Abstract
We extend our previous analyses of mice deficient in selectins by describing the generation and comparative phenotype of mice lacking one, two, or three selectins after sequential ablation of the murine genes encoding P-, E-, and L-selectins. All mice deficient in selectins are viable and fertile as homozygotes. However, mice missing both P- and E-selectins (PE−/−), and mice missing all three selectins (ELP−/−) develop mucocutaneous infections that eventually lead to death. Mice deficient in multiple selectins display varying degrees of leukocytosis, resulting in part from alterations in leukocyte rolling and recruitment. PE−/− mice, ELP−/− mice, and mice missing both P- and L-selectins (PL−/−) show drastic reductions in leukocyte rolling and in extravasation of neutrophils in thioglycollate-induced peritonitis. In a separate inflammatory model (ragweed-induced peritoneal eosinophilia), we demonstrate P-selectin to be both necessary and sufficient for the recruitment of eosinophils. The phenotype of mice missing both E- and L-selectins (EL−/−) is less severe than those seen in the other double knockouts. Comparisons among the double knockouts suggest that P-selectin normally cooperates with both E- and L-selectins. Our results indicate a preeminent role for P-selectin in regulating leukocyte behavior in mice. Data from the ELP−/− mice indicate, however, that all three selectins are important to leukocyte homeostasis and efficient neutrophil recruitment.
The recruitment of leukocytes from the circulation, and their subsequent influx into surrounding tissues at sites of infection or injury is a multistep process that is regulated, in part, by the selectin family of adhesion molecules. These cell surface molecules initiate the first interaction between leukocytes and endothelium that results in the rolling of the leukocytes along the venular wall (1). The selectin gene family, closely linked on mouse chromosome 1, encodes three structurally related proteins that display differential spatial and temporal expression within the vascular system. Endothelial cells express E- and P-selectins, platelets express P-selectin, and leukocytes express L-selectin. Whereas P-selectin is rapidly mobilized to the surface of activated endothelium or platelets, E-selectin expression is induced by inflammatory cytokines. L-selectin is constitutively expressed on most leukocytes.
In the past several years, mice lacking each of the selectins (2–4) have been described and extensively studied. In many instances, the phenotypes associated with the deficiencies make intuitive sense, though E-selectin-deficient (E−/−) mice reportedly show no defects in thioglycollate-induced recruitment of neutrophils, unless P-selectin function also is removed (4). Analyses of mice lacking both endothelial selectins (5, 6) revealed broader than expected roles for these two molecules. Not only do their functions overlap to some degree, as indicated by the single gene knockout (KO) studies, but removing both uncovered unsuspected cooperation between the two. In light of these studies, generating all possible combinations of selectin deficiencies should yield novel insights into how selectins work together to influence leukocyte behavior. We report here a comparative characterization of wild-type (WT) mice and mice deficient in one, two, or three selectins. We show that leukocyte homeostasis and recruitment to inflammatory sites are governed by assorted interactions among all three selectins, dominated by the functions of P-selectin.
MATERIALS AND METHODS
Construction of L-Selectin Targeting Construct.
An amplified λ phage library made from the 129/Sv mouse strain (a gift from S. Tonegawa, Massachusetts Institute of Technology, Cambridge) was screened with a full-length mouse L-selectin cDNA (a gift from T. F. Tedder, Duke University Medical Center, Durham, NC). Genomic clones were subcloned into pBluescript (Stratagene). A 2.1-kb PstI–ApaI fragment (which included all of the lectin domain and most of the epidermal growth factor domain) of one of the clones, was replaced with a 1.7-kb phosphoglycerate kinase promoter puromycin resistance (PGK-PUROr) cassette (see Fig. 1B). The final 8.1-kb construct was linearized with NotI (present in the pBluescript polylinker) for transfection.
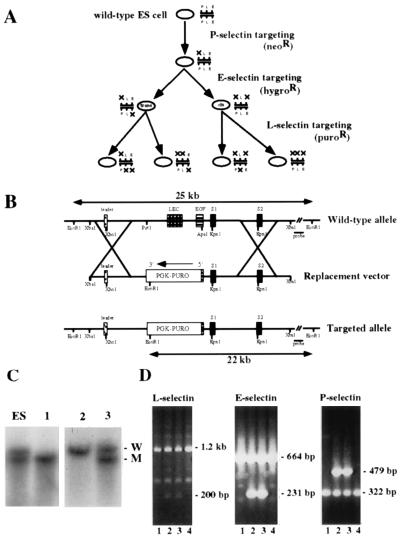
Figure 1.
Targeting strategy and L-selectin genotyping of progeny from double heterozygous crosses. (A) Overall scheme for generating mice deficient in one, two, or three selectins. P-, E-, and L-selectin loci were sequentially mutated by homologous recombination in ES cells by using replacement targeting constructs carrying drug-resistance genes for neomycin, hygromycin, and puromycin, respectively. By targeting the L-selectin locus in two different heterozygous ES cell clones with null mutations in P- and E-selectins, all possible combinations of heterozygous selectin-deficient ES cell clones were generated. Targeting of the P- and E-selectin loci has been described (2, 5). (B) The WT L-selectin locus is shown in the upper line. To construct the targeting vector, the PUROr cassette was inserted into 6.4 kb of L-selectin sequence. The exons encoding the lectin (LEC) domain and most of the epidermal growth factor (EGF) domain were deleted in this process. Resistant clones were screened with a 3′ probe that identifies the targeted 22-kb mutant and 25-kb WT bands upon EcoRI digestion. The XbaI–EcoRI fragment 3′ of the replacement vector is approximately 17 kb long; the XbaI–EcoRV probe comprises the first 1.6 kb of this segment. (C) Southern blot analyses of the L-selectin locus from EcoRI digests. Genomic DNA was extracted from tail biopsies of representative litters derived from mating heterozygous animals known to be carrying the L-selectin mutation. The first lane shows a digest of an ES cell clone, heterozygous for the L-selectin mutation, for comparison. W, WT allele; M, mutated allele. (D) PCR analysis of L-, E-, and P-selectins of progeny from an intercross of animals carrying heterozygous mutations in all three alleles. The targeted E-selectin band is 231 bp in length, the targeted L-selectin band is 200 bp in length, and the targeted P-selectin band is 479 bp in length. The null mutations segregate together during meiosis, indicating the mutations exist in cis. Null progeny from two such crosses were used to establish a homozygous triple-deficient line.
Cell Culture, Transfection, and Selection.
D3 embryonic stem (ES) cells heterozygous for both a P- and an E-selectin null mutation (5) were cultured on mitotically inactivated mouse embryonic fibroblasts in standard ES cell medium (2). ES cells were electroporated (Bio-Rad Gene Pulser, 240 V, 500 μF) with 50 μg of linearized DNA construct and plated on PUROr STO feeder cells (kindly provided by J. Chen, Massachusetts Institute of Technology) in ES cell medium with 2 × 103 units/ml of lymphocyte inhibitory factor. Puromycin (1.5 μM) was added 24 hr posttransfection. Clones were picked 7 days posttransfection and grown without selection drugs on inactivated mouse embryonic fibroblasts.
Southern Blot Analysis to Identify Targeted Clones.
Genomic DNA was isolated from ES cells (2) and digested with EcoRI, and fragments were separated on a 1% agarose gel. The DNA was transferred to Zeta-probe GT membrane (Bio-Rad). A 1.6-kb XbaI–EcoRV genomic fragment immediately downstream to the targeting construct was used as a probe. Blots were hybridized and rinsed as described (5). DNA from positive clones was reprobed with a PUROr cDNA to check for multiple integration events. The integrity of the previous E- and P-selectin mutations was checked by PCR analysis (5).
Generation of Chimeric Mice and Genotyping of Progeny.
Chimeric animals were prepared as described (2), and genomic DNA of the F1 generation was analyzed by using Southern blotting for the L-selectin locus and using PCR for the E- and P-selectin loci (5). Subsequent generations were analyzed at all three gene loci by PCR analyses. The L-selectin PCR assay used forward primers from the lectin domain exon of murine L-selectin (5′- GGG AGC CCA ACA ACA AGA AG -3′) and from the phosphoglycerate kinase promoter of the PUROr cassette (5′- CAC GAG ACT AGT GAG ACG TG -3′) and a reverse primer from the S1 exon domain (5′- ACA CTG GAC CAC ATA CTG ACA CTG -3′), yielding a 1.2-kb WT and a 200-bp mutant fragment. PCR conditions for L-selectin were 30 cycles of 94°C for 1 min, 63°C for 2 min, and 72°C for 3 min.
Animals.
All experiments were conducted on mice of 129/SvJae:C57BL/6 mixed genetic background.
Flow Cytometry.
Leukocytes in whole blood were incubated for 30 min at room temperature with rat monoclonal FITC-conjugated MEL-14 (7) antibody (PharMingen) or FITC-conjugated rat antimouse IgG isotype control mAb (PharMingen) at 1:200. Analysis of 10,000 events was performed on a FACSCAN flow cytometer (Becton-Dickinson).
Northern Blot Analysis.
Mice were treated with lipopolysaccharide (8) from Escherichia coli O55:B5 (Sigma) at 20 μg/g of body weight 4 hr before harvesting lungs and hearts (pooled) for total RNA extraction (TRI Reagent, MRC, Cincinnati). E-selectin transcripts were detected as described (5). The same blot was stripped and reprobed with a mouse β-actin cDNA (Promega) as a control for sample loading.
Western Blot Analysis.
Blood was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing EDTA as anticoagulant. Platelet-rich plasma was isolated by two rounds of low-speed centrifugation. Samples (5 × 109 platelets) were lysed by boiling directly in electrophoresis sample buffer and electrophoresed on a 6% SDS-polyacrylamide gel (9). After transfer to poly(vinylidene difluoride) membrane, the blot was incubated with rabbit polyclonal anti-human P-selectin antibody (CD62P; PharMingen) at 1:200. After ECL detection, the same blot was stripped and incubated with mouse monoclonal anti-vinculin antibody (clone Vin-11–5; Sigma) at 1:1,000, as a control for sample loading.
Blood Counts.
Blood was obtained by retro-orbital venous plexus sampling and processed as described (5). Eosinophils also were enumerated by Phloxine B staining by using Eosinophil Unopettes for Manual Methods (Thomas Scientific, Swedesboro, NJ) following the manufacturer’s instructions.
Intravital Microscopy.
Mice were treated i.p. with 0.5 μg (in 500 μl PBS) murine tumor necrosis factor α (TNF-α; Genzyme, Cambridge, MA), and vessels with an average diameter of 20 μm were analyzed as described (5).
Thioglycollate-Induced Peritonitis.
Mice were injected i.p. with 1 ml of 2.98% thioglycollate (Sigma), and peritoneal lavages and leukocyte determination were performed as described (2).
Ragweed-Induced Peritoneal Eosinophilia.
The methods used for ragweed immunization and challenge, as well as those used for measuring sensitization to the allergen (Evan’s blue reactions) were identical to those described by Broide et al. (10). Peritoneal lavages and eosinophil counts were performed identically to those for thioglycollate-induced peritonitis (described above).
Statistical Analyses.
Data are presented as mean ± SEM. Statistical significance was assessed by two-tailed Student’s t test. All P values reported are of comparisons made to results obtained with WT animals.
RESULTS
Generation of Selectin-Deficient Mice.
Because the genes for all three selectins lie within a 300-kb region on mouse chromosome 1 (11), double- and triple-deficient mice could not be generated by mating single-deficient animals. Therefore, the selectin-deficient animals described here were engineered through a third round of gene targeting by homologous recombination in ES cells that were heterozygous for mutations in both P- and E-selectins (5). Two different clones, one carrying the mutations in cis, the second carrying the mutations in trans, were targeted for the L-selectin null mutation (Fig. 1A).
To make the targeting vector (Fig. 1B), we removed 2.1 kb of the L-selectin gene and replaced it with a puromycin-resistance gene driven by a phosphoglycerate kinase promoter (PGK-PUROr). The replacement vector included 1.8 kb 5′ and 4.6 kb 3′ of genomic DNA flanking the PGK-PUROr.
ES cells were electroporated, and 2,600 resistant clones were picked. Ten homologous recombination events were detected by Southern blot analyses (Fig. 1C). Chimeric animals were generated from these heterozygous clones, six of which transmitted the mutation to their progeny. By following the segregation of the three selectin mutations in subsequent matings (Fig. 1D), and by conducting the appropriate heterozygous intercrosses, eight lines of mice (one WT line and seven different selectin-deficient lines) were established. Homozygous progeny from such intercrosses were bred to establish the wild type and each of the individual null lines. Mice from all deficient lines were viable and fertile as homozygotes, although P-selectin- and E-selectin-double-deficient mice (PE−/−) and E-selectin, L-selectin, and P-selectin-triple-deficient mice (ELP−/−) mice developed mucocutaneous infections that eventually led to death, as reported previously for PE−/− mice (5). All of the other selectin-deficient mice appeared healthy, and they did not develop any obvious infections.
Verification of Null Alleles.
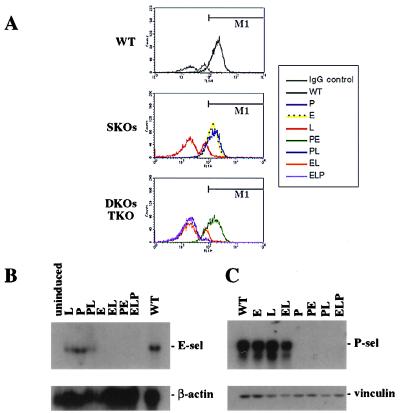
L-selectin expression on circulating leukocytes was evaluated by flow cytometric analysis using FITC-conjugated MEL-14 antibody, a rat mAb directed against the extracellular domain of mouse L-selectin (Fig. 2A). WT (Fig. 2A Top) and single knockouts (SKOs, Fig. 2A Middle) show near WT levels of L-selectin on the leukocytes of E-selectin-deficient (E−/−) and P-selectin-deficient (P−/−) animals, and background levels only in blood from an L-selectin-deficient (L−/−) animal. Double knockouts (DKOs) and the triple knockout (TKO) (Fig. 2A Bottom) show that L-selectin-expressing leukocytes were seen only in blood from a PE−/− KO. L-selectin was not expressed on leukocytes from E-selectin- and L-selectin-double-deficient (EL−/−), P-selectin- and L-selectin-double-deficient (PL−/−), or ELP−/− animals.
Figure 2.
Verification of null alleles. (A) Leukocytes from WT and homozygous selectin-deficient mice were analyzed for L-selectin expression by flow cytofluorometric analysis. Whole blood was collected and leukocytes were stained for L-selectin surface expression. M1 indicates the region of MEL-14-expressing cells. The double peak in the IgG control and L-selectin null genotypes is the result of two cell populations that demonstrate different background binding to the antibodies. (B) WT and homozygous selectin-deficient animals were analysed for expression of E-selectin mRNA by Northern blot analysis. Total RNA was isolated from pooled lungs and hearts of lipopolysaccharide-stimulated animals. Samples were electrophoresed on a 1.0% agarose gel and sequentially probed for E-selectin and β-actin transcripts. Note the underloading of the L and PL lanes. (C) WT and homozygous selectin-deficient animals were analyzed for expression of P-selectin by Western blot analysis. Platelet-rich plasma was isolated from whole blood. Samples were electrophoresed on a 6% SDS-polyacrylamide gel and sequentially stained for P-selectin and vinculin proteins.
To ensure that targeting L-selectin did not substantially alter expression of the E- and P-selectin loci, we evaluated their expression by Northern and Western blot analysis, respectively. We treated mice with lipopolysaccharide to examine E-selectin mRNA (12, 13). Northern blots (Fig. 2B) did not show transcripts in E−/−, EL−/−, PE−/−, or ELP−/− animals. Levels of E-selectin transcripts in the other genotypes were roughly equivalent to those seen in WT animals. P-selectin expression was assayed by Western blot analysis (Fig. 2C) of platelets isolated from each genotype. Protein levels comparable to those in WT animals were seen in platelets from E−/−, L−/−, and EL−/− animals. No expression was seen in the other four strains carrying a P−/− mutation. Thus, each mutation is a null and there is no obvious interference with the expression of the adjacent selectin genes.
Selectin-Deficient Animals Display Varying Degrees of Leukocytosis.
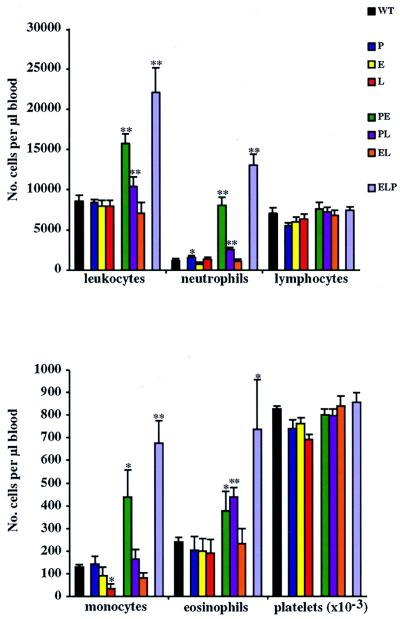
To determine the influence of selectin deficiencies on leukocyte counts, peripheral blood cells were quantitated and leukocyte subpopulations were assessed in young (5–6 weeks old), apparently healthy animals (Fig. 3). As previously reported (5), a general increase in leukocyte counts was observed in PE−/− mice (Fig. 3, green). This increase extended to the subpopulations of neutrophils, monocytes, and eosinophils. In all cases, increases seen in ELP−/− mice (Fig. 3, violet) were even greater. PL−/− mice (Fig. 3, purple) frequently showed increases that were between those seen in the PE−/− and ELP−/− mice. Interestingly, EL−/− mice (Fig. 3, orange) did not show increases in circulating leukocytes; i.e., the absence of E- and/or L-selectin has little affect on leukocyte homeostasis, suggesting these two selectins play a relatively minor role in this process. In contrast, the absence of P-selectin coupled with the absence of either E- or L-selectins enhanced the effects, suggesting that P-selectin contributes in a manner distinct from either of the others. We saw no changes in circulating platelets or lymphocytes (Fig. 3) or in reticulocyte or hemoglobin counts (not shown) in any of the KO animals.
Figure 3.
Peripheral blood counts. Whole blood was collected and a total leukocyte count was obtained on a Coulter counter. Leukocyte subpopulations were determined by differential counts on Diff-Quick-stained smears of the same samples. Ten to 20 animals were used for each genotype. ∗, P < 0.05; ∗∗, P < 0.01, in comparison to WT mice.
Variable Alterations in Leukocyte Rolling Among Various Selectin-Deficient Animals.
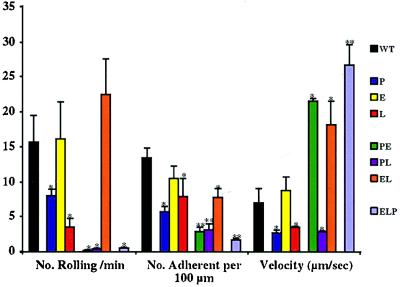
To evaluate the contribution of all selectins to leukocyte rolling under inflammatory conditions, we treated mice with TNF-α 3.5 hr before intravital microscopy of mesenteric venules (Fig. 4). Vessels were hemodynamically similar in all deficiencies, exhibiting no differences in average vessel diameter or in shear rates (not shown). As reported by others, the numbers of leukocytes rolling per min were reduced in P−/− (5) and L−/− animals (3). Rolling was severely suppressed in the venules of PE−/−, PL−/−, and ELP−/− animals. Strikingly, this suppression was not seen in the venules of EL−/− animals (Fig. 4, orange); in these mice, rolling numbers were similar to those of WT animals, showing that P-selectin is sufficient by itself to mediate rolling under the conditions tested. The general trend in EL−/− mice was toward an increase in the number of leukocytes rolling, but we were unable to demonstrate statistical significance. In previous, but similar, studies, we have demonstrated an increase in the number of leukocytes rolling in E−/− mice (unpublished work).
Figure 4.
Leukocyte rolling in TNF-α-stimulated venules. Mice were prepared for intravital microscopy of mesenteric venules 3.5 hr after administration of TNF-α. Venular blood flow and diffractive, rolling leukocytes were videotaped for 20 min. Averages were obtained from at least five animals of each genotype. ∗, P < 0.05; ∗∗, P < 0.01, in comparison to WT mice.
On the other hand, the number of adherent cells was reduced in all genotypes except E−/−. Consistent with the kinetics of the interactions between selectins and their ligands (14–18), rolling in the absence of P- and/or L-selectin was relatively slow and rolling on L-selectin alone, on P-selectin alone, or in the absence of all three selectins was notably faster than WT rolling. However, it should be noted that the “fast rollers” in PE−/−, EL−/−, and ELP−/− mice may represent a small population of cells that is present in the other strains but passes unnoticed because they are a minority population. These cells may be rolling on a nonselectin receptor.
These results suggest that both P- and L-selectins play substantial roles in leukocyte rolling under inflammatory conditions but that only P-selectin can mediate normal levels of rolling in the absence of other selectins. E-selectin appears less important, except in the absence of P-selectin.
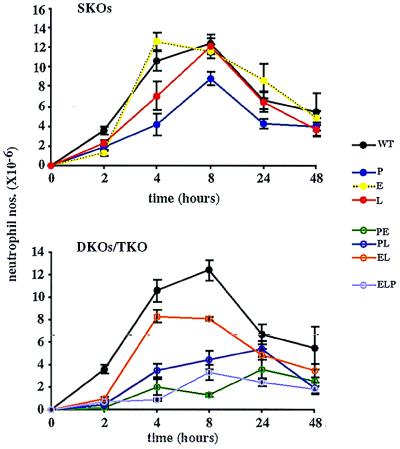
Neutrophil Influx in Thioglycollate-Induced Peritonitis Is Inhibited in Mice Lacking Selectins.
Neutrophil recruitment (0–8 hr). To determine the contributions of the various selectins to the recruitment of neutrophils into the inflamed peritoneum, we injected mice i.p. with thioglycollate and examined the influx of neutrophils to this site at different time intervals (Fig. 5). We saw a delayed neutrophil response at 2 hr in the absence of any one of the selectins (SKOs). However, recruitment in E−/− and L−/− mice recovered to WT levels by 4 and 8 hr, respectively. P−/− mice showed a delayed response even at 8 hr.
Figure 5.
Neutrophil influx after thioglycollate injection. Peritoneal lavages were performed at intervals after thioglycollate administration. Total cell numbers were determined with a hemocytometer, and the percentages of neutrophils were determined from cytospun, Diff-Quick-stained samples. Five to 31 animals of each genotype, for each time point, were used.
An even greater delay in neutrophil influx at 2 hr occurred in the absence of two or three selectins (DKOs/TKO). Recruitment in EL−/− mice showed a dramatic recovery, to near WT levels, at 4 hr, but no additional increase at 8 hr. Influx in PL−/− mice began a steady recovery beyond 2 hr, but neutrophil numbers remained lower than those seen in the single gene deficiencies. Recruitment in PE−/− mice showed a small recovery at 4 hr, but no additional increase. ELP−/− mice did not show any additional neutrophil influx between 2 and 4 hr, but showed some at 8 hr.
Neutrophil clearance (8–48 hr).
Normally, after 8 hr, neutrophil numbers begin to decrease in this model as these cells are cleared from the peritoneum and other cells are recruited to the inflamed site (19). This neutrophil clearance happened with similar kinetics in all but the PE−/− and PL−/− mice. In these two genotypes, neutrophil numbers increased between 8 and 24 hr, which may represent a delay in the normal recruitment of neutrophils in these deficiencies. However, by 48 hr there were no significant differences in the numbers of neutrophils present in the peritoneum for any of the genotypes.
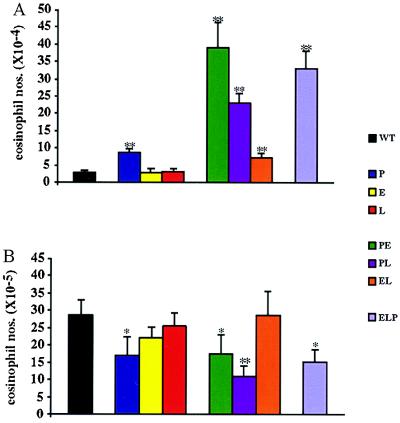
Recruitment of Eosinophils to the Peritoneum Is Impaired When P-Selectin Is Absent.
Before beginning studies of eosinophilic peritonitis, we carefully enumerated circulating levels of eosinophils by using Phloxine B (Fig. 6A). P−/− mice showed a slight, but significant, increase in circulating eosinophils, but WT levels were observed in E−/− and L−/− animals. A similar small increase was seen in EL−/− animals. Large increases were noted in PE−/− and ELP−/− mice, and a smaller increase in PL−/− animals.
Figure 6.
Eosinophil influx after ragweed sensitization and challenge. (A) Peripheral eosinophil counts. Blood was collected by using Eosinophil Unopettes for Manual Methods and eosinophils were counted by using a hemocytometer. (B) Peritoneal eosinophil counts after ragweed challenge. Ragweed-sensitized mice were challenged with an i.p. injection of ragweed allergen. Forty-eight hours later, peritoneal lavages were performed. Total cell numbers were determined with a hemocytometer and the percentages of eosinophils were determined from cytospun, Diff-Quick-stained samples. n = 12–23. ∗, P < 0.05; ∗∗, P < 0.01, in comparison to WT mice. All strains showing decreased levels relative to WT mice were statistically indistinguishable from each other.
To determine the contributions of the various selectins to the recruitment of eosinophils to the inflamed peritoneum, we sensitized mice to ragweed allergen over a 14-day period. On day 20, mice were challenged with an i.p. injection of ragweed allergen and the influx of eosinophils into the peritoneum was examined 48 hr later (Fig. 6B).
Resident levels of peritoneal eosinophils on day 20, before antigen challenge, were very low in all genotypes. All mice showed a significant peritoneal cavity eosinophilia when immunized and then challenged with ragweed allergen as compared with either immunization and/or challenge with PBS (not shown). Similar influxes occurred in WT, E−/−, L−/−, and EL−/− mice. Approximately 2-fold fewer eosinophils were recruited to the peritoneum in P−/−, PE−/−, PL−/−, and ELP−/− mice. The recruited eosinophil numbers for these four genotypes, however, were not statistically different from one another, indicating that the absence of P-selectin was the key difference.
Evan’s blue reactions, performed on day 20, to measure sensitization to ragweed immunization, showed no significant difference from WT reactions in any deficiency (not shown). Although mast cells participate in allergen-induced inflammation (20), we were unable to get accurate measurements of this cell population, likely because of mast cell degranulation during sample collection. Collectively, our data indicate that P-selectin plays a critical role in the recruitment of eosinophils to the inflamed peritoneum and that, in contrast to results with neutrophils, other selectins play little or no role.
DISCUSSION
We present data here that the interactions between leukocytes and endothelial cells are impaired when selectins are missing (Fig. 4). Removing selectins alters the kinetics with which leukocytes interact with inflamed mesenteric venules. An analysis of rolling in ELP−/− mice shows that selectin-independent rolling is very inefficient; the rolling velocity in ELP−/− mice is rapid, the number of cells rolling is greatly reduced, and the adherence of leukocytes to the endothelium is drastically impaired. Although not shown in this study, the receptors mediating the “fast” residual rolling are likely to be α4/vascular cell adhesion molecule 1 (21–23). This phenotype confirms prior reports that selectins are necessary for effective rolling and adhesion and that their functions cannot be replaced by integrins.
Rolling interactions also are impaired in the PE−/− and PL−/− deficiencies, demonstrating that optimal leukocyte rolling depends on the cooperation of P-selectin with either of the other selectins. The number of rolling cells in EL−/− mice is unaltered, though the number of adherent cells is slightly reduced. Nonetheless, in this mutant, more cells adhere than in the other DKOs, or in the TKO. As a whole, these results suggest that E- and L-selectins singly or together play relatively minor roles in rolling and adherence of leukocytes to TNF-α-activated endothelium. In contrast, in the absence of E- and L-selectins, P-selectin plays a more dominant role in mediating leukocyte rolling. Similar observations have been reported recently by Jung and Ley (23) with chimeric animals.
One of the most obvious phenotypes reported here is the leukocytosis that occurs in DKO mice (Fig. 3). The increase is negligible in the EL−/− mice, but elevations are significant in PL−/−, PE−/−, and ELP−/− mice. The leukocyte recruitment defects we observe certainly contribute to increased peripheral leukocyte numbers, though several other mechanisms are likely to be involved. Previously reported for PE−/− mice is an alteration in leukocyte production at the level of the bone marrow (5). The bone marrow from ELP−/− mice is similar to that of PE−/− mice and, whereas the bone marrow from EL−/− mice appears relatively normal, myelopoiesis appears mildly increased in PL−/− mice (not shown). We consistently observe splenomegally in ELP−/− mice that is similar to that in PE−/− mice but we do not see changes in spleen morphology or size in any of the other KOs (not shown). Additionally, leukocyte half-lives likely are altered in the absence of selectins as has been reported in P−/− mice (19). Finally, some of the elevation undoubtedly arises from alterations in cytokine levels. Elevations have been reported in PE−/− mice (5) and are likely to exist in the other multiple selectin-deficient mice.
Our data suggest a key role for P-selectin in mediating leukocyte behavior, and such conclusions are supported by observations reported by Jung and Ley (23). The role of P-selectin is most striking in ragweed-induced recruitment of eosinophils to the peritoneum (Fig. 6). The studies support a crucial role for P-selectin in eosinophil recruitment, as has been suggested for this receptor for some time (10, 24, 25, 26). Although L-selectin can mediate the adhesion and rolling of eosinophils in a number of systems (27, 28), it does not appear to do so in the studies presented here. We show here that neither E- nor L-selectin contribute significantly to eosinophil influx. Our results are consistent with previous studies suggesting that eosinophils do not interact significantly with E-selectin (26, 29, 30). It should be noted, however, that all three selectins are cooperating at some level to influence eosinophil homeostasis; whereas P-selectin is the only selectin whose absence impairs recruitment of these cells to inflamed peritoneum (Fig. 6B), the combined absence of P- and E-selectins, P- and L-selectins, or all three selectins leads to increases in peripheral eosinophils over those seen in the P-selectin single gene deficiency (Fig. 6A).
A complementary role for the endothelial selectins in regulating many aspects of leukocyte behavior has been previously reported, and commented on, by us (5) and others (6). Although an overlapping role for P- and L-selectins has been reported in a few studies (31, 32), our studies indicate these two selectins cooperate to influence many aspects of leukocyte behavior. We show a preeminent role for P-selectin in many processes. A similar conclusion was reached by Jung and Ley (23) in adoptive transfer studies designed to examine the influence of each selectin on the rolling of leukocytes in the cremaster muscle. This preeminence may arise from the fact that P-selectin is longer than either E- or L-selectin and can, therefore, more easily extend above the negatively charged glycocalyx allowing for more efficient interactions between leukocytes and endothelial cells, as demonstrated by Patel et al. (33). The results we present, however, point to diverse interactions among the selectins and reveal broader than expected roles for all three selectins. The conclusions we draw from our analyses in mice may not be fully transferable to humans and other species as the regulation of selectin expression can vary in different situations and organisms.
Acknowledgments
We are grateful to Jane E. Trevithick and Denise Crowley for technical assistance and Glen Paradis for flow cytometry assistance. Special thanks go to Kairbaan Hodivala-Dilke, Julie Lively, and Elizabeth Alcamo for providing helpful discussions and data interpretations. This work was supported by National Institutes of Health Grants PO1 HL41484 (R.O.H.) and HL53756 (D.D.W). R.O.H. is an Investigator of the Howard Hughes Medical Institute. S.D.R. was supported in part by a postdoctoral fellowship from the American Cancer Society (PF-3945).
ABBREVIATIONS
- E−/−
E-selectin-deficient mice
- L−/−
L-selectin-deficient mice
- P−/−
P-selectin-deficient mice
- EL−/−
E-selectin- and L-selectin-double-deficient mice
- PE−/−
P-selectin- and E-selectin-double-deficient mice
- PL−/−
P-selectin- and L-selectin-double-deficient mice
- ELP−/−
E-selectin-, L-selectin-, and P-selectin-triple-deficient mice
- KO
knockout
- SKO
single KO
- DKO
double KO
- TKO
triple KO
- ES
embryonic stem
- TNF-α
tumor necrosis factor α
- WT
wild type
References
- 1.Vestweber D, Blanks J. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 2.Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 3.Arbones M L, Ord D C, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon D J, Tedder T F. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 4.Labow M A, Norton C R, Rumberger J M, Lombard-Gillooly K M, Shuster D J, Hubbard J, Bertko R, Knaack P A, Terry R W, Harbison M L, et al. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.Frenette P S, Mayadas T N, Rayburn H, Hynes R O, Wagner D D. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 6.Bullard D C, Kunkel E J, Kubo H, Hicks M J, Lorenzo I, Doyle N A, Doerschuk C M, Ley K, Beaudet A L. J Exp Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallatin W M, Weissman I L, Butcher E C. Nature (London) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 8.Fries J W, Williams A J, Atkins R C, Newman W, Lipscomb M F, Collins T. Am J Pathol. 1993;143:725–737. [PMC free article] [PubMed] [Google Scholar]
- 9.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Broide D H, Humber D, Sullivan S, Sriramarao P. Blood. 1998;91:2847–2856. [PubMed] [Google Scholar]
- 11.Watson M L, Kingsmore S F, Johnston G I, Siegelman M H, Le Beau M M, Lemons R S, Bora N S, Howard T A, Weissman I L, McEver R P, et al. J Exp Med. 1990;172:263–272. doi: 10.1084/jem.172.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevilacqua M P, Pober J S, Mendrick D L, Cotran R S, Gimbrone M A., Jr Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders W E, Wilson R W, Ballantyne C M, Beaudet A L. Blood. 1992;80:795–800. [PubMed] [Google Scholar]
- 14.Ley K, Zakrzewicz A, Hanski C, Stoolman L M, Kansas G S. Blood. 1995;85:3727–3735. [PubMed] [Google Scholar]
- 15.Jung U, Bullard D C, Tedder T F, Ley K. Am J Physiol. 1996;271:H2740–H2747. doi: 10.1152/ajpheart.1996.271.6.H2740. [DOI] [PubMed] [Google Scholar]
- 16.Kunkel E J, Ley K. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 17.Puri K D, Finger E B, Springer T A. J Immunol. 1997;158:405–413. [PubMed] [Google Scholar]
- 18.Kunkel E J, Chomas J E, Ley K. Circ Res. 1998;82:30–38. doi: 10.1161/01.res.82.1.30. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R C, Mayadas T N, Frenette P S, Mebius R E, Subramaniam M, Lacasce A, Hynes R O, Wagner D D. Blood. 1995;86:1106–1114. [PubMed] [Google Scholar]
- 20.Hom J T, Estridge T. Clin Immunol Immunopathol. 1994;73:305–311. doi: 10.1006/clin.1994.1203. [DOI] [PubMed] [Google Scholar]
- 21.Berlin C, Bargatze R F, Campbell J J, von Andrian U H, Szabo M C, Hasslen S R, Nelson R D, Berg E L, Erlandsen S L, Butcher E C. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 22.Alon R, Kassner P D, Carr M W, Finger E B, Hemler M E, Springer T A. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung U, Ley K. J Immunol. 1999;162:6755–6762. [PubMed] [Google Scholar]
- 24.Symon F A, Walsh G M, Watson S R, Wardlaw A J. J Exp Med. 1994;180:371–376. doi: 10.1084/jem.180.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel K D. Blood. 1998;92:3904–3911. [PubMed] [Google Scholar]
- 26.Teixeira M M, Hellewell P G. J Immunol. 1998;161:2516–2523. [PubMed] [Google Scholar]
- 27.Knol E F, Tackey F, Tedder T F, Klunk D A, Bickel C A, Sterbinsky S A, Bochner B S. J Immunol. 1994;153:2161–2167. [PubMed] [Google Scholar]
- 28.Sriramarao P, von Andrian U H, Butcher E C, Bourdon M A, Broide D H. J Immunol. 1994;153:4238–4246. [PubMed] [Google Scholar]
- 29.Bochner B S, Sterbinsky S A, Bickel C A, Werfel S, Wein M, Newman W. J Immunol. 1994;152:774–782. [PubMed] [Google Scholar]
- 30.Sriramarao P, Norton C R, Borgstrom P, DiScipio R G, Wolitzky B A, Broide D H. J Immunol. 1996;157:4672–4680. [PubMed] [Google Scholar]
- 31.Ley K, Bullard D C, Arbones M L, Bosse R, Vestweber D, Tedder T F, Beaudet A L. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanwar S, Steeber D A, Tedder T F, Hickey M J, Kubes P. J Immunol. 1999;162:2709–2716. [PubMed] [Google Scholar]
- 33.Patel K D, Nollert M U, McEver R P. J Cell Biol. 1995;131:1893–1902. doi: 10.1083/jcb.131.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]