Abstract
Chemoattractants induce neutrophil polarization through localized polymerization of F-actin at the leading edge. The suppression of rear and lateral protrusions is required for efficient chemotaxis and involves the temporal and spatial segregation of signaling molecules. We have previously shown that the intracellular calcium-dependent protease calpain is required for cell migration and is involved in regulating neutrophil chemotaxis. Here, we show that primary neutrophils and neutrophil-like HL-60 cells express both calpain 1 and calpain 2 and that chemoattractants induce the asymmetric recruitment of calpain 2, but not calpain 1, to the leading edge of polarized neutrophils and differentiated HL-60 cells. Using time-lapse microscopy, we show that enrichment of calpain 2 at the leading edge occurs during early pseudopod formation and that its localization is sensitive to changes in the chemotactic gradient. We demonstrate that calpain 2 is recruited to lipid rafts and that cholesterol depletion perturbs calpain 2 localization, suggesting that its enrichment at the front requires proper membrane organization. Finally, we show that catalytic activity of calpain is required to limit pseudopod formation in the direction of chemoattractant and for efficient chemotaxis. Together, our findings identify calpain 2 as a novel component of the frontness signal that promotes polarization during chemotaxis.
INTRODUCTION
Neutrophils are key components of the innate immune system, representing the first responders to inflammatory stimuli such as bacterial infections and tissue injury. The rapid recruitment of neutrophils to inflammatory sites requires a highly specialized form of cell migration in which polarity and movement are directed by spatial cues from a chemotactic gradient. Although the molecular mechanisms that regulate neutrophil chemotaxis remain poorly understood, recent progress indicates that this process is achieved by two distinct, but functionally linked events, including directional sensing and cell polarization (Devreotes and Janetopoulos, 2003; Franca-Koh and Devreotes, 2004).
In professional migratory cells such as neutrophils, G protein-coupled cell surface receptors recognize external chemotactic gradients (Van Haastert and Devreotes, 2004; Huttenlocher, 2005) and are responsible for initiating the translation of spatial information about the chemotactic gradient into an internal gradient of signaling molecules that relay either “front”- or “back”-specific responses. For example, previous work has shown that phosphatidylinositol-3,4,5-trisphosphate (PIP3) is asymmetrically recruited to the membrane adjacent to the highest concentration of chemoattractant (Servant et al., 2000; Kimmel and Parent, 2003) where it stimulates positive feedback mechanisms that promote the frontness signal. Subsequent reinforcement of frontness and backness signals dictate cell polarity, where pseudopod formation is biased according to the direction of the chemotactic gradient, with the leading edge characterized by the asymmetric accumulation of actin, phosphoinositide 3-kinase, Cdc42, and Syk (Weiner et al., 1999; Funamoto et al., 2002; Li et al., 2003; Schymeinsky et al., 2005, 2006) as well as the establishment of lipid microenvironments (Manes et al., 1999; Gomez-Mouton et al., 2001, 2004; Kindzelskii et al., 2004).
We have previously shown that the intracellular, calcium-dependent protease calpain is constitutively active in resting neutrophils and that global inhibition of calpain activity enhances random neutrophil migration or chemokinesis (Lokuta et al., 2003). Interestingly, the increase in chemokinesis caused by calpain inhibition is accompanied by an impaired chemotactic response (Lokuta et al., 2003). Of the 16 identified mammalian calpain isoforms, only calpain 1 (μ-calpain) and calpain 2 (m-calpain) and their shared small regulatory subunit, CSS1, have been implicated as regulators of cell migration (Huttenlocher et al., 1997; Dourdin et al., 2001; Glading et al., 2001; Goll et al., 2003). Recent work has also identified an isoform-specific function of calpain 2 in regulating membrane protrusion at the leading edge of migrating cells (Franco et al., 2004a). Together with the ability of calpain to regulate Rho-GTPase activity (Lokuta et al., 2003), integrin activation (Huttenlocher et al., 1996; Palecek et al., 1998; Rock et al., 2000), and cytoskeletal organization (Dourdin et al., 2001; Bhatt et al., 2002; Franco et al., 2004b), these data raise the possibility that calpains may play a role in establishing frontness during chemotaxis.
In this report, we demonstrate that neutrophils and neutrophil-like HL-60 cells express both calpain 1 (μ-calpain) and calpain 2 (m-calpain) and that these isoforms are asymmetrically distributed to distinct intracellular regions upon chemoattractant stimulation in both neutrophils and HL-60 cells. Using time-lapse microscopy and dimethyl sulfoxide (DMSO)-differentiated HL-60 cells (dHL-60) retrovirally infected with green fluorescent protein (GFP)-tagged wild-type calpain 2, we show that enrichment of calpain 2 at the cell front occurs early upon exposure to a gradient of chemoattractant and is persistent during pseudopod formation and chemotaxis. We also show that the localization of calpain 2 is sensitive to positional changes in the chemotactic gradient. We demonstrate that calpain 2, but not calpain 1, is recruited to lipid raft domains in activated neutrophils. Finally, we show that ectopic expression of calpain 2 in dHL-60 cells enhances chemotaxis, whereas expression of a protease-dead calpain 2 induces multiple lateral pseudopodia and impairs chemotaxis. Together, our data suggest that calpain 2 is a novel component of the frontness signal that limits pseudopod formation to promote neutrophil polarization during chemotaxis.
MATERIALS AND METHODS
Cell Culture and Isolation of Primary Neutrophils
HL-60 cells (UCSF Tissue Culture facility) were cultured and differentiated as described previously (Collins et al., 1978; Hauert et al., 2002). Briefly, HL-60 cells were cultured in Iscove's modified DMEM (American Type Culture Collection, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in 5% CO2 in noncoated T75 flasks. For differentiation, cells were cultured in Iscove's modified DMEM supplemented with 10% nonheat-inactivated FBS, penicillin/streptomycin, and 1.25% DMSO (Sigma-Aldrich, St. Louis, MO) for 7 days. Phoenix cells (a generous gift from Dr. Garry Nolan, Stanford University, Stanford, CA) were maintained in Iscove's modified DMEM with 10% heat-inactivated FBS at 5% CO2. Primary human neutrophils were obtained from healthy donors as described previously (Lokuta et al., 2003).
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from primary neutrophils, undifferentiated and differentiated HL-60 cells, and human embryonic kidney (HEK) 293 cells by using RNA STAT-60 (Tel-Test, Friendswood, TX). After treatment with DNase (Promega, Madison, WI), RT-PCR was performed with 1 μg of RNA and 40 U of Rnasin (Promega) by using the one-step RT-PCR kit (QIAGEN, Valencia, CA) and calpain 1 or calpain 2 gene-specific primers (Witkowski et al., 2002; De Tullio et al., 2003) against a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control. All primers were verified in BLAST searches of the nonredundant database to ensure that each sequence was specific for the target gene. Primers spanned at least one exon boundary of each target sequence to eliminate products from genomic DNA contamination. Gene forward primer (5′-3′) reverse primer (5′-3′): Capn 1, GATGGAGCTACCCGCACAGAC GTGGAGGGCACCACCACATAC; Capn 2, AGGCATACGCCAAGATCAAC GGATGCGGATCAGTTTCTGT; and GAPDH, GAGTCAACGGATTTGGTCGTAT AGTCTTCTGGGTGGCAGTGAT.
The following thermal cycling parameters were used: 50°C for 30 min, 95°C for 15 min, 94°C for 1 min, 55°C for 1 min, 72°C for 1 min (35 cycles), and a final extension at 72°C for 10 min. Each RT-PCR sample was resolved by electrophoresis on a 4.25% nondenaturing polyacrylamide gel, stained with ethidium bromide, and analyzed using a Bio-dock system (UVP, Upland, CA). Data are representative of RT-PCR from three separate RNA preparations.
Protein Extraction, Antibodies, and Immunoblots
Primary neutrophils and dHL-60 cells were either untreated or stimulated with 11.25 nM Complement factor 5a (C5a) (Sigma-Aldrich) or 100 nM N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) (Sigma-Aldrich) for 15 min and treated with lysis buffer (0.5% sodium deoxycholate, 1% NP-40, and 0.1% SDS in phosphate-buffered saline (PBS) supplemented with protease inhibitor cocktail (P-8340; Sigma-Aldrich), phosphatase inhibitor cocktail (P-5726; Sigma-Aldrich), 2 mM phenylmethylsulfonyl fluoride (PMSF), 100 mM sodium orthovanadate, 900 mM benzamidine, and 1 mM phenantroline). Lysates were subjected to three freeze-thaw cycles by using a dry ice methanol bath and solubilized on ice for 30 min. Samples were clarified by centrifugation, and protein concentration was determined using a BCA protein assay (Pierce Chemical, Rockford, IL). Sixty-five micrograms of total protein was used for immunoblots by using standard conditions (Harlow and Lane, 1999). Immunoblots against purified calpain 1 and calpain 2 protein (Calbiochem, San Diego, CA) were used to confirm isoform specificity for each antibody. Primary antibodies were diluted into 2.5% nonfat dry milk and used at the following dilutions: rabbit-α-calpain 1 (Triple Point RP3, 1:500; Abcam, Cambridge, MA), rabbit-α-calpain 2 (Triple Point RP1, 1:500; Abcam), and rabbit-α-calpain 2 (3989, 1:100; Sigma-Aldrich). IRDye 800CW goat α-rabbit (1:10,000; Rockland, Gilbertsville, PA) was used as the secondary antibody. Western blots were imaged with an Odyssey Infrared Imaging system (LI-COR, Lincoln, NE). For Western blot experiments, α-calpain 1 (Triple Point, Abcam) was used for all cells, whereas α-calpain 2 (Triple Point, Abcam) was used for primary neutrophils and α-calpain 2 (Sigma-Aldrich) was used for HL-60 and HEK cells. Detection of calpain 2 in dHL60 cells required the calpain 2 antibody (Sigma-Aldrich) and extensive solubilization of the cell lysates.
Expression Constructs
Wild-type calpain 1 and 2 constructs were generated by subcloning human calpain 1 (a generous gift from Dr. Joan Fox, Cleveland Clinic, Cleveland, OH) and human calpain 2 (a kind gift from Dr. Alan Wells, University of Pittsburgh, Pittsburgh, PA) cDNAs into the pCDNA 3.1 FLAG vector (Invitrogen, Carlsbad, CA). QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA) of the wild-type calpain 2 FLAG construct was used to generate calpain 2 protease dead (PD) FLAG (H262A) (Arthur et al., 1995) by using the primer pair 5′-GCTGGTGAAGGGCGCCGCGTACTCGGTCACCGGAGCC-3′ and 5′-GGCTCCGGTGACCGAGTACGCGGCGCCCTTCACCAGC-3′. Calpain 1, calpain 2, and calpain 2 PD-FLAG were subsequently subcloned into the pMX-IRES GFP retroviral vector (a generous gift from Dr. Clive Svendsen, University of Wisconsin, Madison, WI). The pMX-calpain 2-GFP was generated by subcloning into pEGFP-N1 (Clonetech, Mountain View, CA) and subsequently the empty pMX retroviral vector. QuickChange site directed mutagenesis (Stratagene) of the wild-type pMX-calpain 2-GFP was used to generate pMX-calpain 2 PD-GFP (C105S) (Arthur et al., 1995) by using the primer pair 5′-CCCTAGGTGACTCCTGGCTGCTGGC-3′and 5′-GCCAGCAGCCAGGAGTCACCTAGGG-3′. The accuracy of all constructs was verified by DNA sequencing before use.
Immunofluorescence
Primary neutrophils or dHL-60 cells were resuspended in Dulbecco's phosphate-buffered saline (DPBS) alone, or DPBS containing 11.5 nM C5a or 100 nM fMLP and were allowed to adhere for 10 min to glass coverslips coated with 10 μg/ml fibrinogen. For calpain 1, cells were fixed with 6.6% paraformaldehyde, 0.05% glutaraldehyde in PBS, pH 7.2, quenched with 0.15 M glycine for 15 min, and permeabilized with 0.5% Triton X-100 for 15 min. For calpain 2, cells were fixed and pemeabilized with 6.6% paraformaldehyde, 0.05% glutaraldehyde, and 0.25 mg/ml saponin in PBS, pH 7.2, for 15 min and quenched with 0.15 M glycine for 15 min. Nonspecific binding was blocked with PBS containing 10% heat-inactivated FBS and 0.25 mg/ml saponin at 4°C overnight. Cells were then stained for 30 min with either α-calpain 1 (2H-7; a kind gift from Dr. Ronald Mellgren, University of Toledo, Toledo, OH), α-calpain 2 (Triple Point, Abcam), or α-FLAG (M2; Sigma-Aldrich) and costained with rhodamine-labeled phalloidin (Invitrogen). Rhodamine-labeled goat α-mouse (Invitrogen), fluorescein isothiocyanate (FITC) sheep α-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA), and FITC goat α-rabbit IgG (Jackson ImmunoResearch Laboratories) were used as the secondary antibodies. All antibody incubations were performed at room temperature, and cells were washed extensively in PBS between each incubation step. Cells were mounted in mounting media and viewed on a Nikon Eclipse TE300 inverted fluorescence microscope using a 60× differential interference contrast microscopy objective. Fluorescent images were digitally acquired using a cooled charge-coupled device video camera (Hamamatsu Photonics, Bridgewater, NJ) and processed with MetaMorph version 5.0 (Molecular Devices, Sunnyvale, CA). Localization studies were performed on at least three independent samples.
For immunofluorescence of lipid rafts, primary neutrophils were resuspended in DPBS alone, or DPBS containing 10 mM methyl-β-cyclo-dextran (Sigma-Aldrich) and incubated for 15 min at 37° and 5% CO2. Each sample was stimulated with 100 nM fMLP and allowed to adhere for 10 min to glass coverslips coated with 10 μg/ml fibrinogen. The cells were fixed with 6.6% paraformaldehyde, 0.05% glutaraldehyde, and 0.25 mg/ml saponin in PBS, pH 7.2, for 15 min and quenched for 15 min with 0.15 M glycine. Nonspecific binding was blocked overnight with PBS containing 10% heat-inactivated FBS, 0.25 mg/ml saponin at 4°C. Cells were incubated for 1 h with α-ganglioside marker (GM)-3 antibody (Seikagaku America, Rockville, MD) and α-calpain 2 (Triple Point, Abcam), washed with PBS, incubated with the appropriate secondary antibody for 30 min, and processed as described above.
Retroviral Infection
Phoenix viral packaging cells were transiently transfected by calcium-phosphate precipitation (Jordan et al., 1996), and viral supernatant was harvested and filtered through a 0.45-μm membrane 48 h posttransfection. For infection, 1 × 106 HL-60 cells were resuspended in the viral supernatant supplemented with 1 μg/ml polybrene (Sigma-Aldrich), plated, centrifuged at 1141 × g for 90 min in an Allegra 6R table top centrifuge (Beckman Coulter, Fullerton, CA), and cultured at 32°C for 6 h. The viral supernatant was then replaced with fresh media, and the cells grown overnight at 37°C. The next day, a second spin infection was performed using viral supernatant collected from a second plate of Phoenix cells 72 h posttransfection. Populations of GFP-positive cells were obtained by fluorescence-activated cell sorting (FACS) and verified for expression by Western blotting.
Talin Proteolysis
dHL-60 cells were either untreated or stimulated with 11.25 nM C5a for 2, 5, and 10 min. Cell lysates were taken and analyzed by immunoblot analysis as described above using the 8d4 talin antibody (Sigma-Aldrich). To determine the effect of overexpression of calpain 2 constructs on talin proteolysis, dHL-60 cells expressing either control, calpain 2, or calpain 2 PD-FLAG were either untreated or stimulated with 11.25 nM C5a. Cell lysates were obtained and processed as described. Representative blots are shown from three independent experiments.
Lamellipod Assay
dHL-60 cells that express control vector or wild-type calpain 2 were plated on coverslips coated with 2.5 μg/ml fibrinogen, stimulated with 11 nM C5a for 10 min, fixed, permeabilized, and stained with rhodamine-labeled phalloidin. Fluorescent microscopy images were obtained and analyzed using MetaMorph version 5.0 cell imaging software. For each cell, a line was drawn around the entire periphery of the cell, or the region of F-actin highlighted by rhodamine-labeled phalloidin to determine the length in micrometers. Lamellipod percentage for each cell was calculated by dividing the length of the region containing F-actin by length of the entire cell periphery and multiplying by 100. Data were collected from ≥50 cells from four separate experiments and compared by a two-tailed, paired Student's t test. A value of p ≤ 0.05 was taken as significant.
Chemotaxis Assay
For each experiment, 5 × 105 dHL-60 cells were plated in Gey's media for 10 min on a glass coverslip coated with 2.5 μg/ml fibrinogen (Sigma-Aldrich) and 50 μg/ml fibronectin (purified from human plasma as described previously; Ruoslahti et al., 1982). An Eppendorf FemptoTip was loaded with 58 μM C5a, and a chemotactic gradient was formed by slow release of the chemoattractant from the tip into the media using an Eppendorf FemptoJet microinjection system as described previously (Servant et al., 1999). For experiments involving actin disruption, dHL-60s were pretreated for 15 min in Gey's media containing 3 μM latrunculin A (Sigma-Aldrich). Chemotaxis was recorded using a Nikon Eclipse TE300 inverted fluorescence microscope with a cooled charge-coupled device video camera (Hamamatsu Photonics) by using a 60 or 100× differential interference contrast microscopy (DIC) objective and captured into MetaMorph version 5.0 (Molecular Devices) at 10-s intervals for 10 min. Localization studies were performed on at least three independent samples from multiple cell lines.
Cell Tracking
Cell centroid positions were marked in Scion Image (Scion, Frederick, MD), and cell movement was determined as a function of time in Excel version 8.0 (Microsoft, Redmond, WA). The mean square displacement [d2(t)] was calculated as a function of time and expressed in micrometers per minute for each cell in the field from at least three independent experiments.
Transwell Assay
Analysis of dHL-60 chemotaxis by transwell assay was performed as described previously (Lokuta et al., 2003), except that 11 nM C5a and Gey's media were used as described previously (Hauert et al., 2002). Cells that migrated across the filter were counted and expressed relative to control. The results are from a minimum of three separate experiments.
Lipid Raft Preparations
To analyze detergent-insoluble complexes in flotation gradients, 1 × 108 primary neutrophils were untreated or stimulated with 100 nM fMLP for 15 min in Gey's media. Cells were then lysed in 300 μl of cold TNE buffer (50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 5 mM EDTA, 0.2% Triton X-100, 2 mM PMSF, 100 mM sodium orthovanadate, 900 mM benzamidine, 1 mM phenantroline, and protease inhibitor cocktail [P-8340; Sigma-Aldrich]), sheared three times with a 21-gauge needle, brought to 35% (vol/vol) Optiprep (Nycomed, Oslo, Norway), and received 20 strokes in a dounce homogenizer. The sample was overlaid with 3.5 ml of 30% (vol/vol) Optiprep and 250 μl of TNE buffer in a SW60 tube and then centrifuged 4 h at 170,000 × g at 4°C. After the spin, nine 500-μl fractions were collected from top to bottom, and 70-μl samples were analyzed by SDS-PAGE and Western blot by using the following primary antibodies: α-receptor for activated C kinase (RACK)1 (BD Biosciences PharMingen, San Diego, CA), α-CD43 (1G10), α-calpain 1 (Triple Point, Abcam), and α-calpain 2 (107-82; Sigma-Aldrich). For the SDS-PAGE detection of GM-1, samples were run and transferred using previously described methods (Badizadegan et al., 2000; Rodighiero et al., 2001). Dot blots for CD-45 (BRA-55; Sigma-Aldrich) were carried out using the protocol provided with the Bio-Dot microfiltration apparatus (Bio-Rad) by using a 50-μl sample from each fraction and visualized by Western blot.
Flow Cytometry
Undifferentiated and DMSO-differentiated HL-60 cells expressing either control GFP, pMX-calpain 2-GFP, or pMX-calpain 2 PD-GFP fusion constructs were washed and resuspended in FACS buffer (cold 2% fetal calf serum and 0.2% NaN3 in 1X DPBS). Cells (1 × 106) in 100 μl of FACS buffer were either left unstained or incubated with a 20-μl aliquot of phycoerythrin (PE)-conjugated α-CD54 (intercellular adhesion molecule [ICAM]-1) antibody (5555511; BD Biosciences PharMingen), PE-conjugated α-CD11b (Mac-1) antibody (555388 BD; BD Biosciences PharMingen), or PE-conjugated mouse IgG1 (555749; BD Biosciences PharMingen) for 1 h at room temperature in the dark. Cells were washed twice with FACS buffer, resuspended in 300 μl of FACS buffer, counted using a BD Biosciences FACSCalibur flow cytometer, and analyzed with FlowJo 6.4.6 software (Tree Star, Ashland, OR).
Online Supplemental Material
Time-lapse microscopy of dHL-60 cells that express pMX-calpain 2-GFP migrating toward a pipette tip (Figure 3A) is shown in Supplemental Video 1. Time-lapse microscopy of dHL-60 cells expressing pMX-calpain 2-GFP migrating toward a pipette tip whose position is changed (Figure 3D) is shown in Supplemental Video 2. DIC time-lapse microscopy of dHL-60 cells (Figure 6) that express either control (Supplemental Video 3), calpain 2 wild type (Supplemental Video 4), or calpain 2 PD (Supplemental Video 5) are shown. Time-lapse microscopy of dHL-60 cells that express pMX-calpain 2 PD-GFP migrating toward a pipette tip is shown in Supplemental Video 6. Images for Supplemental Videos 1, 2, and 6 were captured at 10-s intervals. Images for Supplemental Videos 3–5 were captured at 5-s intervals. All time-lapse microscopy images were captured using a 60× objective with a Nikon Eclipse TE300 inverted fluorescence microscope with a cooled charge-coupled device video camera (Hamamatsu Photonics) by using DIC microscopy and captured into MetaMorph version 5.0 (Molecular Devices). Movies were converted into QuickTime movie format and prepared using CinaPak compression.
Figure 3.
Asymmetric distribution of calpain 2 during chemotaxis. dHL-60 cells (dHL-60) that express calpain 2-GFP (Capn2-GFP) were plated onto 2.5 μg/ml fibrinogen and 50 μg/ml fibronectin and exposed to a chemotactic gradient generated by the slow release of C5a from a FemptoTip micropipette. Simultaneous 60× DIC and fluorescent time-lapse images were taken at 10-s intervals by using multidimensional acquisition. The tip of the micropipette is marked with an asterisk (*). (A) Representative DIC and fluorescent images of dHL-60 cells that express calpain 2-GFP. Note the strong signal of calpain 2-GFP at the cell front. Bar, 10 μm. Corresponding time-lapse microscopy of dHL-60 cells that express calpain 2-GFP is shown in Supplemental Video 1. (B) Magnified merged image (right) shows enrichment of calpain 2-GFP (middle) at the leading edge of polarized dHL-60 cells (DIC left). The asterisk is used to indicate the direction of the chemoattractant source. Bar, 1 μm. (C) dHL-60 cells (dHL-60) that express calpain 2-GFP were treated as above and 60× DIC and fluorescent time-lapse images were taken immediately after exposure to the pipette tip (t = 0) and then subsequently at 10-s intervals or as indicated using multidimensional acquisition. Note the early recruitment of calpain 2-GFP to the leading edge during initial pseudopod formation. The asterisk is used to indicate the direction of the chemoattractant source. Bar, 5 μm. (D) dHL-60 cells that express calpain 2-GFP and treated as described above were exposed to a changing gradient of C5a by movement of the micropipette tip. The micropipette position was gradually moved starting between 180″ and 190″ time points (black arrow). White arrowheads denote the change in location of the calpain 2-GFP signal upon changing the micropipette position. Bar, 10 μm. Corresponding time-lapse microscopy is shown in Supplemental Video 2. (E) dHL-60 cells that express calpain 2-GFP were plated as described above and were either treated with control vehicle or with 3 μM latrunculin A for 15 min and exposed to a C5a chemotactic gradient generated from a FemptoTip micropipette. Simultaneous 100× DIC and fluorescent time-lapse images were taken. The asterisk is used to indicate the direction of the chemoattractant source. Bar, 5 μm.
Figure 6.
Calpain 2 is required for efficient chemotaxis to C5a. (A) dHL-60 cells that express control vector (Con), wild-type calpain 2 (Capn 2), or protease-dead calpain 2 (Capn 2 PD) were plated on 2.5 μg/ml fibrinogen and 50 μg/ml fibronectin and exposed to a chemotactic gradient generated by the slow release of C5a from a FemptoTip micropipette. Cells were imaged by time-lapse microscopy at 5-s intervals. Corresponding videos for control (Supplemental Video 3), wild-type calpain 2 (Supplemental Video 4), and protease-dead calpain 2 (Supplemental Video 5) can be viewed in Supplemental Videos. Similar phenotypes were observed in dHL-60 cells that express calpain 2 PD-GFP and can be viewed in Supplemental Video 6. (B) Corresponding Rose plots depicting the cell centroid movement of individual cells are shown from a minimum of three separate experiments as they move toward the micropipette tip, represented at the center of the graph. (C) Cells were imaged by time-lapse microscopy, and the path of each cell was analyzed to determine cell speed (micrometers per minute) as described in Materials and Methods. Data shown are the mean of four experiments ± SEM. Significant difference (p ≤ 0.05 by Student's t test) from appropriate control is indicated by asterisk (*). (D) Transwell assay was performed in the absence of stimuli (−) or with C5a (+) in the bottom chamber using dHL-60 cell lines that express control vector (Con), calpain 2 (Capn 2), or protease-dead calpain 2 (Capn 2 PD). Migration is shown relative to control cells. Results are representative from three different experiments. In the absence of stimuli, the average percentage of total cells that migrated ranged from 0.2 to 0.4% for all of the cell lines, compared with C5a stimulation: 14.2% (Con), 21.7% (Capn 2), and 6.5% (Capn 2 PD). Asterisks (*) indicate significant difference compared with control (p ≤ 0.05 by Student's t test).
RESULTS
Expression of Calpain 1 and Calpain 2 in Neutrophils and HL-60 Cells
Previous studies using casein zymography indicate that calpain 1 is the predominant active isoform expressed in primary neutrophils (Lokuta et al., 2003). To determine expression of the two ubiquitous calpain isoforms, calpain 1 and calpain 2 in neutrophils and the neutrophil-like HL-60 cell line, we used a series of isoform-specific reagents in RT-PCR and immunoblot analysis. We now show that primary neutrophils and the neutrophil-like HL-60 cell line express both calpain 1 and calpain 2 (Figure 1, A and B). Expression of calpain 2, but not calpain 1, in dHL-60 cells was difficult to detect by immunoblotting and required special conditions for detection (see Materials and Methods).
Figure 1.
Expression and localization of calpain 1 and calpain 2 in primary neutrophils. RT-PCR (A) and immunoblotting (B) were used to determine the expression of calpain 1 and calpain 2 in primary neutrophils (PMNs) as described in Materials and Methods. (A) Control RNA from HEK 293 cells was compared with RNA from PMNs and undifferentiated and differentiated HL-60 cells (uHL and dHL) by using gene-specific primers against human calpain 1 (Capn 1) and calpain 2 (Capn 2). GAPDH loading controls indicate equal sample was loaded in each lane. (B) Cell lysates were collected from PMNs, HL-60 cells (uHL and dHL), and HEKs as described in Materials and Methods and probed using isoform-specific antibodies. For Western blot experiments, α-calpain 2 (Triple Point, Abcam) was used for neutrophils (box) and α-calpain 2 (Sigma-Aldrich) was used for HL-60 and HEK cells. Molecular weight markers (M) indicate proper predicted size. (C) Resting and fMLP-treated human neutrophils were plated on coverslips coated with 2.5 μg/ml fibrinogen for 10 min, fixed, stained for calpain 1 (left), costained with rhodamine-phalloidin for F-actin (middle), and overlaid (right). Magnified merged image shows no colocalization (yellow; note arrow) between F-actin (red) and calpain 1 (green). Bar, 10 μm. (D) Resting and fMLP-treated human neutrophils were treated as described in C and stained for calpain 2 (left), F-actin (middle), and overlaid (right). Magnified merged image shows strong colocalization (yellow; note arrow) between F-actin (red) and calpain 2 (green). Bar, 10 μm. (E) C5a-stimulated dHL-60 cells were plated on coverslips coated with 2.5 μg/ml fibrinogen for 10 min, fixed, and stained for endogenous calpain 1 (left) or calpain 2 (right). Arrows show localization of calpain 1 toward the cell rear and calpain 2 at the leading edge. Bar, 5 μm.
Asymmetric Distribution of Calpain 1 and Calpain 2 in Neutrophils
We next determined the localization of endogenous calpain 1 and calpain 2 in both resting and stimulated primary neutrophils by using immunofluorescence. In resting cells, both calpain 1 and calpain 2 showed a diffuse intracellular distribution and did not colocalize with F-actin at the cell periphery (Figure 1, C and D). However, treatment with a uniform concentration of chemoattractant resulted in the asymmetric distribution of calpain 1 and calpain 2 (Figure 1, C and D). Calpain 1 localization remained relatively diffuse and did not colocalize with F-actin at the leading edge of the cell (Figure 1C). In contrast, calpain 2 was enriched at the leading edge of the cell where it colocalized with F-actin (Figure 1D). These findings demonstrate that calpain 1 and calpain 2 have distinct subcellular distributions in neutrophils in response to chemoattractant, with calpain 2 enriched at the cell front.
Calpain 2 Localizes to the Leading Edge of Neutrophil-like HL-60 Cells
To complement our work with primary neutrophils, we have also used the neutrophil-like HL-60 cell line (Collins et al., 1977). HL-60 cells are a promyelocytic leukemia cell line that can be differentiated into a neutrophil-like state by culturing with 1.25% DMSO (Collins et al., 1978). On differentiation, HL-60 cells become sensitive to myeloid-specific histochemical stains and display a similar morphology, polarity, and protein expression profile as primary neutrophils (Collins et al., 1979; Gallagher et al., 1979; Hauert et al., 2002). Most importantly, dHL-60 cells are responsive to various chemoattractrants (Collins et al., 1979; Gallagher et al., 1979; Hauert et al., 2002), which makes them a valid, widely used model system to study neutrophil chemotaxis (Servant et al., 2000; Weiner et al., 2002; Gomez-Mouton et al., 2004; Van Keymeulen et al., 2006; Wong et al., 2006). Using immunofluorescence, we found that treatment with a uniform concentration of chemoattractant triggers the asymmetric distribution of endogenous calpain 1 and calpain 2 in dHL-60 cells (Figure 1E) similar to primary neutrophils (Figure 1D) with calpain 2 enriched at the leading edge.
To further assess the role of calpain 2 in neutrophil chemotaxis, stable HL-60 cell lines that express C-terminal FLAG-tagged wild-type or protease-dead (PD) calpain 2 coupled to ribosome entry site (IRES)-driven expression of GFP were generated (Figure 2A). Cell populations were sorted by flow cytometry, differentiated with DMSO, and protein expression was confirmed by immunoblotting (Figure 2B). In accordance with our findings for the localization of endogenous calpain 2 in primary neutrophils and dHL-60 cells (Figure 1, D and E), FLAG-tagged calpain 2 also localized to the leading edge in response to chemoattractant stimulation, whereas calpain 1 staining was diffuse and localized away from the leading edge (Figure 2C). Calpain 2 PD also localized to membrane protrusions but did not display as strong enrichment at the leading edge compared with wild-type calpain 2 (Figure 2C).
Figure 2.
Calpain 2 regulates talin proteolysis in dHL-60 cells. (A) Schematic of constructs. Stable HL-60 cell lines were generated that express FLAG-tagged versions of wild-type calpain 2 and a calpain 2 PD. Roman numerals designate the domains of calpain 2, and the active site residues within the protease domain are noted. The six EF hands in calpain 2 are shaded (dark gray). The protease-dead construct was generated by mutating the active site histidine (H262A denoted with an asterisk). (B) Expression of FLAG-tagged calpain 2 (WT) and protease-dead calpain 2 (PD) in dHL-60 cells was detected by immunoblot with α-FLAG. (C) dHL-60 cells that express wild-type calpain 1 (Capn 1), wild-type calpain 2 (Capn 2), or protease-dead calpain 2 (Capn 2 PD) were plated on coverslips coated with 2.5 μg/ml fibrinogen, stimulated with 11 nM C5a for 10 min, fixed, and stained with α-FLAG as indicated. Bar, 5 μm. (D) dHL60 cells were either not stimulated (0) or treated with C5a over a 10-min time course as indicated. Cell lysates were taken and examined for talin proteolysis by immunoblot analysis. A representative blot from three separate experiments is shown. Maximal talin cleavage was observed with 10 min of C5a stimulation. (E) Talin proteolysis was examined in dHL-60 cells that express control vector (Con), Capn 2, or Capn 2 PD. Cell lysates were obtained from dHL-60 cells that were not stimulated (NS) or treated with C5a for 10 min (C5a). A representative blot from three separate experiments is shown.
To determine whether calpain activity was affected by expression of wild type and calpain 2 PD, analysis of talin proteolysis was performed in dHL-60 cells treated with C5a. Calpain 2-mediated cleavage of the cytoskeletal protein talin (Franco et al., 2004b) occurs upon treatment of neutrophils with chemoattractant (Sampath et al., 1998). The proteolytic cleavage of talin produces a 190-kDa product, which is increased in dHL-60 cells treated with C5a compared with unstimulated cells and is also increased over a time course of chemoattractant stimulation in dHL-60 cells (Figure 2D). Furthermore, talin proteolysis was increased in dHL-60 cells that express wild-type calpain 2 compared with control cells and dHL-60 cells that express calpain 2 PD (Figure 2E). These findings suggest that overexpression of calpain 2 enhances calpain activity as a function of talin proteolysis and that the protease activity of calpain 2 is required for this effect.
Calpain 2 Localization Is a Dynamic Marker of Frontness during Chemotaxis
To examine calpain 2 localization in live cell imaging studies, we generated stable HL-60 cell lines that express either C-terminal GFP-tagged wild-type or protease-dead (PD) calpain 2 (Figure 3). Cell populations were sorted by flow cytometry, differentiated with DMSO, and expression of each construct was confirmed by immunoblotting (Supplemental Figure 1A). Using MAC-1 surface expression as a marker of differentiation in a FACS-based assay (Carrigan et al., 2005), we demonstrated that DMSO-induced differentiation of HL-60 cells results in the upregulation of Mac-1 (gray) (Carrigan et al., 2005) as well as ICAM-1 (black) and that overexpression of wild-type calpain 2 or PD did not alter the up-regulation of these receptors at the cell surface (Supplemental Figure 1, B and C). This indicates that overexpression of wild-type calpain 2 or PD did not alter differentiation. Using time-lapse fluorescent microscopy in a micropipette-based assay, dHL-60 cells expressing calpain 2-GFP demonstrated asymmetric localization of calpain 2 toward the chemoattractant source (Figure 3 and Supplemental Video 1). Calpain 2 signal from the GFP channel overlayed with DIC images of the cell revealed that the majority of calpain 2-GFP was localized toward the leading edge (Figure 3B). This was in contrast to cells that expressed GFP alone, which demonstrated a diffuse distribution of GFP (data not shown).
To determine the temporal relationship between chemoattractant stimulation and calpain 2 redistribution, the localization of calpain 2-GFP was observed upon initial exposure to chemoattractant via the micropipette assay. Initially, cells displayed a spherical morphology (Figure 3C). However, within approximately the first minute after chemoattractant exposure a leading pseudopod formed and the cells acquired a polarized morphology (Figure 3C). In comparison, after 10 s of chemoattractant exposure, calpain 2-GFP was enriched in the direction of chemoattractant. The enrichment of calpain 2 toward the chemoattractant source occurred as the leading pseudopod was established, but before the acquisition of a polarized morphology (Figure 3C). Additionally, as the cell began to chemotax toward the highest concentration of chemoattractant, calpain 2-GFP remained localized at the leading edge (Figure 3C). These findings demonstrate that asymmetric distribution of calpain 2 occurs rapidly after chemoattractant exposure and suggests that calpain 2 may be a component of the initial signaling events that define frontness. To test this hypothesis, we examined whether positional changes in the chemoattractant gradient would alter the localization of calpain 2-GFP. We found that moving the position of the C5a-loaded micropipette resulted in immediate redistribution of calpain 2-GFP toward the highest concentration of chemoattractant (Figure 3D and Supplemental Video 2). This redistribution of calpain 2-GFP was followed by a change in the direction of migration and a further enrichment of calpain 2-GFP at the newly defined leading edge. These data demonstrate that localization of calpain 2 to the cell front is a dynamic process that is sensitive to the area of the cell experiencing the highest concentration of chemoattractant.
Our findings suggest that calpain 2 may be involved in a positive feedback loop that reinforces frontness. To test this hypothesis, we examined the role of actin assembly in the recruitment of calpain 2 to the cell front. After pretreatment with latrunculin A, calpain 2-GFP failed to translocate toward the highest concentration of chemoattractant, and cell polarization was abrogated (Figure 3E). These findings indicate that calpain 2 translocation occurs in an actin-dependent manner and suggests calpain 2 functions downstream of actin polymerization to reinforce frontness.
Calpain 2 Localizes to GM-3–rich Lipid Rafts at the Leading Edge
Previous studies have demonstrated that leukocytes establish GM-1–rich lipid rafts at the rear and GM-3–rich rafts at the leading edge of the cell in response to chemoattractant stimulation. These lipid rafts serve as organizing centers during chemotaxis, sequestering signaling proteins to specific regions of the cell (Manes et al., 1999; Gomez-Mouton et al., 2001; Pierini et al., 2003; Gomez-Mouton et al., 2004; Kindzelskii et al., 2004). Our data indicate that calpain 2 is recruited to the cell front upon chemoattractant stimulation, suggesting that calpain 2 activity may be sequestered by localization to lipid rafts. To determine whether calpain 2 localizes to lipid rafts, detergent-resistant membranes (DRMs) were isolated in resting and stimulated primary neutrophils. Isolation of DRMs from resting neutrophils showed that both calpain 1 and calpain 2 partition with soluble cellular components such as RACK1 and CD45 into nonraft fractions (Figure 4A). Chemoattractant stimulation with fMLP induced the partitioning of calpain 2, but not calpain 1, into the DRM fraction with lipid raft markers CD43 and GM-1 (Figure 4A). Although calpain 1 did not move into the lipid raft fraction, it became diffusely distributed throughout the soluble fractions after neutrophil activation. Biochemical characterization does not distinguish between front and rear raft fractions because both GM-1–rich lipid rafts and GM-3–rich rafts partition to the same fraction in standard lipid raft preparations. To determine whether calpain 2 is associated with GM-3–rich rafts at the leading edge of the cell, we performed immunostaining. The results revealed colocalization of calpain 2 with GM-3 lipid rafts at the leading edge of the cell after chemoattractant stimulation (Figure 4B), suggesting that calpain 2 specifically translocates to GM-3–containing lipid rafts at the leading edge of neutrophils. In addition, treatment with the lipid raft disrupting drug methyl- β-cyclo-dextran disrupted calpain 2 localization and prevented its colocalization with F-actin after chemoattractant stimulation (Figure 4C). Together, these findings demonstrate that calpain 2, but not calpain 1, partitions to cholesterol-rich membrane domains, and this localization may function to restrict calpain 2 activity to specific regions of the leading edge of the cell during chemotaxis.
Figure 4.
Chemoattractant induces translocation of calpain 2 to lipid rafts. DRM domains were isolated from unstimulated and fMLP-stimulated primary neutrophils by using density gradients. Fractions were collected from raft fractions (1 and 2) and from detergent-soluble fractions (3–9) and analyzed by immunoblot by using the indicated antibodies as described in Materials and Methods. Both SDS-PAGE (top) and dot blots (bottom) were performed to detect the presence of the lipid raft markers. The blots are representative of a minimum of four separate experiments. (B) Primary neutrophils were plated on coverslips coated with 2.5 μg/ml fibrinogen after treatment with 10 nM fMLP for 10 min before fixation and stained for GM-3 (red), calpain 2 (green), and overlay (yellow). Bar, 5 μm. (C) Primary neutrophils were pretreated with either vehicle or 10 mM β-methyl-cyclo-dextran for 15 min, stimulated with 10 nM fMLP for 10 min, and plated on coverslips coated with 2.5 μg/ml fibrinogen. The cells were then fixed and stained for F-actin (red), calpain 2 (green), and overlay (yellow) as described in Materials and Methods. Bar, 5 μm.
Calpain 2 Limits Pseudopod Formation during Chemotaxis
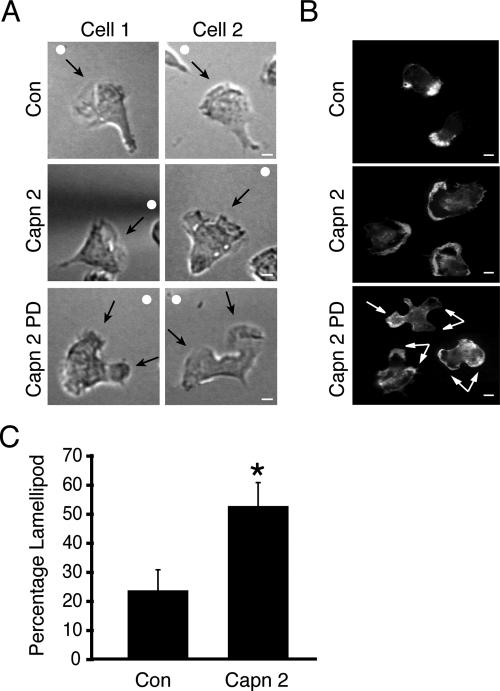
Recent work has shown that calpain 2 plays an important role in regulating membrane protrusion at the leading edge of fibroblasts (Franco et al., 2004a). Because calpain 2 is asymmetrically recruited to the cell front in response to chemoattractant and is found in lipid rafts, we were interested to determine whether calpain 2 activity regulates pseudopod formation in response to a gradient of chemoattractant. dHL-60 cells that express wild-type calpain 2 exhibited a characteristic polar morphology with a single leading edge upon chemoattractant stimulation compared with control cells (Figure 5A). In contrast, cells expressing calpain 2 PD displayed an altered cell polarity with excessive pseudopod formation and impaired formation of a defined leading edge (Figure 5A). Staining of the dHL-60 control and wild-type calpain 2-expressing cells revealed a localized area of F-actin in a single dominant lamellipodium. In contrast, F-actin was enriched at multiple protrusions in calpain 2 PD-expressing cells (Figure 5B). In addition, expression of wild-type calpain 2 resulted in a greater than twofold increase in the lamellipod size compared with control cells where the frontal lamellipod made up 20–30% of total cell size (Figure 5C). These data suggest that cell polarity is enhanced as a result of overexpression of calpain 2. Together, our findings suggest that calpain 2 is important for limiting pseudopodia formation in the direction of chemoattractant and establishing polarity in response to chemotactic gradients.
Figure 5.
Calpain 2 protease activity regulates lamellipodium formation. (A) dHL-60 cells that express control vector (Con), wild-type calpain 2 (Capn 2), or protease-dead calpain 2 (Capn 2 PD) were plated onto 2.5 μg/ml fibrinogen and 50 μg/ml fibronectin and exposed to a C5a chemotactic gradient. Images were captured using 60× DIC. A white circle (○) marks the direction of the chemoattractant source, and arrows indicate the leading edge of the cell. Bar, 5 μm. (B) dHL-60 cells (dHL-60) that express control vector (Con), Capn 2, and Capn 2 PD were plated on coverslips coated with 2.5 μg/ml fibrinogen, stimulated with 11 nM C5a for 10 min, fixed, and stained with rhodamine-labeled phalloidin as described in Materials and Methods. Arrows are used to indicate the leading edge of the cell. Double arrows were used to highlight multiple F-actin based protrusions in cells that express calpain 2 PD. Bar, 5 μm. (C) Cell imaging software was used to determine lamellipod percentage of dHL-60 cells that express control vector (Con) or calpain 2 wild type (Capn2) as described in Materials and Methods. Error bars show SEM from ≥50 cells from four separate experiments. Asterisks indicate significant difference as compared with control (p ≤ 0.05 by Student's t test).
Catalytic Activity of Calpain 2 Is Required for Efficient Chemotaxis
To further elucidate the function of calpain 2 in neutrophil chemotaxis, live cell imaging studies and transwell assay were performed. Control and calpain 2 dHL-60 cell lines rapidly adopted a polarized morphology in the direction of the pipette tip, with pseudopodia that were restricted to the leading edge (Figure 6A and Supplemental Videos 3 [control] and 4 [calpain 2]). The calpain 2 cells adopted a more polarized morphology than control cells, as depicted by a more defined leading edge in the direction of the chemoattractant source and suppression of rear and lateral pseudopodia (Figure 6A and Supplemental Video 4). In contrast, dHL-60 cell lines that expressed calpain 2 PD displayed a compromised ability to develop a defined leading edge in response to C5a, displaying a variable phenotype inclusive of multiple lateral pseudopodia, bifurcated lamellipods, and increased membrane protrusions, which hindered the efficiency of chemotaxis (Figure 6A and Supplemental Video 5). Cells that express wild-type calpain 2 showed a highly directed migration with more robust recruitment of cells to the pipette tip as depicted in Rose plots and observed in videos (Figure 6B and Supplemental Video 4). dHL-60 cells that express calpain 2 exhibited significantly increased cell speeds (14 ± 0.91 μm/min) compared with control cells (9.3 ± 0.41 μm/min) (Figure 6C). In contrast, dHL-60 cells that expressed calpain 2 PD demonstrated reduced cell speed (5.5 ± 0.52 μm/min) and directional migration (Figure 6C and Supplemental Video 5). Similar phenotypes were observed in dHL-60 cells that express calpain 2 PD-GFP (Supplemental Video 6). We have attempted small interfering RNA-based experiments to further examine calpain 2 function during dHL-60 cell chemotaxis; however, we have been unsuccessful in these endeavors.
We also examined the effects of calpain 2 expression on the migration of dHL-60 cells to C5a by using a transwell assay. Ectopic expression of calpain 2 enhanced chemotactic migration of dHL-60 cells to C5a compared with control cells (Figure 6D). In contrast, expression of calpain 2 PD reduced chemotaxis of dHL-60 cells to C5a (Figure 6D). Because expression of a protease-dead calpain 2 reduced HL-60 chemotaxis, increased membrane protrusions, and impaired establishment of a defined leading edge, our findings suggest that calpain 2 plays an important role in establishing frontness and is necessary for effective chemotaxis (Figure 6D).
DISCUSSION
Calpains have recently emerged as key regulators of fibroblast migration; however, little is known about their role in neutrophil chemotaxis. In this study, we have examined the expression and localization of calpain isoforms during neutrophil chemotaxis and found that calpain 1 and calpain 2 are asymmetrically distributed in response to chemoattractant stimulation, with calpain 2 recruited to the lipid rafts at the cell front. Using time-lapse microscopy, we have examined the localization of calpain 2 during chemotaxis, and our results demonstrate that chemoattractant stimulation triggers the rapid redistribution of calpain 2 during early pseudopod formation to the leading edge and that its localization is sensitive to positional changes in the chemotactic gradient. We also show that the catalytic activity of calpain is involved in biasing pseudopod formation in the direction of chemoattractant and that this activity is needed for effective chemotaxis. Therefore, this study identifies a novel “frontness” targeting property of calpain 2 that is distinct from calpain 1 during neutrophil chemotaxis.
We previously showed that inhibition of calpain 1, but not calpain 2, in resting neutrophils induces random motility in the absence of exogenous activators and that calpain 2 is not required for random migration induced by uniform chemoattractant stimulation (Lokuta et al., 2003). Interestingly, the increase in random migration induced by calpain inhibition is accompanied by a diminished chemotactic response, and our findings now suggest that calpain 2 is a key isoform involved in chemotaxis. In accordance with their distinct functional roles, calpain 2, but not calpain 1, localizes to the leading edge during chemotaxis. The spatial segregation of signaling molecules to different subcellular regions during neutrophil chemotaxis is not uncommon. For example, members of the small Rho-GTPase family, such as Rac and Cdc42, have been shown to localize to the leading edge (Srinivasan et al., 2003), whereas Rho translocates to the cell rear (Xu et al., 2003). Accordingly, Rac and Cdc42 are essential for polarization and directional migration, whereas Rho is responsible for rear retraction (Niggli, 1999; Alblas et al., 2001). Therefore, spatial restriction of calpain 2 to the cell front suggests that calpain 2 plays a role in the amplification of frontness signaling at the leading edge during neutrophil chemotaxis.
Further analysis of calpain 2 function during chemotaxis indicates that calpain 2 promotes frontness by stabilizing lamellipod formation in the direction of chemoattractant (Figures 3, 5, and 6). Calpain 2 is an immediate-early marker of the cell front, and its localization is sensitive to positional changes in the chemotactic gradient. In addition, dHL-60 cells that overexpress calpain 2 generate a prominent pseudopod at the leading edge, demonstrate persistent polarity in a gradient of chemoattractant, and display enhanced chemotaxis. In contrast, expression of protease-dead calpain 2 impairs neutrophil chemotaxis by compromising pseudopod formation at the leading edge. Our findings in neutrophils are consistent with a recent study that demonstrates a key role for calpain 2 in regulating membrane protrusion and leading edge formation in fibroblasts (Franco et al., 2004a). It is intriguing to speculate that calpain 2 may function in a pathway analogous to PIP3 and Cdc42, which localize to the cell front and function not only to promote pseudopod formation at the leading edge but also to regulate Rho-mediated contractility at the cell rear (Van Keymeulen et al., 2006).
Previous studies have shown that stimulation of T-cells with phorbol 12-myristate 13-acetate partitions calpain activity to the membrane (Rock et al., 2000) and that calpain 2, but not calpain 1, associates with lipid rafts in T-cells. In accordance with these findings, our work demonstrates that calpain 2, but not calpain 1, also targets to GM-3–rich lipid rafts at the leading edge of activated neutrophils. Targeting of calpain 2 to lipid rafts may serve to restrict calpain 2 activity at the leading edge and most likely acts as a scaffold to bring calpain 2 in proximity with key regulators of calpain activity such as calcium, phosphoinositides, and pH (Guroff, 1964; Saido et al., 1992; Arthur and Crawford, 1996; Melloni et al., 1996; Shiraha et al., 2002; Glading et al., 2004). Recent work suggests that the calcium channel TrpC localizes to lipid raft domains at the leading edge (Kindzelskii et al., 2004), which would place it in proximity to calpain 2 and possibly provide a mechanism to regulate calpain 2 activity locally. Furthermore, both calpain 2 and phosphoinositides localize to the leading edge and several groups have shown that binding of phospholipids to the C2-like region in calpain domain III can lower the calcium concentration required for calpain activation (Saido et al., 1992; Melloni et al., 1996; Tompa et al., 2001; Shao et al., 2006). Finally, previous studies have shown the localization of the mammalian Na+-H+ exchanger NHE1 to the leading edge and have implicated the exchanger in regulating calpain activity (Denker and Barber, 2002). A recent study using Dictyostelium discoideum demonstrated that a NHE1 homologue, DdNHE1, also localizes to the leading edge and is required for cell polarization during chemotaxis in a cAMP gradient (Patel and Barber, 2005). Together, spatial restriction of calpain 2 within lipid rafts may provide a mechanism to allow for the localized activation of calpain 2 at the leading edge during neutrophil chemotaxis.
A critical but unanswered question is the identity of the key effectors of calpain 2 activity at the cell front. Recent studies indicate that calpain 2-mediated talin proteolysis is a key mechanism that mediates adhesion dynamics in fibroblasts (Franco et al., 2004b). Talin also localizes to the leading edge during neutrophil chemotaxis and is associated with lipid rafts in activated neutrophils (Yan and Berton, 1998). Calpain-mediated proteolysis of talin also occurs in neutrophils stimulated with chemoattractant and has been implicated in regulating the association of β2 integrin with the actin cytoskeleton (Sampath et al., 1998). Accordingly, our findings suggest that ectopic expression of calpain 2 in dHL-60 cells increases talin proteolysis in response to chemoattractant and that this proteolysis is reduced by expression of calpain 2 PD. However, despite the well-respected relationship between calpain and talin, it is likely that additional calpain substrates play a role in calpain 2 effector pathways during chemotaxis.
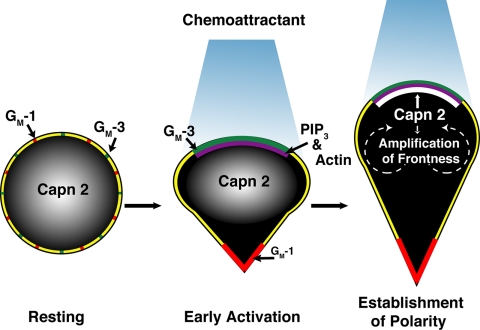
In summary, we show that calpain 1 and calpain 2 are asymmetrically distributed upon chemoattractant stimulation with calpain 2, but not calpain 1, targeted to lipid rafts at the cell front. We also show that calpain 2 is recruited during early pseudopod formation to the area of the cell experiencing the highest concentration of chemoattractant and that calpain 2 localization is defined according to spatial cues from the chemotactic gradient. Finally, we demonstrate that calpain 2 activity is necessary for limiting pseudopod formation and for efficient chemotaxis. Based on our data, we propose that calpain 2 is a novel component of the signaling pathways at the leading edge that bias pseudopod formation in the direction of chemoattractant to promote frontness during neutrophil chemotaxis (Figure 7). We hypothesize that the localization of calpain 2 to lipid rafts at the leading edge provides a scaffold to restrict calpain 2 activity to highly localized regions at the leading edge and place it in proximity to specific effector pathways. Additional work is necessary to determine the calpain 2 mediated effector pathways during neutrophil chemotaxis and to understand the significance of calpain 1 targeting in neutrophil chemotaxis.
Figure 7.
Model of calpain function during chemotaxis. Unstimulated neutrophils display a round nonpolarized morphology with a uniform distribution of membrane lipid rafts (red and green) and calpain 2 (white). Asymmetric recruitment of PIP3 (purple) occurs with chemoattractant stimulation and represents an early marker of gradient sensing. Subsequently, asymmetric F-actin staining is observed, and membrane lipid raft markers also show an asymmetric distribution. An early event at the leading edge also includes the recruitment of calpain 2 to GM-3–rich lipid rafts. We propose that calpain 2 is involved in a positive feedback loop that limits lamellipod formation to the leading edge and amplifies the frontness signal during chemotaxis.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Mary Lokuta for experimental assistance, insightful discussions, and critical reading of the manuscript; Jun Zhu for construction of the calpain 2-GFP retroviral fusion constructs; Ben Perrin, Kate Cooper, Henry Bourne, Verena Niggli, and Robert Sitrin for scientific discussions and advice; Garry Nolan for Phoenix cells; Joan Fox and Alan Wells for calpain 1 and calpain 2 cDNAs; Clive Svendsen for the IRES-GFP retroviral vector; Ron Mellgren for the 2H-7 calpain 1 antibody; and Kathy Schell, Joel Puchalski, and Dagna Sheerar for expertise at the Flow Cytometry Facility at the University of Wisconsin. This work was supported by National Institutes of Health Grants R01 GM-074827 (to A.H.) and American Heart Association grant-in-aid (to A.H.). P.N. was supported by an American Heart Association pre-doctoral fellowship, and M.A.S. is supported by postdoctoral fellowship 0625751Z from the American Heart Association.
Abbreviations used:
- C5a
Complement factor 5a
- DIC
differential interference contrast
- dHL-60
differentiated HL-60
- fMLP
N-formyl- l-methionyl-l-leucyl-l-phenylalanine
- GM
ganglioside marker-1
- PD
protease dead
- PMN
polymorphonuclear cell
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- RACK
receptor for activated C kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0876) on December 27, 2006.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Alblas J., Ulfman L., Hordijk P., Koenderman L. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Mol. Biol. Cell. 2001;12:2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. S., Crawford C. Investigation of the interaction of m-calpain with phospholipids:calpain-phospholipid interactions. Biochim. Biophys. Acta. 1996;1293:201–206. doi: 10.1016/0167-4838(95)00243-x. [DOI] [PubMed] [Google Scholar]
- Arthur J. S., Gauthier S., Elce J. S. Active site residues in m-calpain: identification by site-directed mutagenesis. FEBS Lett. 1995;368:397–400. doi: 10.1016/0014-5793(95)00691-2. [DOI] [PubMed] [Google Scholar]
- Badizadegan K., Dickinson B. L., Wheeler H. E., Blumberg R. S., Holmes R. K., Lencer W. I. Heterogeneity of detergent-insoluble membranes from human intestine containing caveolin-1 and ganglioside G(M1) Am. J. Physiol. 2000;278:G895–G904. doi: 10.1152/ajpgi.2000.278.6.G895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A., Kaverina I., Otey C., Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- Carrigan S. O., Weppler A. L., Issekutz A. C., Stadnyk A. W. Neutrophil differentiated HL-60 cells model Mac-1 (CD11b/CD18)-independent neutrophil transepithelial migration. Immunology. 2005;115:108–117. doi: 10.1111/j.1365-2567.2005.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977;270:347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J. Exp. Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tullio R., Stifanese R., Salamino F., Pontremoli S., Melloni E. Characterization of a new p94-like calpain form in human lymphocytes. Biochem. J. 2003;375:689–696. doi: 10.1042/BJ20030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker S. P., Barber D. L. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P., Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- Dourdin N., Bhatt A. K., Dutt P., Greer P. A., Arthur J. S., Elce J. S., Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J. Biol. Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- Franca-Koh J., Devreotes P. N. Moving forward: mechanisms of chemoattractant gradient sensing. Physiology. 2004;19:300–308. doi: 10.1152/physiol.00017.2004. [DOI] [PubMed] [Google Scholar]
- Franco S. J., Perrin B. J., Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp. Cell Res. 2004a;299:179–187. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Franco S. J., Rodgers M. A., Perrin B. J., Han J., Bennin D. A., Critchley D. R., Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004b;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Funamoto S., Meili R., Lee S., Parry L., Firtel R. A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Gallagher R., et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- Glading A., Bodnar R. J., Reynolds I. J., Shiraha H., Satish L., Potter D. A., Blair H. C., Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A., Uberall F., Keyse S. M., Lauffenburger D. A., Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J. Biol. Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- Goll D. E., Thompson V. F., Li H., Wei W., Cong J. The calpain system. Physiol. Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gomez-Mouton C., Abad J. L., Mira E., Lacalle R. A., Gallardo E., Jimenez-Baranda S., Illa I., Bernad A., Manes S., Martinez A. C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mouton C., Lacalle R. A., Mira E., Jimenez-Baranda S., Barber D. F., Carrera A. C., Martinez A. C., Manes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J. Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G. A neutral, calcium-activated proteinase from the soluble fraction of rat brain. J. Biol. Chem. 1964;239:149–155. [PubMed] [Google Scholar]
- Harlow E., Lane D. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. Using Antibodies: A Laboratory Manual. [Google Scholar]
- Hauert A. B., Martinelli S., Marone C., Niggli V. Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis. Int. J. Biochem. Cell Biol. 2002;34:838–854. doi: 10.1016/s1357-2725(02)00010-9. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A. Cell polarization mechanisms during directed cell migration. Nat. Cell Biol. 2005;7:336–337. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A., Ginsberg M. H., Horwitz A. F. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J. Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A., Palecek S. P., Lu Q., Zhang W., Mellgren R. L., Lauffenburger D. A., Ginsberg M. H., Horwitz A. F. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Jordan M., Schallhorn A., Wurm F. M. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Parent C. A. The signal to move: D. discoideum go orienteering. Science. 2003;300:1525–1527. doi: 10.1126/science.1085439. [DOI] [PubMed] [Google Scholar]
- Kindzelskii A. L., Sitrin R. G., Petty H. R. Cutting edge: optical microspectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of neutrophils: apparent role in calcium signaling. J. Immunol. 2004;172:4681–4685. doi: 10.4049/jimmunol.172.8.4681. [DOI] [PubMed] [Google Scholar]
- Li Z., et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- Lokuta M. A., Nuzzi P. A., Huttenlocher A. Calpain regulates neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA. 2003;100:4006–4011. doi: 10.1073/pnas.0636533100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S., Mira E., Gomez-Mouton C., Lacalle R. A., Keller P., Labrador J. P., Martinez A. C. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Michetti M., Salamino F., Minafra R., Pontremoli S. Modulation of the calpain autoproteolysis by calpastatin and phospholipids. Biochem. Biophys. Res. Commun. 1996;229:193–197. doi: 10.1006/bbrc.1996.1779. [DOI] [PubMed] [Google Scholar]
- Niggli V. Rho-kinase in human neutrophils: a role in signaling for myosin light chain phosphorylation and cell migration. FEBS Lett. 1999;445:69–72. doi: 10.1016/s0014-5793(99)00098-8. [DOI] [PubMed] [Google Scholar]
- Palecek S. P., Huttenlocher A., Horwitz A. F., Lauffenburger D. A. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 1998;111:929–940. doi: 10.1242/jcs.111.7.929. [DOI] [PubMed] [Google Scholar]
- Patel H., Barber D. L. A developmentally regulated Na-H exchanger in Dictyostelium discoideum is necessary for cell polarity during chemotaxis. J. Cell Biol. 2005;169:321–329. doi: 10.1083/jcb.200412145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini L. M., Eddy R. J., Fuortes M., Seveau S., Casulo C., Maxfield F. R. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- Rock M. T., Dix A. R., Brooks W. H., Roszman T. L. Beta1 integrin-mediated T cell adhesion and cell spreading are regulated by calpain. Exp. Cell Res. 2000;261:260–270. doi: 10.1006/excr.2000.5048. [DOI] [PubMed] [Google Scholar]
- Rodighiero C., Fujinaga Y., Hirst T. R., Lencer W. I. A cholera toxin B-subunit variant that binds ganglioside G(M1) but fails to induce toxicity. J. Biol. Chem. 2001;276:36939–36945. doi: 10.1074/jbc.M104245200. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82:803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Shibata M., Takenawa T., Murofushi H., Suzuki K. Positive regulation of μ-calpain action by polyphosphoinositides. J. Biol. Chem. 1992;267:24585–24590. [PubMed] [Google Scholar]
- Sampath R., Gallagher P. J., Pavalko F. M. Cytoskeletal interactions with the leukocyte integrin beta2 cytoplasmic tail. Activation-dependent regulation of associations with talin and alpha-actinin. J. Biol. Chem. 1998;273:33588–33594. doi: 10.1074/jbc.273.50.33588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymeinsky J., Sindrilaru A., Frommhold D., Sperandio M., Gerstl R., Then C., Mocsai A., Scharffetter-Kochanek K., Walzog B. The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for β2 integrin (CD11/CD18)-mediated neutrophil migration. Blood. 2006;108:3919–3927. doi: 10.1182/blood-2005-12-030387. [DOI] [PubMed] [Google Scholar]
- Schymeinsky J., Then C., Walzog B. The non-receptor tyrosine kinase Syk regulates lamellipodium formation and site-directed migration of human leukocytes. J. Cell Physiol. 2005;204:614–622. doi: 10.1002/jcp.20323. [DOI] [PubMed] [Google Scholar]
- Servant G., Weiner O. D., Herzmark P., Balla T., Sedat J. W., Bourne H. R. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G., Weiner O. D., Neptune E. R., Sedat J. W., Bourne H. R. Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol. Biol. Cell. 1999;10:1163–1178. doi: 10.1091/mbc.10.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Chou J., Baty C. J., Burke N. A., Watkins S. C., Stolz D. B., Wells A. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol. Cell Biol. 2006;26:5481–5496. doi: 10.1128/MCB.02243-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraha H., Glading A., Chou J., Jia Z., Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol. Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Wang F., Glavas S., Ott A., Hofmann F., Aktories K., Kalman D., Bourne H. R. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa P., Emori Y., Sorimachi H., Suzuki K., Friedrich P. Domain III of calpain is a ca2+-regulated phospholipid-binding domain. Biochem. Biophys. Res. Commun. 2001;280:1333–1339. doi: 10.1006/bbrc.2001.4279. [DOI] [PubMed] [Google Scholar]
- Van Haastert P.J.M., Devreotes P. N. Chemotaxis: signalling the way forward. Nat. Rev. Mol. Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A., Wong K., Knight Z. A., Govaerts C., Hahn K. M., Shokat K. M., Bourne H. R. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J. Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner O. D., Neilsen P. O., Prestwich G. D., Kirschner M. W., Cantley L. C., Bourne H. R. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner O. D., Servant G., Welch M. D., Mitchison T. J., Sedat J. W., Bourne H. R. Spatial control of actin polymerization during neutrophil chemotaxis. Nat. Cell Biol. 1999;1:75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski J. M., Zmuda-Trzebiatowska E., Swiercz J. M., Cichorek M., Ciepluch H., Lewandowski K., Bryl E., Hellmann A. Modulation of the activity of calcium-activated neutral proteases (calpains) in chronic lymphocytic leukemia (B-CLL) cells. Blood. 2002;100:1802–1809. doi: 10.1182/blood-2001-11-0073. [DOI] [PubMed] [Google Scholar]
- Wong K., Pertz O., Hahn K., Bourne H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc. Natl. Acad. Sci. USA. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T., Bourne H. R. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yan S. R., Berton G. Antibody-induced engagement of beta2 integrins in human neutrophils causes a rapid redistribution of cytoskeletal proteins, Src-family tyrosine kinases, and p72syk that precedes de novo actin polymerization. J. Leukoc. Biol. 1998;64:401–408. doi: 10.1002/jlb.64.3.401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.