Abstract
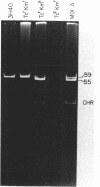
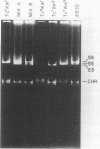
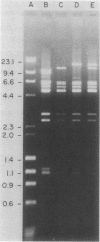
Campylobacter jejuni 3H40 and 4B20 harbored 59-kilobase (kb) self-transmissible plasmids encoding resistance to kanamycin and tetracycline. Although the two antibiotic resistances were more frequently inherited together, some transconjugants and ethidium bromide segregants which were resistant to only one of these antibiotics were recovered. The kanamycin-susceptible, tetracycline-resistant segregants carried plasmids 4 kb smaller than the 59-kb plasmids of their parents, whereas the kanamycin-resistant, tetracycline-susceptible segregants contained no detectable plasmid DNA. Restriction endonuclease maps of deleted forms of the 59-kb plasmids revealed that deletions and rearrangements of 4-kb lengths of DNA were associated with loss of kanamycin resistance. Translocation of the kanamycin resistance determinant between plasmid and chromosomal DNA was demonstrated. Such phenomena have not been previously described in C. jejuni spp. and are consistent with the interpretation that the kanamycin resistance determinant is encoded by a translocatable element.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bopp C. A., Birkness K. A., Wachsmuth I. K., Barrett T. J. In vitro antimicrobial susceptibility, plasmid analysis, and serotyping of epidemic-associated Campylobacter jejuni. J Clin Microbiol. 1985 Jan;21(1):4–7. doi: 10.1128/jcm.21.1.4-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Munroe D. L. Occurrence of plasmids and antibiotic resistance among Campylobacter jejuni and Campylobacter coli isolated from healthy and diarrheic animals. J Clin Microbiol. 1985 Sep;22(3):339–346. doi: 10.1128/jcm.22.3.339-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Patten V., Bednorz D. Susceptibility of Campylobacter fetus to twenty-two antimicrobial agents. Antimicrob Agents Chemother. 1978 Mar;13(3):416–418. doi: 10.1128/aac.13.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart C. J., Ward G. E., Kurtz H. J. In vitro activities of 47 antimicrobial agents against three Campylobacter spp. from pigs. Antimicrob Agents Chemother. 1985 Jan;27(1):55–59. doi: 10.1128/aac.27.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H., Schenk E. A. Pathogenic properties of Campylobacter jejuni: assay and correlation with clinical manifestations. Infect Immun. 1985 Oct;50(1):43–49. doi: 10.1128/iai.50.1.43-49.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E. Plasmid-mediated tetracycline resistance in Campylobacter jejuni: expression in Escherichia coli and identification of homology with streptococcal class M determinant. J Bacteriol. 1986 Mar;165(3):1037–1039. doi: 10.1128/jb.165.3.1037-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Bronsdon M. A., Gordon K. P., Plorde J. J. Isolation of plasmids encoding tetracycline resistance from Campylobacter jejuni strains isolated from simians. Antimicrob Agents Chemother. 1983 Feb;23(2):320–322. doi: 10.1128/aac.23.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Williams S., Gordon K. P., Nolan C., Plorde J. J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985 Jan;27(1):37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. L., Reller L. B., Blaser M. J. Comparison of antimicrobial susceptibility patterns of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1984 Sep;26(3):351–353. doi: 10.1128/aac.26.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]