Abstract
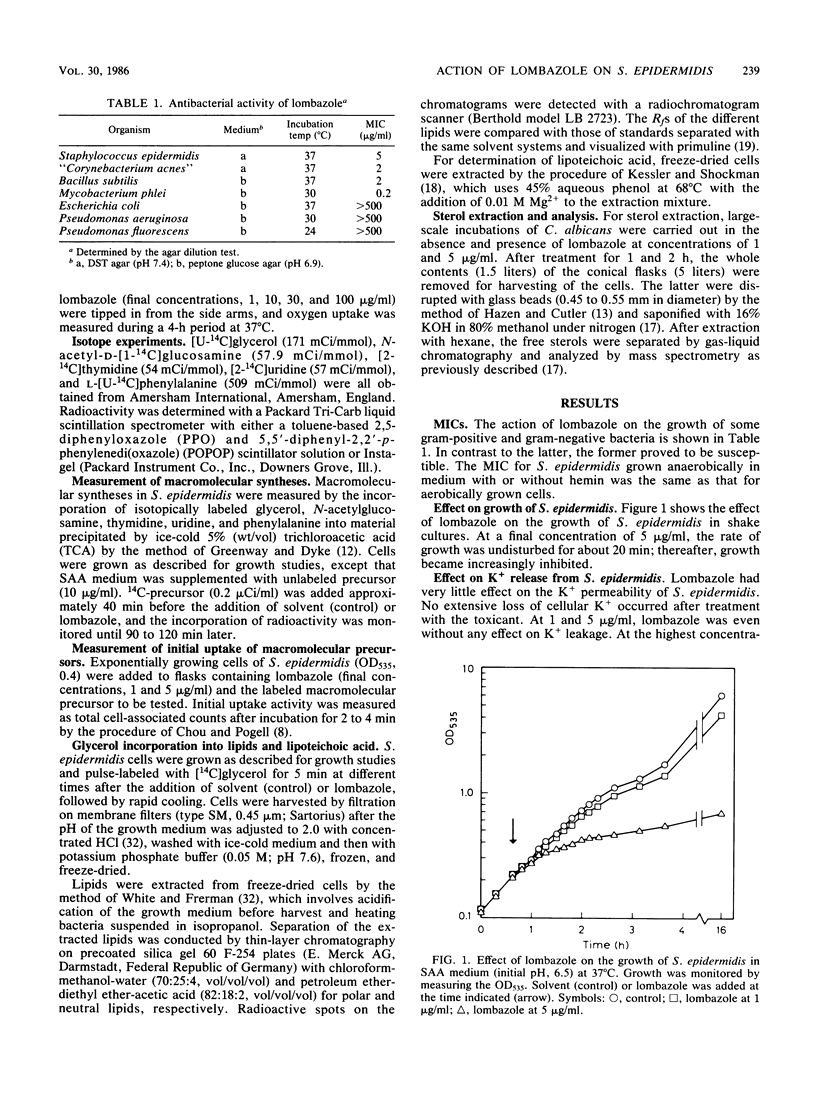
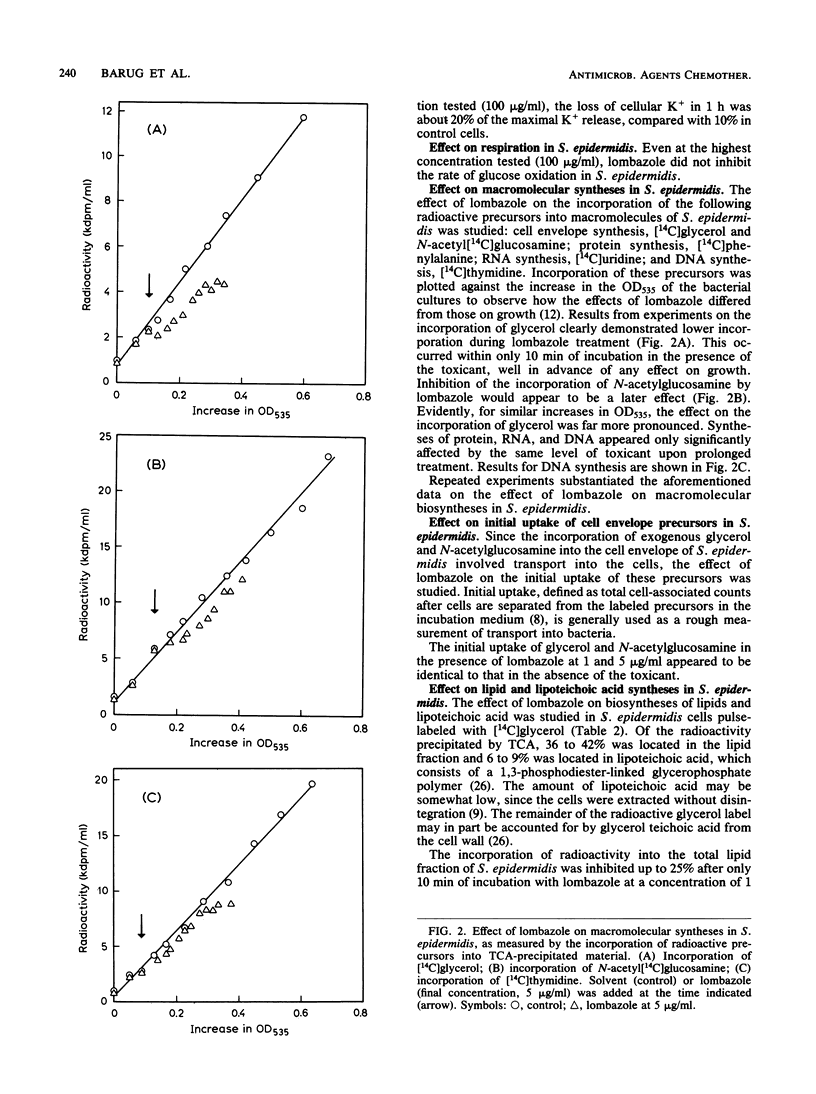
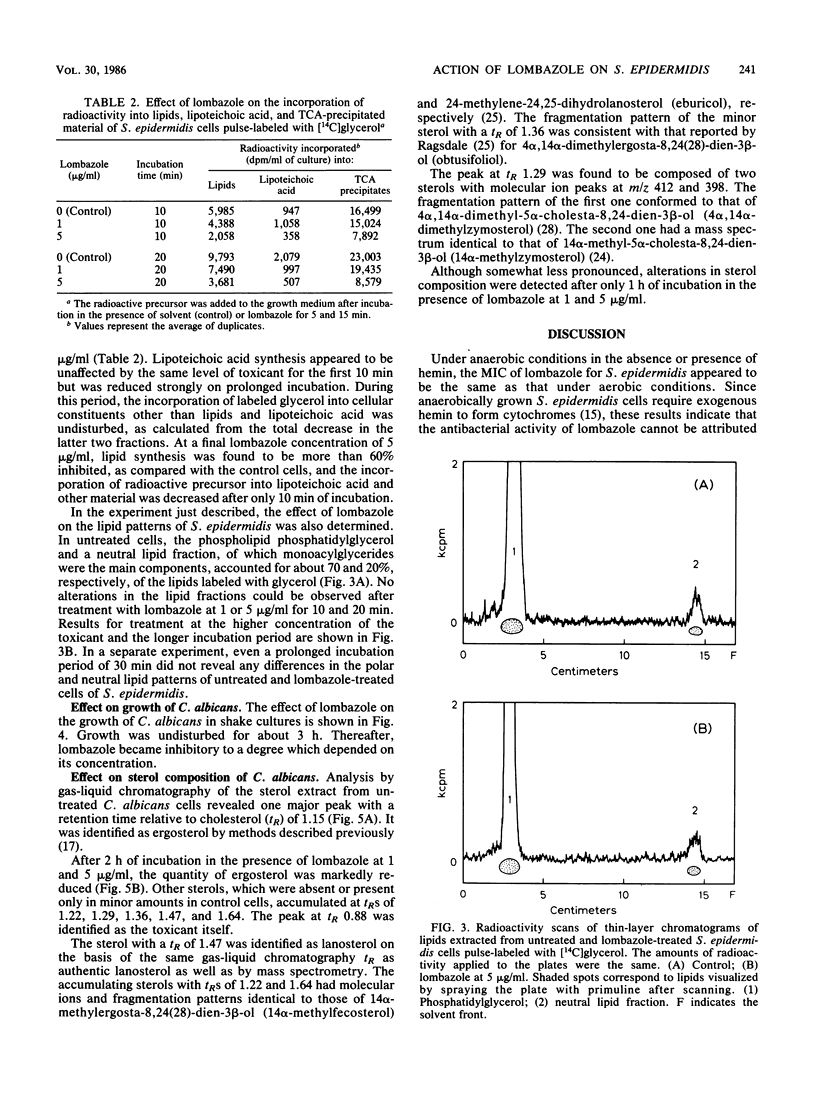
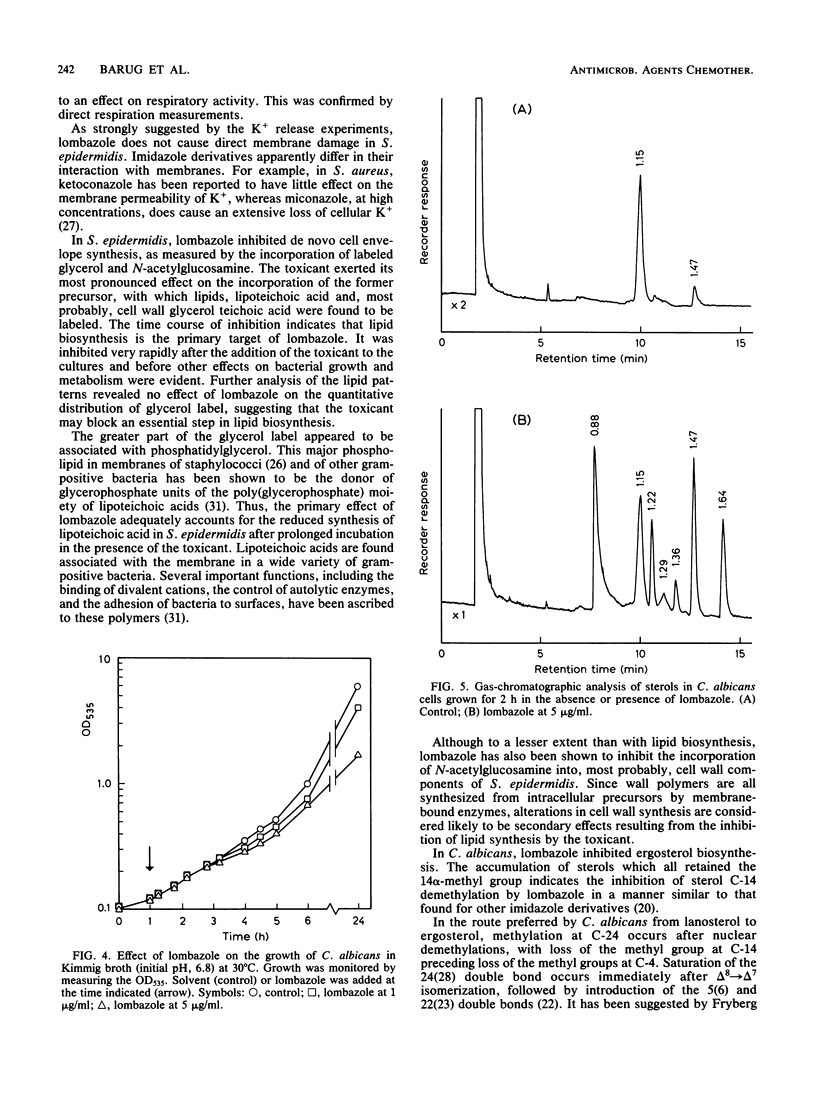
Lombazole had no effect on respiration at any tested concentration and had little effect on the K+ permeability of Staphylococcus epidermidis. Of the major metabolic processes investigated in this bacterium, only de novo synthesis of the cell envelope was inhibited by lombazole well in advance of an effect on growth. The time course of inhibition indicated that lombazole exerted its primary effect via inhibition of lipid synthesis; other induced changes, such as reduced synthesis of lipoteichoic acid and cell wall components, were considered to be secondary effects. Although the precise site of action in S. epidermidis has to be established, the absence of alterations in lipid patterns after treatment with lombazole suggests the toxicant may affect an essential step in lipid biosynthesis. In Candida albicans, lombazole inhibited the sterol C-14 demethylation step in the ergosterol biosynthesis pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barug D., Bastiaanse H. B. An evaluation of the antifungal effect of bifonazole on Torulopsis glabrata and Candida albicans under various in vitro test conditions. Arzneimittelforschung. 1983;33(4):524–528. [PubMed] [Google Scholar]
- Barug D., de Groot K. Effect of the imidazole derivative lombazole on the ultrastructure of Staphylococcus epidermidis and Candida albicans. Antimicrob Agents Chemother. 1985 Nov;28(5):643–647. doi: 10.1128/aac.28.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs W. H., Andrews F. A., Sarosi G. A. Action of imidazole-containing antifungal drugs. Life Sci. 1981 Jan 12;28(2):111–118. doi: 10.1016/0024-3205(81)90542-7. [DOI] [PubMed] [Google Scholar]
- Berg D., Regel E., Harenberg H. E., Plempel M. Bifonazole and clotrimazole. Their mode of action and the possible reason for the fungicidal behaviour of bifonazole. Arzneimittelforschung. 1984;34(2):139–146. [PubMed] [Google Scholar]
- Chou W. G., Pogell B. M. Mode of action of pamamycin in Staphylococcus aureus. Antimicrob Agents Chemother. 1981 Oct;20(4):443–454. doi: 10.1128/aac.20.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983 Jul 1;133(3):523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Sterol biosynthesis in antibiotic sensitive and resistant Candida. Arch Biochem Biophys. 1976 Mar;173(1):171–177. doi: 10.1016/0003-9861(76)90247-2. [DOI] [PubMed] [Google Scholar]
- Greenway D. L., Dyke K. G. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979 Nov;115(1):233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Cutler J. E. Optimal conditions for breaking medically important yeasts by an inexpensive and simple method. Mycopathologia. 1982 Nov 19;80(2):113–116. [PubMed] [Google Scholar]
- JACOBS N. J., CONTI S. F. EFFECT OF HEMIN ON THE FORMATION OF THE CYTOCHROME SYSTEM OF ANAEROBICALLY GROWN STAPHYLOCOCCUS EPIDERMIDIS. J Bacteriol. 1965 Mar;89:675–679. doi: 10.1128/jb.89.3.675-679.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev. 1977 Jun;41(2):391–418. doi: 10.1128/br.41.2.391-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S. K. Thin-layer chromatography of neutral glycosphingolipids and gangliosides. Methods Enzymol. 1981;72:185–204. doi: 10.1016/s0076-6879(81)72012-3. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 1. Differential scanning calorimetric studies. Biochemistry. 1985 May 7;24(10):2431–2439. doi: 10.1021/bi00331a007. [DOI] [PubMed] [Google Scholar]
- Pierce A. M., Mueller R. B., Unrau A. M., Oehlschlager A. C. Metabolism of delta24-sterols by yeast mutants blocked in removal of the C-14 methyl group. Can J Biochem. 1978 Aug;56(8):794–800. doi: 10.1139/o78-121. [DOI] [PubMed] [Google Scholar]
- Ragsdale N. N. Specific effects of triarimol on sterol biosynthesis in Ustilago maydis. Biochim Biophys Acta. 1975 Jan 24;380(1):81–96. doi: 10.1016/0005-2760(75)90047-8. [DOI] [PubMed] [Google Scholar]
- Trocha P. J., Jasne S. J., Sprinson D. B. Yeast mutants blocked in removing the methyl group of lanosterol at C-14. Separation of sterols by high-pressure liquid chromatography. Biochemistry. 1977 Oct 18;16(21):4721–4726. doi: 10.1021/bi00640a029. [DOI] [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


