Abstract
Lens development requires the precise coordination of cell division and differentiation. The mechanisms by which the differentiation program is initiated after cell cycle arrest remains not well understood. Cyclin-dependent kinase inhibitors (CKIs), such as p15 and p21, have been suggested to be critical components that inhibit G1 progression and therefore, their activation is necessary for quiescence and important for the onset of differentiation. Regulation of p15 and p21 is principally governed by transforming growth factor (TGF)-β–signaling pathway. We have identified that Cdh1/APC, a critical ubiquitin protein ligase, plays an important role in regulating lens differentiation by facilitating TGF-β–induced degradation of SnoN, a transcriptional corepressor that needs to be removed for transcriptional activation of p15 and p21. The depletion of Cdh1 by RNA interference attenuates the TGF-β–mediated induction of p15 and p21 and significantly blocks lens differentiation. Expression of nondegradable SnoN also noticeably attenuates lens induction. Furthermore, we have shown that Cdh1 and SnoN form a complex at the onset of lens differentiation. In vivo histological analysis confirms our biochemical and genetic results. Thus, Cdh1/APC is crucial to the coordination of cell cycle progression and the initiation of lens differentiation through mediating TGF-β–signaling-induced destruction of SnoN.
INTRODUCTION
Proper lens differentiation requires precise temporal control of the cell cycle and the coordination of cell cycle exit with differentiative cues and signaling pathways (Zhu and Skoultchi, 2001). Studies of lens development have led to establishment of lens induction as a popular model system to study differentiation (Lovicu and McAvoy, 2005), especially in the identification of signaling pathways that together orchestrate the coordination of proliferation with differentiation in lens development (Zhu and Skoultchi, 2001; Lovicu and McAvoy, 2005). Epithelial lens differentiation into lens fiber cell is dramatically inhibited in p27 and p57 doubly deficient mice (Zhang et al., 1998). In addition, mice lacking both p21 and p57 fail to form myotubes for muscle development (Zhang et al., 1999). Thus, accumulating evidence suggests that specific inhibitors of cyclin-dependent kinase inhibitors (CKIs), such as p57, p27, p21, and p15 (Skapek et al., 1995), play a crucial role in differentiation by inducing cell cycle arrest necessary for the initiation of terminal differentiation (Dyer and Cepko, 2001; Zhu and Skoultchi, 2001; Liu et al., 2004). In fact, members of the INK family, such as p15, and the Cip/Kip family, such as p21 interact with cyclin D/CDK4/6 and cyclin E/CDK2 to block their kinase activity, thereby, liberating retinoblastoma protein (Rb) and its related family members to initiate terminal differentiation (Dyer and Cepko, 2001). CKIs are implicated in lens development (Zhu and Skoultchi, 2001; Liu et al., 2004); however, the mechanisms by which these CKIs are regulated remain poorly understood. Moreover, how the regulation is organized by defined signals awaits further investigation.
Previous studies have implicated the ubiquitin proteasomal system as an important regulatory mechanism in lens differentiation, and this role could involve regulating CKI expression by TGF-β (Wang-Su et al., 2003; Guo et al., 2004, 2006; Hosler et al., 2006). One of the critical signaling cascades implicated in lens development is the TGF-β pathway (Lovicu et al., 2004; de Iongh et al., 2005). Binding of the TGF-β ligand to membrane-bound type II receptors results in the activation of the Smads cascade that facilitates the transactivation of TGF-β–responsive genes (Massague and Gomis, 2006). SnoN, a critical transcriptional corepressor of the TGF-β pathway, has been proposed to be a functional switch controlling the expression of p15 and p21 (Zhu et al., 2005, 2006). Previous genetic and cellular studies have discovered that SnoN plays a pivotal role in development, including Drosophila and murine eye development (Davis et al., 2001; Shinagawa and Ishii, 2003), neurulation and skeletal muscle development (Berk et al., 1997; Shinagawa and Ishii, 2003), and neuronal axonal morphogenesis (Konishi et al., 2004). The biochemical mechanism by which SnoN is regulated in lens development remains unknown.
We have demonstrated that Cdh1/APC, an ubiquitin proteasomal ligase, mediates the TGF-β–signaling cascade by the destruction of SnoN, thereby, resulting in the transcriptional activation of TGF-β–responsive genes (Wan et al., 2001). We have also observed the expression of Cdh1 during eye development (Wan and Kirschner, 2001). In this study, we examined whether Cdh1/APC is a critical component in facilitating the TGF-β–signaling cascade that ensures cell cycle arrest for the initiation of terminal differentiation during lens epithelial to lens fiber cell transformation. Identification of Cdh1/APC as a key regulator that governs CKIs, such as p15 and p21, by controlling SnoN protein stability will significantly enhance our understanding of lens development.
To investigate the role of Cdh1/APC in lens development, we have systematically dissected Cdh1/APC modulation of the TGF-β–signaling cascade that contributes to the initiation of lens differentiation. We have used an in vitro induction model system of early lens epithelial differentiation in combination with in vivo murine histological analysis. We have conducted loss of function assays by examining the effect of RNA interference of Cdh1 and Smad3 upon lens differentiation. We have identified Cdh1/APC targets in the TGF-β–signaling pathway during lens development. In addition, we biochemically addressed the mechanism by which Cdh1/APC modulates the TGF-β–signaling pathway upon degradation of SnoN. Taken together, we have demonstrated that the critical role of Cdh1/APC during lens development is to coordinate cellular proliferation and differentiation by facilitating TGF-β–induced expression of p15 and p21, thereby resulting in cell cycle arrest and terminal differentiation.
MATERIALS AND METHODS
Cell Culture
The murine lens epithelial cells, alpha TN4 (gift from Dr. B. J. Wagner with permission from Dr. Paul Russell) were grown in 100-mm tissue culture plates in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 μg/ml streptomycin, and 100 μg/ml penicillin in a 5% CO2 environment at 37°C.
Induction of Lens Epithelial Cells into Lentoids
Alpha TN4 cells can be induced into lentoids. Cells were washed with 1× PBS and then treated briefly with 0.05% trypsin for 60 s followed by the induction protocol modified from the Russell lab (Kidd et al., 1994) and the Gershengorn lab (Hardikar et al., 2003), which is to incubate the cells in serum-free DMEM/F12 medium supplemented with insulin, transferrin, and selenium (Invitrogen, Carlsbad, CA).
Antibodies
Protein lysates of uninduced or induced (24 h) alpha TN4 cells were prepared by gathering cell pellets and resuspending them in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris Cl, ph 7.4, 1 mM EDTA, 1 mM EGTA, 0.5% IGEPAL CA-630, 0.1% SDS, 1% sodium deoxycholate), with fresh protease inhibitors (Boehringer Mannheim, Indianapolis, IN) and 1 mM dithiothreitol added before use. Each concentration of uninduced and induced lysates were analyzed by immunoblotting. Antibodies to APC2 (AbCam, Cambridge, United Kingdom), Cdh1 (Oncogene, Boston, MA), cyclin A (AbCam), cyclin B (Chemicon, Temecula, CA; Sigma, St. Louis, MO), Cdc2-phosphorylated (AbCam), Wee1 (AbCam), Cdc25A (AbCam), Cdc20 (AbCam), Cdc27 (Stratagene, La Jolla, CA), Mad2 (Novus, Littleton, CO), proliferating cell nuclear antigen (Stratagene), Mad3 (AbCam), p27 (Santa Cruz Biotechnology, Santa Cruz, CA; R&D Systems, Minneapolis, MN), p21 (BD Biosciences PharMingen, San Diego, CA; R&D Systems), p15 (AbCam), p16 (AbCam), Rb (AbCam), p53 (Calbiochem), Brca1 (AbCam), Bard1 (AbCam), Brca2 (AbCam), alpha-B crystallin (Novus), Map kinase (R&D Systems), lipoxygenase (Cayman, Ann Arbor, MI), fibroblast growth factor (FGF2; R&D Systems), insulin-like growth factor (IGF; Stratagene), SnoN (Santa Cruz; Cascade Bioscience, Windsor, MA), TGF-β I (AbCam, R&D Systems), Smad3 (Santa Cruz), Skp2 (Santa Cruz; Zymed, South San Francisco, CA), Casein Kinase II (AbCam), and Casein Kinase I (AbCam) were obtained from the listed resources. Equal concentration were loaded and analyzed by Western blot. Protein abundance was determined by using NIH imaging software.
Neutralization of the TGF-β Ligand in Lentoid Induction Medium
Cells in 100-mm tissue culture plates were incubated with 20 μg/ml neutralizing anti-TGF-β1, 2, and 3 monocolonal antibody (R&D Systems; Ray et al., 2005).
Quantification of the TGF-β Ligand
Cells were washed with PBS and then induced at different initial time points. Particulates were removed by centrifugation at 1000 rpm for 2 min. Sample lysates were then activated with HCl according manufacturer's instructions followed by analysis using TGF-β immunoassay (MB100B, R&D Systems).
Quantification of Lentoids
Subconfluent cells (1 × 106 cells) were induced to differentiate into lentoids in 100-mm cultured dish. After 24–30 h, the number of lentoids formed were counted. Lentoids had to be more than 30 cells to be counted (Kidd et al., 1994; Singh et al., 2002).
Construction of Cdh1-small interfering iRNA Stable Cell Lines
Small interfering iRNA (siRNA) were purchased from Dharmacon (Boulder, CO). siRNA-Cdh1 retrovirus was packaged by transfecting the siRNA into subconfluent phoenix packaging cells using Oligofectamine (Invitrogen) according to manufacturer's instructions. Three constructs have been engineered: 1) pSUPER-Cdh1-N (amino acid 266-286), 2) pSUPER-Cdh1-C (amino acid 566-586), and 3) pSuper-Control (firefly luciferase siRNA; Wei et al., 2004). Alpha TN4 cells were infected with the virus, and positive clones were selected in the presence of puromycin-containing (400 mM) medium.
Construction of Smad3 siRNA and Transient Transfection
siRNAs were synthesized by Dharmacon. The sequence of anti-Smad3 siRNA was 5′-AAUGGUGCGAGAAGGCGGUCAdTfT-3′ (Ray et al., 2005). The siRNAs (150–300 nM) are transfected twice into targeted cells to achieve noticeable depletion of Smad3.
RNA Extraction and RT-PCR
Total RNA was extracted using the SV Total RNA Isolation System (Promega, Madison, WI). SnoN RT-PCR was performed using the following primers: SnoN sense primer (5′-GAAAACCTCCAGTCTAAGTTCTCCTTAGTT-3′) and antisense primer (5′-ATGAAGCTGGTCTGAAGTACACCTTGAACA-3′). The expected size was ∼500 base pairs.
In Vitro Degradation and Ubiquitylation of SnoN
Approximately 10 ng of 35S-labeled SnoN and SnoNΔdb were added to 20 μl fresh alpha TN4 extracts supplemented with degradation cocktail (1.25 mg/ml ubiquitin, 1× energy regeneration, and 0.1 mg/ml cycloheximide). Aliquots were removed at different times and resolved by SDS-PAGE and autoradiography (Wan et al., 2001). The method for in vitro ubiquitylation was previously described (Wan and Kirschner, 2001).
Coimmunoprecipitation of Cdh1 and SnoN
Alpha TN4 cells were cotransfected with a combination of expression vectors encoding Myc-Cdh1 and HA-SnoN. The transfected cells were preincubated with proteasomal inhibitor MG-132 (10 μM) or vehicle (dimethyl sulfoxide) for 1 h followed by induction with serum-free medium with ITS. Myc-Cdh1 complexes were pulled down by anti-Myc matrix (Roche, Indianapolis, IN). Interaction between SnoN and Cdh1 was determined by protein immunoblotting with anti-HA and anti-Myc antibodies.
Constructs
pREX-SnoN-IRES-GFP and pREX-SnoNΔdb-IRES-GFP were engineered by PCR using SnoN and SnoNΔdb templates (Wan and Kirschner, 2001; Wan et al., 2001) and the following primers: SnoN HA sense 5′-AAAGAATTCACCATGGGATATCCATATGATGTTCCTGATTATGCTGGGGAAAACCTCCAGACAAATTTCTC-3′; SnoN antisense 5′-GGC TGA TTA TGA CT AGA GTC GCG GC-3′ and then cloned into pREX-IRES-GFP, a retroviral expression vector.
Retroviral Experiment with Nondegradable SnoN
Retroviral vector of SnoN or SnoNΔdb were transfected into phoenix packaging cells. The retrovirus was then used to infect alpha TN4 cells followed by screening for green fluorescent protein (GFP)-positive cells. The SnoN- and SnoNΔdb-positive cells were then tested for lentoid formation after induction.
Immunohistochemical Analysis
Murine embryonic eyes at E14.5 were isolated, fixed in 4% paraformaldehyde overnight, and then paraffin-embedded. The eyes were then sectioned horizontally at 5 μm. After antigen retrieval with Reveal (Biocare Medical, Concord, CA) sections were blocked with Sniper (Biocare Medical) and stained with the following primary antibodies: anti-phospho-histone 3 (1:1K, Upstate Biotechnology, Lake Placid, NY); anti-Ki67 (1:2K; Dako, Carpinteria, CA); anti-TGF-β (1:1000; R&D), anti-Smad 3 (1:5000; Santa Cruz); anti-Cdc27 (1:2000; Stratagene); anti Cdh1 (1:2000; Oncogene); anti-SnoN (1:1000; Cascade Bioscience), anti-p21 (1:1000; R&D). Biotinylated-conjugated secondary antibodies were used for detection. Bound immunoperoxidase was visualized by incubation with 3,3′-diaminobenzidine with hydrogen peroxide for 6 min.
RESULTS
Protein Expression Profile of Lentoid Differentiation
Several critical signaling pathways have been suggested to play crucial roles in the process of lens terminal differentiation (Lovicu and McAvoy, 2005). Extracellular signals integrate the cell cycle with transcriptional programs that result in the formation of the functional lens (Dyer and Cepko, 2001; Zhu and Skoultchi, 2001). To systematically analyze the critical molecules that are involved in lens differentiation including cell cycle regulators, signaling molecules, and transcriptional regulators, we have conducted an analysis of the protein expression profile during lens formation utilizing an established cell culture–based lens induction model (Kidd et al., 1994).
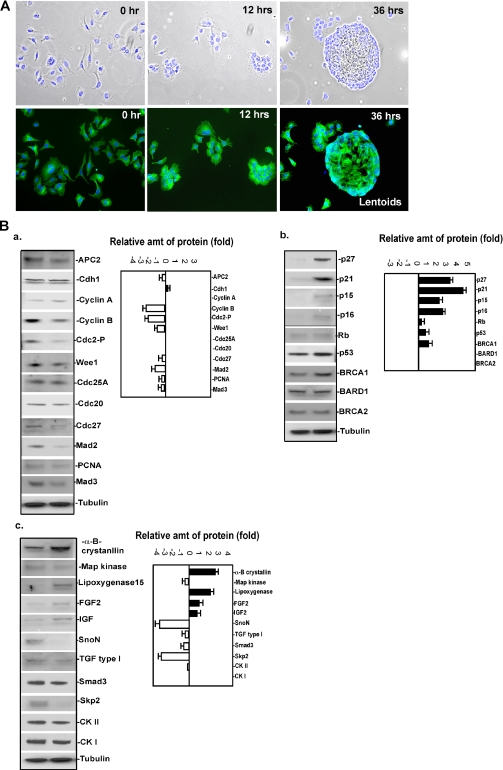
Alpha TN4 are murine epithelial cells that after induction with serum-free medium containing insulin, transferrin, and selenium exhibit increased expression of alpha B-crystallin coupled with elongation and cell cycle arrest, as well as changes in cellular adhesion that is reminiscent of cells during embryonic lens formation (Kidd et al., 1994). After induction, alpha TN4 cells differentiate into lens-like structures called lentoids in ∼24–36 h, as shown in Figure 1A. To monitor the profiles of certain cell cycle regulators, signaling molecules and transcriptional regulators, which potentially are involved in lens differentiation, we measured the abundance of candidate proteins using immunoblotting at baseline and after 24 h of induction. As shown in Figure 1B, several proteins exhibited drastic alteration after lens differentiation. Among the proteins tested that promote cell cycle progression, the protein levels of cyclin B, Cdk2, and Wee1 were significantly decreased in differentiated lentoids. In contrast, immunoblots of proteins that inhibit cell cycle progression, including p27, p21, p15, and p16, were dramatically increased after induction. The immunoblots of proteins implicated in signaling pathways and differentiation, including SnoN, Skp2, and alpha B-crystallin, also varied with lentoid formation. This protein expression profiling provided valuable clues to dissect the underlying mechanism of lens differentiation, especially from regulation by protein turnover.
Figure 1.
Analysis of protein expression profile during lentoid formation in an in vitro system. (A) An in vitro system for lens induction. Alpha TN4 cells were induced with serum-free medium with ITS. Top panels, merged images of phase contrast and DAPI (blue) stained cells; bottom panels, merged images of tubulin- (green) and DAPI (blue)-stained cells. The cells were examined at 0, 12, and 36 h. At 36 h, cells show a loss of surface adhesion and conglomerate into lentoids. (B) Analysis of protein expression profiles during lentoid formation. Protein levels for three groups of proteins including cell cycle positive regulators, cell cycle inhibitors, and differentiation-related molecules were measured at baseline, before induction, and 24 h after induction. The fold increased or decreased after induction was calculated by comparing the abundance relative to baseline and 24 h after induction. Equal amounts of total protein were subjected to immunoblot analysis, as evidenced by equal concentrations of tubulin.
SnoN, a Transcriptional Corepressor of p21 and p15, Is Dramatically Altered during Lens Differentiation
Several of the previously tested proteins (SnoN, p21, and p15) that show alterations after lens induction have been implicated in the TGF-β–signaling pathway, which is essential for lens and other organ development (Konishi et al., 2004; Guo et al., 2006; Hosler et al., 2006). Recent evidence suggests the crucial role of SnoN is to antagonize TGF-β–signaling pathways and therefore maintain a balance between cellular proliferation and cell cycle arrest (Sun et al., 1999; Stroschein et al., 2001; Wan et al., 2001). To achieve TGF-β–induced cell cycle arrest, SnoN has to be degraded through the ubiquitin proteasomal system to enable the expression of p15 and p21. To assess the functional significance of SnoN, p15, and p21 as signaling molecules in the TGF-β pathway contributing to lens differentiation, we examined the SnoN-p15/p21 cascade during early lens epithelial cell differentiation.
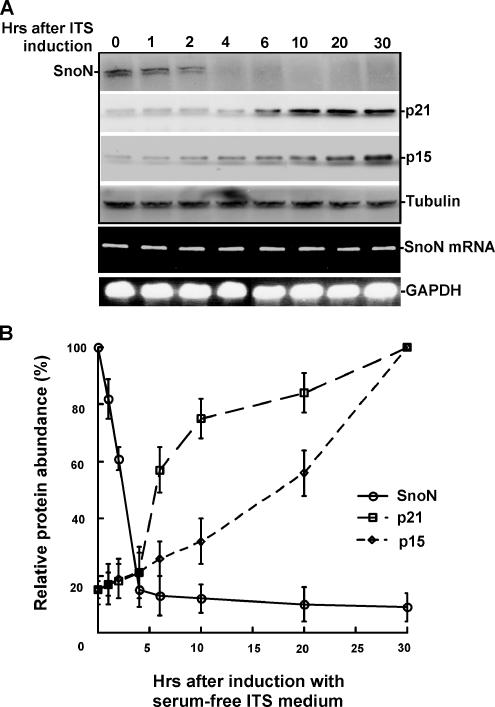
To further examine the decrease in SnoN protein levels during lens differentiation and the accumulation of p21 and p15 that results from SnoN degradation, we measured the protein levels of p21, p15, and SnoN at different times after induction as indicated in Figure 2A. As shown in Figure 2, SnoN protein levels decreased approximately 2 h after induction, whereas both p21 and p15 gradually accumulated at the same time. To test if the change in SnoN protein level was due to posttranslational alterations, we also examined SnoN mRNA levels during the time course. As shown in Figure 2, A and B, no noticeable change of SnoN mRNA levels was observed after induction. As shown in Supplementary Figure 1A, we also monitored the protein levels of alpha B crystallin after the induction of alpha TN4 showing increased expression suggesting cellular differentiation. Our results suggest that the regulation of SnoN occurs posttranslationally, and the observed reduction in protein level is likely regulated by the ubiquitin proteasomal pathway.
Figure 2.
Time course for the degradation of SnoN and accumulation of p21 and p15 after lens induction. (A) Protein levels for SnoN, p21, and p15 were measured by immunoblot assay at the time points indicated during the 30 h after induction. SnoN was dramatically degraded in 2–4 h after induction, whereas p21 and p15 gradually accumulated. Equal amounts of total protein were subjected to immunoblot analysis, as evidenced by equal concentrations of tubulin. SnoN mRNA levels were monitored by RT-PCR during the 30 h after induction. (B) Quantification of the protein levels for SnoN, p21, and p15 during lens differentiation.
The TGF-β–signaling Cascade Plays a Critical Role in Lens Differentiation
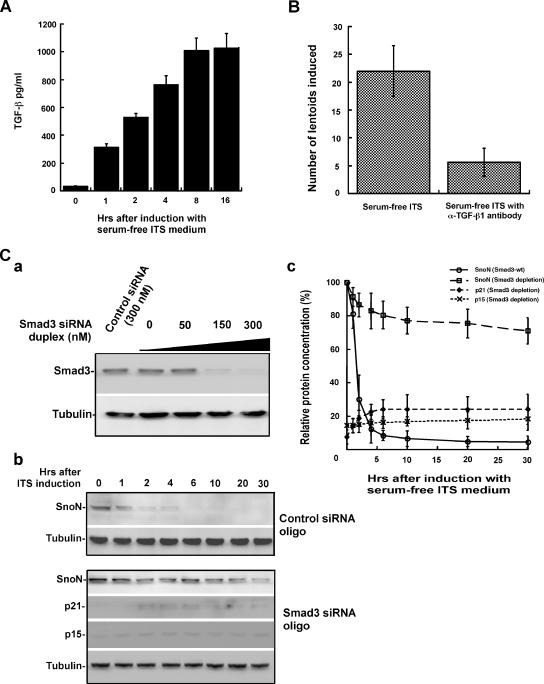
Given that SnoN, p21, and p15 are all part of the TGF-β–signaling pathway, we next assessed whether TGF-β is a critical signal mediated by the SnoN-p21/p15 cascade in lens differentiation in the alpha TN4 induction model (Datto et al., 1995; Li et al., 1995; Sun et al., 1999; Feng et al., 2000; Pardali et al., 2000; Wan et al., 2001; Lovicu and McAvoy, 2005). We first measured the expression of the TGF-β1 ligand in the culture medium secreted by the cells during lentoid induction. As shown in Figure 3A, serum-free ITS-inducing medium significantly increased the concentration of TGF-β in the medium, revealing that TGF-β is secreted in response to the induction medium and suggesting that the TGF-β–signaling cascade is needed to coordinate possibly with other signaling pathways the initiation of the lens differentiating program (Lovicu and McAvoy, 2005). To further identify the role of TGF-β in lens differentiation, we depleted the TGF-β ligand in the induction medium by addition of anti-TGF-β1 antibody (Ray et al., 2005). As shown in Figure 3B, the depletion of the TGF-β ligand significantly blocked the differentiation of alpha TN4 into lentoids.
Figure 3.
The TGF-β–signaling pathway plays an important role in lens differentiation. (A) Measurement of stimulation of TGF-β ligand secretion in response to serum-free ITS induction. (B) Depletion of the TGF-β ligand by antibody results in abrogation of lentoid formation. The number of lentoids comprising more than 30 cells were counted. (C) Depletion of Smad3 by siRNA duplex attenuates SnoN degradation and accumulation of p21 and p15 during lentoid formation. (a) Smad3 is efficiently depleted by transfection of Smad3 siRNA duplex. (b) Depletion of Smad3 by siRNA duplex attenuates SnoN degradation and accumulation of p21 and p15 during lentoid formation. (c) Quantification of the protein levels for SnoN, p21, and p15 during lens differentiation. The quantification of p21 and p15 is compared with their maximal increase after induction in wild-type alpha TN4.
Given that Smad3 is an important mediator of the TGF-β pathway, we depleted endogenous Smad3 by RNA interference and measured alterations of SnoN, p21, and p15 during lentoid induction. As shown in Figure 3C, a–c, depletion of Smad3 significantly attenuated SnoN degradation concomitant with the elevation of p21 and p15 levels. The formation of lentoids was also significantly inhibited (data not shown) similar to that observed after the depletion of the TGF-β ligand (Figure 3B). Taken together, these results suggest that TGF-β signaling is crucial for lens differentiation.
Cdh1/APC, an E3 Ligase, Targets SnoN for Degradation during Lens Differentiation
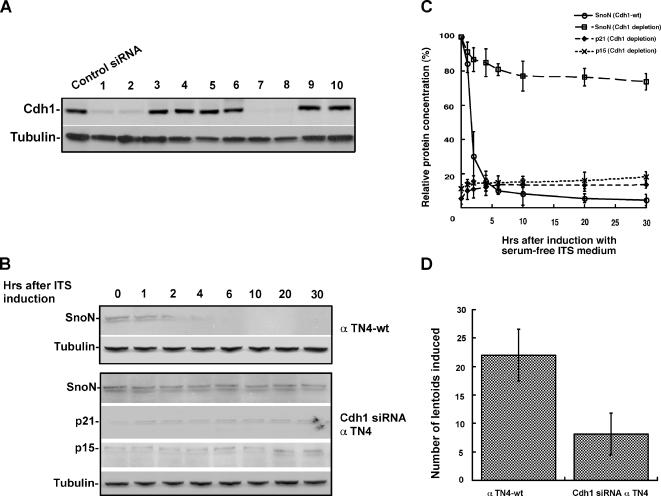
Previous studies suggested that Cdh1/APC, a critical E3 ligase governs SnoN degradation in response to the TGF-β–signaling cascade (Wan et al., 2001). When TGF-β is not available, SnoN functions as a transcriptional corepressor inhibiting TGF-β–induced gene expression. On stimulation with TGF-β, SnoN is rapidly degraded by Cdh1/APC, therefore facilitating the expression TGF-β–induced genes, such as p21 and p15 (Sun et al., 1999; Liu et al., 2001; Wan et al., 2001; Zhu et al., 2005). To demonstrate that Cdh1/APC is a crucial mediator of the TGF-β–signaling pathway in early lens epithelial differentiation, we stably depleted Cdh1 by siRNA knockdown in alpha TN4 cells (Figure 4A). As shown in Figure 4B and C, depletion of Cdh1 significantly abolished characteristic changes in SnoN-p21/p15 during lens cell induction and resulted in abrogation of lentoid formation, as indicated in Figure 4D. In addition, we analyzed bromodeoxyuridine (BrdU) incorporation in alpha TN4 cells before and after induction in both wild-type and Cdh1 siRNA cells. As shown in Supplementary Figure 2, wild-type alpha TN4 cells showed ∼25% positive BrdU-stained cells before induction and <5% after induction, whereas no significant drop was seen for Cdh1 siRNA cells. These data suggest the importance of Cdh1/APC as the critical E3 ligase facilitating lens differentiation.
Figure 4.
Cdh1/APC is involved in lens differentiation. (A) Selection of stable Cdh1 siRNA clones in alpha TN4 cells. (B) Knockdown of Cdh1 by siRNA attenuates SnoN degradation and p21 and p15 accumulation. (C) Quantification of the protein levels for SnoN, p21, and p15 during lens differentiation. (D) Knockdown of Cdh1 significantly blocked lentoid formation. The number of lentoids comprising more than 30 cells were counted.
SnoN Is a Substrate Targeted by Cdh1/APC during Lens Differentiation
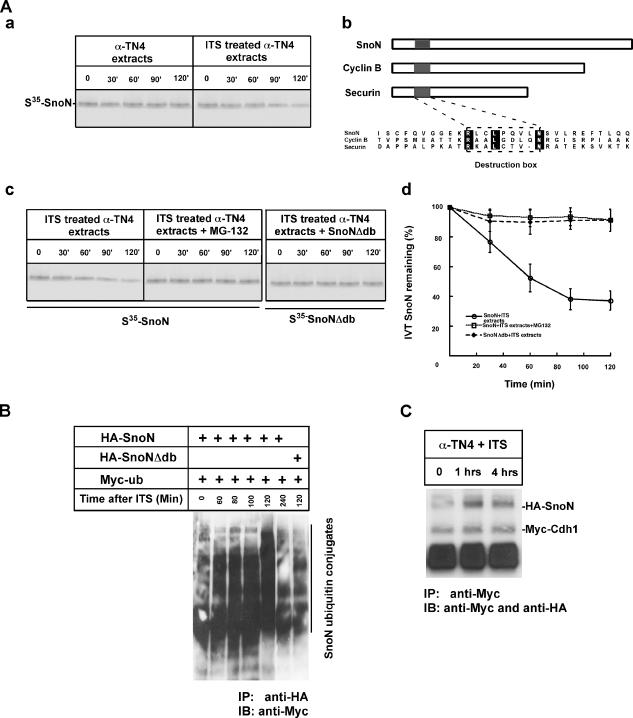
Most of the Cdh1/APC substrates are targeted through a molecular degron, D-box (destruction box), which contains RXXLXXXXN/D (King et al., 1996; Pfleger et al., 2001; Wan et al., 2001). To determine if degradation of SnoN observed during lens cell differentiation is dependent on a D-box, we established a cell-free degradation assay (Wan et al., 2001; Figure 5Aa) and engineered a D-box deletion mutant of SnoN. As shown in Figures 5, B–D, deletion of the D-box domain in SnoN significantly attenuated its degradation in extracts prepared from cells induced for 2 h. In addition, supplementation with MG-132, a proteasomal inhibitor, significantly blocked the degradation of SnoN in the same induction extract. This result suggests that the D-box serves as a critical recognition motif mediating SnoN degradation by Cdh1/APC during lens differentiation.
Figure 5.
SnoN is a substrate targeted by Cdh1/APC during lens differentiation. (A) SnoN is degraded in a functional extract prepared from the cells cultured with serum-free ITS induction medium. (a) 35S-labeled in vitro–synthesized SnoN was subjected to extracts prepared from cells without or with serum-free ITS induction. SnoN is stable in uninduced extract, whereas SnoN is degraded in extract prepared from cells induced with serum-free ITS. (b) Alignments of multiple APC substrates including SnoN, cyclin B, and securin. Destruction box, recognition motif in the substrates are indicated. (c) MG132 significantly blocked SnoN degradation. D-box deletion mutant of SnoN failed to be degraded in extracts prepared from cells with serum-free ITS. (d) Quantification of the protein levels for SnoN, p21, and p15 during lens differentiation. (B) Ubiquitylation assay of SnoN during lens differentiation. Ubiquitin tagged with Myc epitope together with SnoN or D-box–deleted SnoN were together transfected into alpha TN4 cells. Ubiquitylation of SnoN was detected by immunoprecipitation of SnoN complex with anti-HA antibody followed by immunoblotting with anti-Myc. As indicated, SnoN is dramatically ubiquitylated after induction although there is a basal ubiquitylation of SnoN before induction. Deletion of the D-box motif significantly decreased SnoN ubiquitylation during lens differentiation. (C) Physical interaction analysis of SnoN and Cdh1 during lens differentiation. Cotransfection of HA-tagged SnoN and Myc-tagged Cdh1 into alpha TN4 cells. Interaction of SnoN and Cdh1 was measured by coimmunoprecipitation at different times as indicated after serum-free ITS induction. SnoN and Cdh1 significantly interacted with each other after 1 h after induction.
To show ubiquitin-mediated degradation of SnoN in alpha TN4 cells induced by the addition of serum-free ITS medium during lens differentiation, we conducted an ubiquitylation assay by cotransfection of Myc-tagged ubiquitin with HA-tagged wild-type SnoN or the D-box deleted mutant. Cotransfected alpha TN4 cells were supplemented with serum-free ITS medium and subsequently harvested at the indicated time after induction (Figure 5B). Ubiquitylation of SnoN was detected by immunoprecipitation of the SnoN complex with anti-HA antibody followed by immunoblotting with anti-Myc. As shown in Figure 5B, SnoN is dramatically ubiquitylated after induction, although there is a basal level of ubiquitylation of SnoN before induction. Deletion of the D-box motif significantly decreased SnoN ubiquitylation during lens differentiation, further suggesting that the reduction in SnoN protein levels during lens differentiation is via an ubiquitylation pathway.
To demonstrate that Cdh1 physically interacts with SnoN when SnoN is ubiquitylated by Cdh1/APC during lens differentiation, we tested the physical interaction between Cdh1 and SnoN by cotransfection of HA-tagged SnoN and Myc-tagged Cdh1. As shown in Figure 5C, SnoN-Cdh1 interaction was detected by coimmunoprecipitation of the transfected extract 1 h after induction. Taken together, the results suggest that Cdh1/APC catalyzes SnoN ubiquitylation and Cdh1 and SnoN physically interact during lens differentiation.
Expression of Nondegradable SnoN Blocks Lens Differentiation
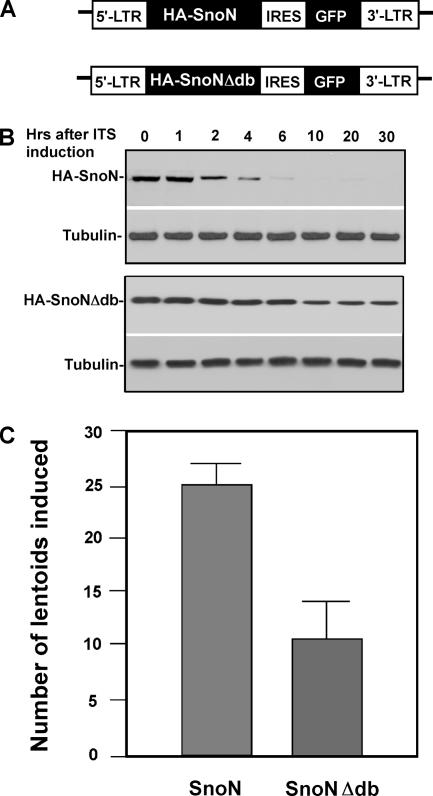
The above experiments demonstrate that degradation of SnoN is necessary for the morphological changes seen in lentoid formation. We next explored whether the expression of a nondegradable SnoN would interfere with lens differentiation. Previous data suggest that SnoN is targeted by Cdh1 via the recognition motif, D-box, because deletion of the D-box in SnoN stabilizes SnoN and therefore blocks TGF-β–induced expression of p21 and p15. To test whether expression of nondegradable SnoN would result in abrogation of lens differentiation, we engineered constructs for SnoN and SnoNΔdb based on a retroviral expression system (Liu et al., 1997). In this system, GFP under the control of IRES (internal ribosomal entry site) is linked to SnoN or SnoNΔdb, which allows for rapid sorting of GFP-positive cells, which thus are SnoN or SnoNΔdb stably expressing cells (Figure 6A). Protein levels of expressed WT-SnoN and nondegradable SnoN were measured (Figure 6B), where the protein levels of WT-SnoN dropped, whereas the nondegradable SnoN remained stable after induction. As shown in Figure 6C, stable expression of SnoNΔdb significantly attenuated the number of lentoids formed, suggesting that SnoN degradation is necessary for lens differentiation.
Figure 6.
Expression of nondegradable SnoN inhibits lentoid formation. (A) Diagram of retroviral vector expressing wild-type SnoN and nondegradable SnoN. (B) Protein levels of stably expressed WT SnoN and nondegradable SnoN after the lentoid induction. (C) Expression of nondegradable SnoN significantly inhibits lentoid formation.
In Vivo Analysis of the Role of Cdh1/APC Mediating the TGF-β–signaling Cascade in Lens Differentiation
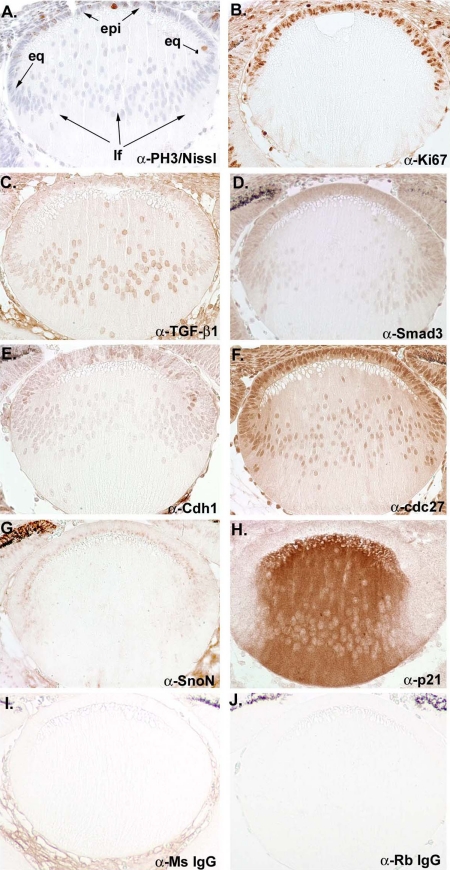
Our biochemical and genetic analyses suggest that Cdh1/APC is a critical E3 ligase mediating the TGF-β–signaling pathway by degrading SnoN in lens differentiation. To validate our results based on the lentoid induction model, we examined the expression of several critical molecules of the Cdh1/APC and the TGF-β pathways histologically in vivo. Embryonic murine lens (E14.5) were immunohistochemically labeled to detect the expression of critical proteins including TGF-β1, Smad3, Cdh1, Cdc27 (a core subunit of APC), SnoN, and p21. In Figure 7, A and B, immunostaining with the mitotic marker anti-PH3 (phosphohistone 3) and anti-Ki67 (nucleolar protein; expressed in S through M phases) label cells that are undergoing proliferation, which in this instance are mostly seen in the epithelial (epi) layer of the lens, including the equatorial region (eq). A very limited number of cells were seen to proliferate in lens fibers (lf). As shown in Figure 7, C–H, components of the TGF-β pathway including TGF-β1 and Smad3 are principally expressed in the epithelial and equatorial region of the lens. As cells are maturing into lens fiber cells, limited TGF-β and Smad3 were detected, suggesting that their effective range is at the epithelial and equatorial region of the lens. Similarly, Cdc27 and Cdh1 are mainly seen in the anterior epithelial and equatorial region and limited in the lens fiber region. The expression of SnoN is also chiefly seen in the anterior epithelial and equatorial region, but not in the lens fiber cells. In contrast, p21 is predominantly detected in the lens fiber region. In Figure 7, I and J, we also included immunohistological staining using anti-mouse alone and anti-rabbit alone as negative control. To confirm the specificity of immunohistological staining, we isolated epithelial and fiber cells and analyzed relative levels of the protein studied in Figure 7 by Western blotting. As shown in Supplementary Figure 1B, the results of protein analysis of Western blots are in principle consistent with the result from the immunohistological analysis. Taken together, the immunohistological analysis of expression of the elements of the TGF-β/Cdh1/APC pathway in murine embryonic lens supports the conclusions of our biochemical and genetic analyses (Figure 8).
Figure 7.
In vivo analysis of the role of Cdh1/APC mediating the TGF-β–signaling pathway in lens differentiation. (A and B) Immunostaining of PH3 and Ki67, proliferation markers, in murine embryonic lens at E14.5. (C and D) Immunostaining of TGF-β1 and Smad3 in murine embryonic lens at E14.5 (E and F) Immunostaining of cdc27 (core subunit of APC) and Cdh1. (G and H) Immunostaining of SnoN and p21 in murine embryonic lens at E14.5. (I and G) Negative control for immunostaining using anti-mouse and anti-rabbit IgG.
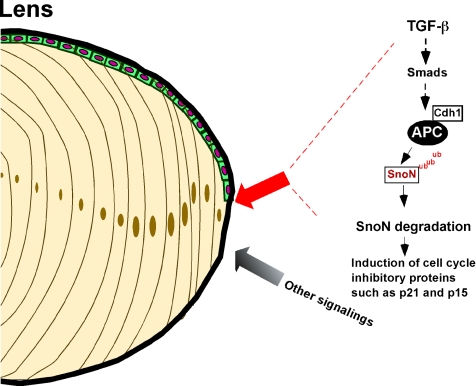
Figure 8.
Model for Cdh1/APC in mediating the TGF-β–signaling pathway in lens differentiation. Cdh1/APC, a critical ubiquitin protein ligase, plays an important role in terminal lens differentiation through mediating the TGF-β–signaling casade.
DISCUSSION
The lens is a relatively simple multicellular organ, which provides a convenient model system to study regulatory mechanisms of development. Previous studies from us and others suggested an important role of ubiquitin-proteasome system in governing the coordination of cell cycle arrest and cellular differentiation (Guo et al., 2006). Orchestration of cell cycle arrest at the precise time is fundamental to ensure sequential events necessary for the terminal differentiation (Dyer and Cepko, 2001; Zhu and Skoultchi, 2001). P21 and p15 are two essential cell cycle inhibitors that contribute to the cell cycle arrest via down-regulation of cyclin D/CDK4/6 activity (Reynisdottir et al., 1995). The major path to up-regulate p21 and p15 as mediated by TGF-β signaling is normally suppressed by SnoN, a transcriptional corepressor (Fausto et al., 1986; Dyer and Cepko, 2001; Cheng and Scadden, 2002). The results from this study uncover a novel regulatory mechanism by which activation of p21 and p15 expression is attained, which is that Cdh1/APC, a ubiquitin E3 ligase, targets SnoN for degradation in response to the TGF-β–signaling cascade. The failure to achieve arrest through this pathway will prevent subsequent cellular differentiation.
Results Utilizing the Cell Culture System Correlates with the Current Model
Current understanding of lens differentiation is that multiple factors are necessarily involved, including FGF, Wnt, and TGF-β (de Iongh et al., 2001, 2005). FGF has been implicated in the formation of primary lens fiber, whereas Wnt/β-catenin is involved in the development of lens epithelia. Subsequently FGF and TGF-β are suggested to promote the formation of the secondary lens fiber from lens epithelia. To address the mechanism of how the epithelial cells differentiate into secondary lens fiber cells, we used a lens epithelial cell culture system.
Given that differentiation to lens fiber is a multistep process, ideally, an in vitro system that meets the entire differential process from cellular migration to lens fiber would facilitate a comprehensive investigation. Alpha TN4 cells are murine epithelial cells that upon induction exhibit the phenotype of early epithelial lens differentiation as reflected by increased expression of alpha B-crystallin, elongation, cell cycle arrest, and changes in cellular adhesion property (Kidd et al., 1994). This cell line has been broadly used as a cultured model system to study lens (Kidd et al., 1994; Krausz et al., 1996; Singh et al., 2002; Belusko et al., 2003; Kitano et al., 2006). On the other hand, a caveat of this cell-cultured system is that it does not fully differentiate into lens fiber cells. Similar issue is present in the study of differentiation in other cell-cultured model systems, such as C2C12 (muscle), PC12 (neurons), and PANC1 (pancreatic islets cells), but nevertheless adequate within their limitation to provide a vehicle to study differentiation. In this present study, focusing on the early juncture between cell cycle arrest and the initiation of the differentiation program, alpha TN4 cells are suitable to be used to investigate early lens differentiation. Moreover, after the cell-cultured study, we correlated our finding with the in vivo histological study.
Focusing on the TGF-β–signaling pathway, we have revealed that the ubiquitin-proteasomal system, in particular the critical E3 ligase APC, is integral to facilitating the expression of CKIs through the destruction of SnoN. This finding confirms the notion that TGF-β is an important player involving in lens epithelial differentiation and moreover, this regulation utilizes the ubiquitin-proteasomal system to achieve its effect. The implication of TGF-β in lens differentiation does not suggest that it alone is sufficient for the process. Present understanding is that lens differentiation requires multiple extracellular signaling proteins (e.g., FGF, Wnt, BMP, insulin) in concert to achieve the differential program. Our study implicates TGF-β in the orchestration of cell cycle arrest followed by the initiation of the differential program. Subsequent signaling cascades are believed to be necessary for continued differentiation. Further investigations are needed to dissect the multiple signaling cascades in the process of lens differentiation.
Cdh1/APC, a Critical E3 Ligase, Mediates the TGF-β–signaling Cascade, Resulting in Orchestrating CKIs during Lens Differentiation
Regulated proteolysis has been recognized as a prime candidate for the regulation of cellular differentiation, particularly in lens development (Guo et al., 2006). APC, a multifunctional E3 ligase, has been demonstrated to control cell cycle progression and some developmental events (Peters, 2002; Araki et al., 2005; Stegmuller et al., 2006).
The function of APC has been initially examined in the control of chromatid separation during mitosis via degradation of the anaphase inhibitor, securin (Feng et al., 2000). Activation of APC is regulated by WD40 family members including Cdc20 and Cdh1, where Cdc20 activates APC in mitosis and Cdh1 is associated with APC during G1 (Visintin et al., 1997; Fang et al., 1998). Previous biochemical studies have shown that APC-catalyzed substrate for ubiquitylation is mediated by recognizing molecular module-destruction box (King et al., 1996; Yu et al., 1996). Utilizing an RNAi interference approach, we have demonstrated that Cdh1/APC is required for lens differentiation based on the lentoid induction model. Furthermore, we have identified that SnoN is the substrate for Cdh1/APC during lens differentiation. Using immunoblotting analyses, we have shown that degradation of SnoN by Cdh1/APC results in the accumulation of p21 and p15, which contributes arrest of cell cycle necessary for differentiation.
Using biochemical and immunocytochemical analyses, we have elucidated the mechanism by which Cdh1/APC targets SnoN for ubiquitylation. On activation of the TGF-β–signaling pathway, Cdh1/APC is activated. Subsequently, Cdh1 recognizes the D-box motif in SnoN and brings the substrate (SnoN) to near physical proximity to the E3 ligase (APC), thereby facilitating the addition of ubiquitin chain to the substrate SnoN by APC.
Although we demonstrated that activation of APC by TGF-β results in the degradation of SnoN during lens differentiation, the exact mechanism to account for the TGF-β–mediated APC activation still remains to be elucidated. In addition, how the TGF-β signal integrates with other signaling pathways, which has been reported to play roles in lens differentiation, such as FGF, IGF, and Wnt remains unclear (de Iongh et al., 2001, 2005). Using siRNA knockdown, we have demonstrated that Smad3 serves a critical role in mediating the TGF-β–signaling cascade. However, we do not know exactly how Smad3 signaling pathway is linked to Cdh1/APC for its activation. Currently, we have engineered alpha TN4 cells that stably express Smad3-TAP (tandem affinity purification) and Cdh1-TAP to systematically identify potential interacting proteins by purification of the Smad3 and/or Cdh1 complex, respectively. Identification of key interacting proteins in Smad3 and/or Cdh1 molecular complexes would significantly enhance our understanding by which Cdh1/APC is activated during lens differentiation.
In Vivo Validation of the Role of Cdh1/APC Mediating the TGF-β–signaling Cascade in Lens Differentiation
Establishment of lentoid induction system has allowed for genetic and biochemical characterizations of the molecular mechanism(s) that regulates the transition from proliferating epithelial cells to the early stages of lens differentiation (Kidd et al., 1994; Singh et al., 2002). In this simplified lens differentiation system, alpha TN4 epithelial cells act as precursor cells that would change upon induction into cells exhibiting phenotype of early differentiation that physiologically in lens would reflect cells at the initial stage of becoming secondary fiber cells. The critical induction condition utilized to drive the formation of lentoids is supplementation of ITS in serum-free medium. Insulin, transferrin, and selenium are the three major functional components in the ITS serum-free induction medium. Our measurements have shown that TGF-β is one of the induced factors in response to supplementation of ITS in the lentoid induction system. Although our biochemical and genetic analyses demonstrate the role of the TGF-β–signaling pathway in the regulation of lentoid formation based on the alpha TN4 model, the induction mechanism could be more complex in vivo. To correlate our findings to physiological lens differentiation, we have validated our working model by detecting expression of critical proteins that we have tested in alpha TN4 model in embryonic murine lens, at stage E14.5 (Chen et al., 2004). As expected, the immunohistochemical analyses of the murine lens confirm in principle, that the critical molecules of the TGF-β pathway and Cdh1/APC system are present in the anterior epithelial and equatorial regions of the lens, whereas the CKIs such as p21 are only localized in the lens fiber cells.
Although the expression pattern of the above tested molecules confirms their predicted roles, how they function and coordinate with each other at the in vivo level still remains unknown. Animal studies of certain TGF-β–signaling molecules and some CKIs in the aspect of differentiation of hematopoietic system and hepatocellular system have been done; however, the intracellular function of the TGF-β–signaling cascade in physiological lens formation has not been tested yet. Further, conditional evaluation of Cdh1, APC, SnoN, or some TGF-β–signaling molecules such as TGF-β receptors or certain Smads proteins need to be conducted in animal model by targeted knockout or transgenic study. Combination of a cultured cell-based induction system and animal study will provide a complete understanding of mechanism by which lens differentiation is controlled.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the members of the Wan lab for their technical assistance. We thank Drs. B. J. Wagner and Paul Russell for providing the alpha TN4 cell line and advising on induction of lens differentiation. We also appreciate members of Dr. Michael Epperly's lab in our analysis of TGF-β ligand secretion. This work is supported National Institutes of Health Grants CA115943 and GM070681. Y.W. is a scholar of the American Cancer Society V Cancer Research Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0809) on January 10, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Araki M., Yu H., Asano M. A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo. Genes Dev. 2005;19:2458–2465. doi: 10.1101/gad.1361905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belusko P. B., Nakajima T., Azuma M., Shearer T. R. Expression changes in mRNAs and mitochondrial damage in lens epithelial cells with selenite. Biochim. Biophys. Acta. 2003;1623:135–142. doi: 10.1016/j.bbagen.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Berk M., Desai S. Y., Heyman H. C., Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes Dev. 1997;11:2029–2039. doi: 10.1101/gad.11.16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Stump R. J., Lovicu F. J., McAvoy J. W. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int. J. Dev. Biol. 2004;48:867–877. doi: 10.1387/ijdb.041882yc. [DOI] [PubMed] [Google Scholar]
- Cheng T., Scadden D. T. Cell cycle entry of hematopoietic stem and progenitor cells controlled by distinct cyclin-dependent kinase inhibitors. Int. J. Hematol. 2002;75:460–465. doi: 10.1007/BF02982107. [DOI] [PubMed] [Google Scholar]
- Datto M. B., Li Y., Panus J. F., Howe D. J., Xiong Y., Wang X. F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl. Acad. Sci. USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Shen W., Sandler Y. I., Heanue T. A., Mardon G. Characterization of mouse Dach2, a homologue of Drosophila dachshund. Mech. Dev. 2001;102:169–179. doi: 10.1016/s0925-4773(01)00307-0. [DOI] [PubMed] [Google Scholar]
- de Iongh R. U., Lovicu F. J., Overbeek P. A., Schneider M. D., Joya J., Hardeman E. D., McAvoy J. W. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- de Iongh R. U., Wederell E., Lovicu F. J., McAvoy J. W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Dyer M. A., Cepko C. L. Regulating proliferation during retinal development. Nat. Rev. Neurosci. 2001;2:333–342. doi: 10.1038/35072555. [DOI] [PubMed] [Google Scholar]
- Fang G., Yu H., Kirschner M. W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N., Mead J. E., Braun L., Thompson N. L., Panzica M., Goyette M., Bell G. I., Shank P. R. Proto-oncogene expression and growth factors during liver regeneration. Symp. Fundam. Cancer Res. 1986;39:69–86. [PubMed] [Google Scholar]
- Feng X. H., Lin X., Derynck R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. EMBO J. 2000;19:5178–5193. doi: 10.1093/emboj/19.19.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Shang F., Liu Q., Urim L., West-Mays J., Taylor A. Differential regulation of components of the ubiquitin-proteasome pathway during lens cell differentiation. Invest. Ophthalmol. Vis. Sci. 2004;45:1194–1201. doi: 10.1167/iovs.03-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Shang F., Liu Q., Urim L., Zhang M., Taylor A. Ubiquitin-proteasome pathway function is required for lens cell proliferation and differentiation. Invest. Ophthalmol. Vis. Sci. 2006;47:2569–2575. doi: 10.1167/iovs.05-0261. [DOI] [PubMed] [Google Scholar]
- Hardikar A. A., Marcus-Samuels B., Geras-Raaka E., Raaka B. M., Gershengorn M. C. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc. Natl. Acad. Sci. USA. 2003;100:7117–7122. doi: 10.1073/pnas.1232230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler M. R., Wang-Su S. T., Wagner B. J. Role of the proteasome in TGF-beta signaling in lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2006;47:2045–2052. doi: 10.1167/iovs.05-0650. [DOI] [PubMed] [Google Scholar]
- Kidd G. L., Reddan J. R., Russell P. Differentiation and angiogenic growth factor message in two mammalian lens epithelial cell lines. Differentiation. 1994;56:67–74. doi: 10.1046/j.1432-0436.1994.56120067.x. [DOI] [PubMed] [Google Scholar]
- King R. W., Glotzer M., Kirschner M. W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano A., Saika S., Yamanaka O., Reinach P. S., Ikeda K., Okada Y., Shirai K., Ohnishi Y. Genipin suppression of fibrogenic behaviors of the alpha-TN4 lens epithelial cell line. J. Cataract Refract. Surg. 2006;32:1727–1735. doi: 10.1016/j.jcrs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Konishi Y., Stegmuller J., Matsuda T., Bonni S., Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- Krausz E., Augusteyn R. C., Quinlan R. A., Reddan J. R., Russell P., Sax C. M., Graw J. Expression of Crystallins, Pax6, Filensin, CP49, MIP, and MP20 in lens-derived cell lines. Invest. Ophthalmol. Vis. Sci. 1996;37:2120–2128. [PubMed] [Google Scholar]
- Li J. M., Nichols M. A., Chandrasekharan S., Xiong Y., Wang X. F. Transforming growth factor beta activates the promoter of cyclin-dependent kinase inhibitor p15INK4B. through an Sp1 consensus site. J. Biol. Chem. 1995;270:26750–26753. doi: 10.1074/jbc.270.45.26750. [DOI] [PubMed] [Google Scholar]
- Liu Q., Shang F., Guo W., Hobbs M., Valverde P., Reddy V., Taylor A. Regulation of the ubiquitin proteasome pathway in human lens epithelial cells during the cell cycle. Exp. Eye Res. 2004;78:197–205. doi: 10.1016/j.exer.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Liu X., Sun Y., Constantinescu S. N., Karam E., Weinberg R. A., Lodish H. F. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl. Acad. Sci. USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Sun Y., Weinberg R. A., Lodish H. F. Ski/Sno and TGF-beta signaling. Cytokine Growth Factor Rev. 2001;12:1–8. doi: 10.1016/s1359-6101(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Lovicu F. J., Ang S., Chorazyczewska M., McAvoy J. W. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGF beta-induced anterior subcapsular cataract. Dev. Neurosci. 2004;26:446–455. doi: 10.1159/000082286. [DOI] [PubMed] [Google Scholar]
- Lovicu F. J., McAvoy J. W. Growth factor regulation of lens development. Dev. Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Massague J., Gomis R. R. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Pardali K., Kurisaki A., Moren A., ten Dijke P., Kardassis D., Moustakas A. Role of Smad proteins and transcription factor Sp1 in p21(Waf1/Cip1) regulation by transforming growth factor-beta. J. Biol. Chem. 2000;275:29244–29256. doi: 10.1074/jbc.M909467199. [DOI] [PubMed] [Google Scholar]
- Peters J. M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Pfleger C. M., Lee E., Kirschner M. W. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 2001;15:2396–2407. doi: 10.1101/gad.918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D., et al. Transforming growth factor beta facilitates beta-TrCP-mediated degradation of Cdc25A in a Smad3-dependent manner. Mol. Cell. Biol. 2005;25:3338–3347. doi: 10.1128/MCB.25.8.3338-3347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdottir I., Polyak K., Iavarone A., Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- Shinagawa T., Ishii S. Generation of Ski-knockdown mice by expressing a long double-strand RNA from an RNA polymerase II promoter. Genes Dev. 2003;17:1340–1345. doi: 10.1101/gad.1073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Awasthi N., Egwuagu C. E., Wagner B. J. Immunoproteasome expression in a nonimmune tissue, the ocular lens. Arch. Biochem. Biophys. 2002;405:147–153. doi: 10.1016/s0003-9861(02)00341-7. [DOI] [PubMed] [Google Scholar]
- Skapek S. X., Rhee J., Spicer D. B., Lassar A. B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Stegmuller J., Konishi Y., Huynh M. A., Yuan Z., Dibacco S., Bonni A. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron. 2006;50:389–400. doi: 10.1016/j.neuron.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Stroschein S. L., Bonni S., Wrana J. L., Luo K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 2001;15:2822–2836. doi: 10.1101/gad.912901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liu X., Ng-Eaton E., Lodish H. F., Weinberg R. A. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling. Proc. Natl. Acad. Sci. USA. 1999;96:12442–12447. doi: 10.1073/pnas.96.22.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R., Prinz S., Amon A. CDC20 and CDH1, a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Wan Y., Kirschner M. W. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc. Natl. Acad. Sci. USA. 2001;98:13066–13071. doi: 10.1073/pnas.231487598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Liu X., Kirschner M. W. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol. Cell. 2001;8:1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- Wang-Su S. T., et al. Proteome analysis of lens epithelia, fibers, and the HLEB-3 cell line. Invest. Ophthalmol. Vis. Sci. 2003;44:4829–4836. doi: 10.1167/iovs.03-0556. [DOI] [PubMed] [Google Scholar]
- Wei W., Ayad N. G., Wan Y., Zhang G. J., Kirschner M. W., Kaelin W. G., Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- Yu H., King R. W., Peters J. M., Kirschner M. W. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr. Biol. 1996;6:455–466. doi: 10.1016/s0960-9822(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Zhang L., Sato E., Amagasaki K., Nakao A., Naganuma H. Participation of an abnormality in the transforming growth factor-beta signaling pathway in resistance of malignant glioma cells to growth inhibition inducedby that factor. J. Neurosurg. 2006;105:119–128. doi: 10.3171/jns.2006.105.1.119. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wong C., DePinho R. A., Harper J. W., Elledge S. J. Cooperation between the Cdk inhibitors p27(K.IP1) and p57(K.IP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wong C., Liu D., Finegold M., Harper J. W., Elledge S. J. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Skoultchi A. I. Coordinating cell proliferation and differentiation. Curr. Opin. Genet. Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]
- Zhu Q., Pearson-White S., Luo K. Requirement for the SnoN oncoprotein in transforming growth factor beta-induced oncogenic transformation of fibroblast cells. Mol. Cell. Biol. 2005;25:10731–10744. doi: 10.1128/MCB.25.24.10731-10744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.