAC is fully functional. Thus, providing time in a checkpoint-independent and transient manner appears to be sufficient for Mad2-depleted cells to either reactivate or maintain SAC activity and respond correctly to microtubule depolymerization. Given that this sustained SAC activity cannot be observed after depletion of other SAC proteins such as BubR1, we hypothesize that the APC/C inhibitory signal provided by Mad2 is specifically required during early stages of prometaphase to ensure maintenance of SAC activity. Subsequently, SAC activity could be ensured by other checkpoint proteins such as BubR1 that could then accumulate strongly at kinetochores. These observations are in full accordance with previous biochemical studies that identified at the G2-M transition the MCC, a multisubunit complex containing BubR1-Mad2-Bub3-Cdc20 (Sudakin et al., 2001). The MCC was shown to be the most powerful APC/C inhibitor (Sudakin et al., 2001; Tang et al., 2001; Sudakin and Yen, 2004). Interestingly, formation of the MCC does not require unattached kinetochores given that it is present well before the NEBD. Taken together, these observations have suggested a “two-step” model for the activation and maintenance of SAC activity (Chan et al., 2005). This model proposes a first step involving the formation of the MCC as cells reach the G2/M transition, allowing cyclin accumulation and mitotic entry. Subsequently, in a second step after NEBD, SAC proteins can bind unattached kinetochores and produce additional inhibitory complexes that sustain SAC activity until all kinetochore pairs are properly attached and congression is achieved. Subsequent studies both in yeast (Fraschini et al., 2001) and Drosophila (Lopes et al., 2005) provide strong support for this model. Our results reported here, provide a further refinement of this model in that the second step can be separated into two events: one at NEBD when cytoplasmic Mad2 is required to extend prometaphase and provide enough time so that in a second event, SAC proteins such as BubR1 and Bub3 can fully engage checkpoint activity. Further studies on the role of Mad2 and other SAC proteins in the inhibitory activity of the MCC before and during early stages of mitosis will be required to unravel how the different levels of regulation are organized. Nevertheless, our observations provide new insights into how the signals provided by different SAC proteins might contribute to a fully integrated checkpoint response.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Soren Steffensen, Nicolas Malmanche, and Paula Sampaio for their contribution to the design of the experiments involving the transient mitotic arrest and to André Maia for invaluable help during the course of this work and valuable comments during manuscript preparation. We are also in debt to all other members of the laboratory for comments and constructive criticism and to Augusta Monteiro for excellent technical assistance. B.O. holds a fellowship from the Training and Mobility of Researchers (TMR) program of the European Union (EU). The laboratory of C.E.S. is supported by the Fundação para a Ciência e a Tecnologia of Portugal and by a TMR grant of the EU.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-07-0587) on December 20, 2006.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Basu J., Bousbaa H., Logarinho E., Li Z., Williams B. C., Lopes C., Sunkel C. E., Goldberg M. L. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 1999;146:13–28. doi: 10.1083/jcb.146.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj R., Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- Bhat M. A., Philp A. V., Glover D. M., Bellen H. J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with Topoisomerase II. Cell. 1996;87:1103–1114. doi: 10.1016/s0092-8674(00)81804-8. [DOI] [PubMed] [Google Scholar]
- Blower M. D., Karpen G. H. The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat. Cell Biol. 2001;3:730–739. doi: 10.1038/35087045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffin E., Lefebvre C., Huang J., Gagou M. E., Karess R. E. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr. Biol. 2005;15:856–861. doi: 10.1016/j.cub.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Canman J. C., Salmon E. D., Fang G. Inducing precocious anaphase in cultured mammalian cells. Cell Motil. Cytoskelet. 2002;52:61–65. doi: 10.1002/cm.10032. [DOI] [PubMed] [Google Scholar]
- Chan G. K., Liu S. T., Yen T. J. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–598. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Chen R. H. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 2002;158:487–496. doi: 10.1083/jcb.200204048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Brady D. M., Smith D., Murray A. W., Hardwick K. G. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol. Biol. Cell. 1999;10:2607–2618. doi: 10.1091/mbc.10.8.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Shevchenko A., Mann M., Murray A. W. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 1998;143:283–295. doi: 10.1083/jcb.143.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Waters J. C., Salmon E. D., Murray A. W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Chung E., Chen R. H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- Coelho P. A., Queiroz-Machado J., Sunkel C. E. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 2003;116:4763–4776. doi: 10.1242/jcs.00799. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O., Koshland D. The metaphase-to-anaphase transition: avoiding a mid-life crisis. Curr. Opin. Cell Biol. 1997;9:800–806. doi: 10.1016/s0955-0674(97)80080-4. [DOI] [PubMed] [Google Scholar]
- Fraschini R., Beretta A., Sironi L., Musacchio A., Lucchini G., Piatti S. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 2001;20:6648–6659. doi: 10.1093/emboj/20.23.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschik P., Criqui M. C., Parmentier Y., Derevier A., Fleck J. Cell cycle-dependent proteolysis in plants. Identification Of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor mg132. Plant Cell. 1998;10:2063–2076. doi: 10.1105/tpc.10.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G. J., Chen R. H., Murray A. W. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J. Cell Biol. 1998;141:1193–1205. doi: 10.1083/jcb.141.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Ahmad K., Platero J. S., van Steensel B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc. Natl. Acad. Sci. USA. 2000;97:716–721. doi: 10.1073/pnas.97.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., Moree B., Farrar E. M., Stewart S., Fang G., Salmon E. D. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 2004;14:953–964. doi: 10.1016/j.cub.2004.05.053. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Trotis L., Roberts B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Jablonski S. A., Chan G. K., Cooke C. A., Earnshaw W. C., Yen T. J. The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma. 1998;107:386–396. doi: 10.1007/s004120050322. [DOI] [PubMed] [Google Scholar]
- Johnson V. L., Scott M. I., Holt S. V., Hussein D., Taylor S. S. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- Jones J. T., Myers J. W., Ferrell J. E., Meyer T. Probing the precision of the mitotic clock with a live-cell fluorescent biosensor. Nat. Biotechnol. 2004;22:306–312. doi: 10.1038/nbt941. [DOI] [PubMed] [Google Scholar]
- Kapoor T. M., Mayer T. U., Coughlin M. L., Mitchison T. J. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G. J., Kim Y., Weaver B. A., Mao Y., McLeod I., Yates J. R., 3rd, Tagaya M., Cleveland D. W. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 2005;169:49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson M. A., Kapoor T. M. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat. Cell Biol. 2005;7:93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- Li R., Murray A. W. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Logarinho E., Bousbaa H., Dias J. M., Lopes C., Amorim I., Antunes-Martins A., Sunkel C. E. Different spindle checkpoint proteins monitor microtubule attachment and tension at kinetochores in Drosophila cells. J. Cell Sci. 2004;117:1757–1771. doi: 10.1242/jcs.01033. [DOI] [PubMed] [Google Scholar]
- Lopes C. S., Sampaio P., Williams B., Goldberg M., Sunkel C. E. The Drosophila Bub3 protein is required for the mitotic checkpoint and for normal accumulation of cyclins during G2 and early stages of mitosis. J. Cell Sci. 2005;118:187–198. doi: 10.1242/jcs.01602. [DOI] [PubMed] [Google Scholar]
- Maeshima K., Laemmli U. K. A two-step scaffolding model for mitotic chromosome assembly. Dev. Cell. 2003;4:467–480. doi: 10.1016/s1534-5807(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Maiato H., Sunkel C. E., Earnshaw W. C. Dissecting mitosis by RNAi in Drosophila tissue culture cells. Biol. Proced. Online. 2003;5:153–161. doi: 10.1251/bpo57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P., Draviam V. M., Sorger P. K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Meraldi P., Sorger P. K. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 2005;24:1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A., Cole R. W., Rieder C. L. DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr. Biol. 2002;12:1797–1806. doi: 10.1016/s0960-9822(02)01226-5. [DOI] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N. K., Murray A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Hardwick K. G. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B., Ward S. C., Gorbsky G. J. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 1995;130:929–939. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R. A., Coelho P. A., Sunkel C. E. The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis. Mol. Cell. Biol. 2005;25:8971–8984. doi: 10.1128/MCB.25.20.8971-8984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Cole R. W., Khodjakov A., Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W. Making membranes green: construction and characterization of GFP-fusion proteins targeted to discrete plasma membrane domains. Biotechniques. 2002;32:1044–1046. doi: 10.2144/02325st05. 1048, 1050–1051. [DOI] [PubMed] [Google Scholar]
- Sironi L., Melixetian M., Faretta M., Prosperini E., Helin K., Musacchio A. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 2001;20:6371–6382. doi: 10.1093/emboj/20.22.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V., Chan G. K., Yen T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V., Yen T. J. Purification of the mitotic checkpoint complex, an inhibitor of the APC/C from HeLa cells. Methods Mol. Biol. 2004;281:199–212. doi: 10.1385/1-59259-811-0:199. [DOI] [PubMed] [Google Scholar]
- Swedlow J. R., Sedat J. W., Agard D. A. Multiple chromosomal populations of topoisomerase II detected in vivo by time-lapse, three-dimensional wide-field microscopy. Cell. 1993;73:97–108. doi: 10.1016/0092-8674(93)90163-k. [DOI] [PubMed] [Google Scholar]
- Tang Z., Bharadwaj R., Li B., Yu H. Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Ha E., McKeon F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 1998;142:1–11. doi: 10.1083/jcb.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. D., et al. The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr. Biol. 2000;10:1463–1466. doi: 10.1016/s0960-9822(00)00806-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.