Abstract
During human embryo implantation, trophectoderm mediates adhesion of the blastocyst to the uterine epithelium. The rapid growth of the embryo and invasion of the maternal tissue suggest adhesion-induced activation of the embryonal cells. We show here that ligation of trophinin, a homophilic cell adhesion molecule expressed on trophoblastic cells, induces tyrosine phosphorylation in trophinin-expressing trophoblastic HT-H cells. The phosphorylation could be induced in HT-H cells with the binding of trophinin-expressing cells or anti trophinin antibodies. Trophinin-dependent tyrosine phosphorylation was associated with actin reorganization. We also isolated trophinin-binding peptides from phage libraries. These peptides exhibited the consensus sequence GWRQ and seemed to reproduce the effects of trophinin-mediated cell adhesion. Upon binding of a GWRQ peptide, HT-H cells became highly proliferative and motile. HT-H cells expressed ErbB family receptors and bound EGF and heparin-binding EGF-like growth factor (HB-EGF), but ErbB family receptor phosphorylation in these cells required GWRQ. In the absence of GWRQ, trophinin interacted with the cytoplasmic protein bystin, which binds to ErbB4 and blocks its autophosphorylation. In HT-H cells, GWRQ peptide dissociated trophinin from bystin, and ErbB4 was activated. Culturing monkey blastocysts in the presence of the peptide increased total number and motility of the trophectoderm cells. These results suggest that trophinin-mediated cell adhesion functions as a molecular switch for trophectoderm activation in human embryo implantation.
Keywords: BYSL, ErbB4, pregnancy, receptor tyrosine kinase, stem cells
A fertilized mammalian egg autonomously develops into a blastocyst, which must be successfully implanted in the uterus to develop further into a fetus. In higher primates, including humans, trophectoderm cells that come in contact with the uterine epithelium rapidly grow and invade maternal tissue, suggesting that cell adhesion may signal trophectoderm to form an active trophoblast.
Embryo implantation is unique to mammals, and this process differs significantly among mammalian species (1, 2). Some mechanisms underlying human embryo implantation are likely unique to humans; for example, ectopic implantation is relatively common in humans (3) but absent or rare in other mammalian species (4). Although significant progress has been made in the study of mouse embryo implantation (2, 5, 6), less is known about human embryo implantation, because there are significant differences in this process between mice and humans. Furthermore, technical and ethical difficulties in studying implantation-stage human embryos limit our understanding of this process at the molecular level.
Morphological observations of human and monkey embryo implantation sites indicate that blastocyst trophectoderm cells that have adhered to the uterus, proliferate and invade, whereas trophectoderm not in contact with the uterine epithelium remains a monolayer (7–9). This finding suggests that the initial adhesion triggers activation of cells in trophectoderm. However, the mechanism underlying this activation is unknown.
We previously identified trophinin as a homophilic cell adhesion molecule that mediates adhesion between trophoblastic cells and endometrial epithelial cells (10–13). When both of these cell types express trophinin, they adhere to each other. Trophinin is expressed in trophectoderm cells in monkey blastocysts (10) and in extra-embryonic and maternal cells at monkey and human embryo implantation sites (10, 11, 13). Here we describe a short peptide that binds to the trophinin extracellular domain and triggers a response that mimics the changes caused by trophinin-mediated cell adhesion. We use this peptide to establish a molecular link between trophinin-mediated cell adhesion and trophoblast activation.
Results
Trophinin-Mediated Cell Adhesion Activates Trophoblastic Cells.
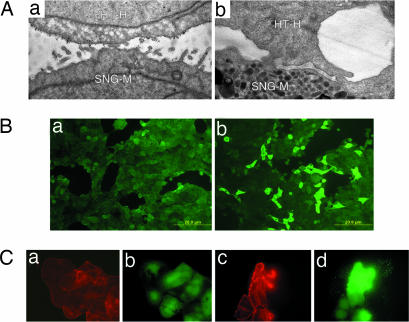
As shown in our previous studies, trophoblastic embryonal carcinoma HT-H cells adhered with high affinity to the apical surface of trophinin-expressing endometrial adenocarcinoma cells (10, 14). The adhesion is trophinin-mediated and is accompanied by morphological changes in both cell types (Fig. 1A), suggesting that trophinin-mediated cell adhesion triggers a signal transduction in these cells. When a single cell suspension of HT-H cells was added to an HT-H cell monolayer, some HT-H cells in the monolayer showed significant elevation of tyrosine phosphorylation (24.5 ± 2.06% of positive cells vs. control 0.8 ± 0.75%, n = 5) (Fig. 1B).
Fig. 1.
Activation of HT-H cells by trophinin-mediated cell adhesion. (A) Electron micrographs of HT-H cells 10 min (a) and 6 h (b) after adhesion to trophinin-expressing endometrial SNG-M cells (10). Gold particles represent anti-trophinin antibodies. (B) An HT-H monolayer was incubated in medium containing Na3VO4, overlaid without (a) or with (b) HT-H cell suspension and stimulated for phosphorylation at 37°C for 30 min. Adhered HT-H cells were removed by gentle pipetting, and the HT-H monolayer was stained with anti-phosphotyrosine (pY) antibody to detect tyrosine-phosphorylated proteins. (C) HT-H cells treated for anti-trophinin antibody cross-linking and phosphorylation stimulation. HT-H cells were reacted with the second antibody only (a and b) and with anti-trophinin antibody followed by a second antibody (c and d). The cells were stained with rhodamine–phalloidin for F-actin (red) and an anti-pY antibody (green).
To mimic trophinin-mediated cell adhesion, we treated HT-H cells with a monoclonal antibody against the cell surface domain of trophinin (11) and then cross-linked with a second antibody (ref. 15 and see Methods). HT-H cells cross-linked with anti-trophinin antibody showed enhanced levels of F-actin formation and tyrosine phosphorylation (Fig. 1C), suggesting that engaging trophinin on the cell surface elicits a signal that is transmitted into the cytoplasm.
A Peptide Mimics Trophinin-Mediated Cell Adhesion.
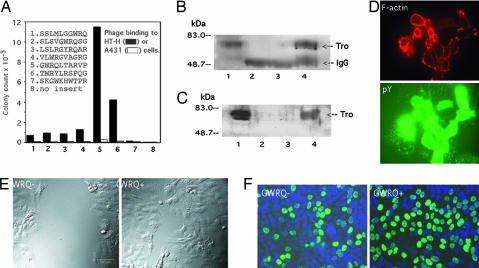
A previous study suggested that trophinin-mediated cell adhesion is based on a unique structure created by trophinin clustering on the cell surface (10). To explore the possibility that a small molecule could mimic trophinin-mediated cell adhesion, we screened a peptide-displaying phage library for trophinin-binding peptides. A screen using HT-H cells as a target identified a series of peptides containing the consensus sequence GWRQ (Fig. 2A). Specificity of GWRQ for trophinin was ascertained in several ways. First, phage clones displaying the GWRQ peptide bound to the trophinin-positive HT-H cells, but not to trophinin-negative A431 cells (Fig. 2A). Second, anti-trophinin antibody inhibited the binding of the GWRQ phage and of a synthetic GWRQ peptide to HT-H cells [supporting information (SI) Fig. 7]. Third, the GWRQ phage and GWRQ peptide both captured trophinin from the HT-H cells (Fig. 2 B and C).
Fig. 2.
Identification of the GWRQ peptide and its effect on HT-H cells. (A) Cell binding of individual phage clones from screening of a 10-mer peptide phage library for binding to HT-H cells. The screen identified a series of peptides with the consensus sequence GWRQ. Note that the strongest binder is a representative of peptides that contain the GWRQ motif at the N terminus. (B) Western blot with anti-trophinin antibody. Lane 1 shows untreated HT-H cell lysate. Lanes 2–4 show immunoprecipitates from HT-H lysates obtained as follows: lane 2, incubated with GWRQ phage, followed by immunoprecipitation with rabbit IgG; lane 3, incubated with control phage, followed by immunoprecipitation with rabbit antiphage antibody; and lane 4, incubated with GWRQ phage, followed by immunoprecipitation with antiphage antibody. (C) Western blot with anti-trophinin antibody. Lane 1 shows untreated HT-H cell lysate. Lanes 2–4 show pull-down products from HT-H lysates obtained as follows: lane 2, incubated with biotinylated GWRQ peptide and collected by control beads; lane 3, incubated with biotinylated control peptide and collected by avidin beads; lane 4, incubated with biotinylated GWRQ peptide and collected by avidin beads. (D) Activation of HT-H cells by synthetic GWRQ peptide. HT-H cells were treated in the same manner as shown in Fig. 1C, except that GWRQ peptide (5 μg/ml at 4°C for 15 min) was used in place of anti-trophinin antibody. (E) Effect of GWRQ on HT-H cells on wound-healing. HT-H cells at 5 h of recovery with or without GWRQ peptide. (F) Cell proliferation assay. Mitotic activity of HT-H cells was determined by BrdU incorporation 24 h after GWRQ treatment.
When GWRQ peptide was added to HT-H cell cultures, actin polymerization and tyrosine phosphorylation were induced (Fig. 2D), whereas control peptides did not cause these effects (data not shown). This finding suggests that the GWRQ peptide activates HT-H cells in a manner similar to the anti-trophinin antibody (Fig. 1C). Both monomeric and octameric GWRQ peptides activated HT-H cells, but >100-times the amount of monomeric GWRQ was required to achieve the same level of activation as octameric GWRQ (SI Fig. 8). Thus in further experiments reported here, we used only octameric GWRQ or GWRQ-MAPS. Because tyrosine phosphorylation and actin polymerization are associated with cell motility, we tested GWRQ in motility assays. Both a wound-healing assay (Fig. 2E) and an invasion assay (SI Fig. 9) showed that HT-H cells cultured with GWRQ were highly motile compared with control cultures. GWRQ also increased the proliferation of HT-H cells (52.9 ± 7.8% of cells positive for BrdU incorporation vs. 36.1 ± 9.2% in control cultures, n = 6, P < 0.05) (Fig. 2F).
GWRQ Peptide Causes Trophoblast Spreading in Cultured Monkey Blastocysts.
To evaluate the biological relevance of the GWRQ effects, we tested the peptide in monkey blastocyst cultures by growing fertilized monkey eggs to the blastocyst stage (16, 17) with or without GWRQ. The embryos remained spherical in the absence of GWRQ, (Fig. 3A), whereas trophectoderm cells migrated out of the cell mass in GWRQ-treated cultures (Fig. 3B). Furthermore, these cell numbers were higher in blastocysts cultured with GWRQ. It should be noted that only two monkey blastocysts were examined, one cultured with GWRQ and one control embryo cultured without GWRQ. Due to the limited sample size, these embryo-based results should be considered preliminary. Nevertheless, these results suggest that GWRQ peptide enhances both cell motility and proliferation in the monkey blastocyst.
Fig. 3.
Effect of GWRQ peptide on monkey blastocysts. Rhesus monkey blastocysts were cultured in standard media (A) or in the presence of GWRQ peptide (B). Phase contrast (Left) and DAPI staining (Right) are shown.
GWRQ Peptide Controls EGF Receptor Activation.
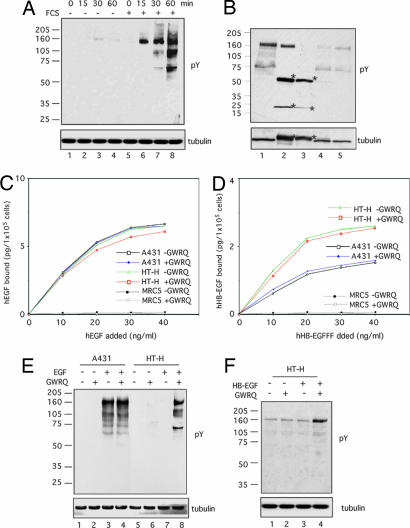
Because all of the changes induced by GWRQ are characteristic of cellular responses to growth factors present in FCS, we compared the effect of GWRQ on HT-H cells cultured with or without FCS. Western blot analysis revealed that GWRQ increased levels of tyrosine-phosphorylated proteins in the presence of serum, but not in serum-depleted cultures (Fig. 4A). Thus, the activation of HT-H by GWRQ is serum-dependent.
Fig. 4.
Effect of GWRQ peptide on EGFR activation in HT-H cells. (A) Western blot of tyrosine-phosphorylated proteins. HT-H cells were cultured in medium with or without FCS and were treated with GWRQ peptide at 4°C for 15 min, followed by phosphorylation stimulation at 37°C for a period of 0–60 min. (B) Western blot of tyrosine-phosphorylated proteins immunoprecipitated by a mixture of antibodies against ErbB family EGF receptors. HT-H cells were treated with GWRQ peptide at 4°C for 15 min, followed by an incubation at 37°C for 30 min in the presence of FCS. Samples loaded are as follows: lane 1, total cell lysate; lane 2, immunoprecipitates by a mixture of antibodies for ErbB1, -2, -3, and -4; lane 3, immunoprecipitates by control antibodies; lane 4, supernatant of immunoprecipitates by a mixture of antibodies for ErbB1, -2, -3, and -4; and lane 5, supernatant of immunoprecipitate by control antibodies. Asterisks indicate IgG. (C) Binding of recombinant human EGF to HT-H, A431, and MRC-5 (human fibroblast) cells treated with or without GWRQ peptide. Data were obtained by duplicate measurements, of which the errors were within 15%. (D) Binding of recombinant human HB-EGF to HT-H, A431, and MRC-5 cells treated with or without GWRQ peptide. Data were obtained from duplicate measurements, and errors were within 15%. (E) Western blot of tyrosine-phosphorylated proteins from serum-starved HT-H and A431 cells treated with EGF and/or GWRQ peptide and incubated at 37°C for 30 min. Control peptides showed no effect on these cells. (F) Western blot of tyrosine-phosphorylated proteins from serum-starved HT-H cells treated with or without HB-EGF and/or GWRQ peptide at 4°C for 15 min, followed by incubation at 37°C for 30 min.
The major tyrosine-phosphorylated protein in GWRQ-treated HT-H cells migrated at ≈160 kDa (Fig. 4A), suggesting that these bands are ErbB family EGF receptors. HT-H cells express all four ErbB family receptors (SI Fig. 10 A). Immunoprecipitation of GWRQ-activated HT-H cell lysates with a mixture of antibodies to ErbB1–4 precipitated almost all tyrosine-phosphorylated 160 kDa proteins (SI Fig. 10 B), including ErbB4 (SI Fig. 10 B and C). Addition of recombinant EGF and heparin-binding EGF-like growth factor (HB-EGF) to HT-H cells cultured in FCS-containing medium did not significantly alter levels of tyrosine-phosphorylated proteins (SI Fig. 10 D). Western blots with phosphospecific antibodies also showed activation of MAPK (p38), mitogen-activated protein kinase kinase (MEK) 3/6, S6K (p70), JNK, STAT3, Rb, and Src in GWRQ-treated HT-H cells (SI Fig. 11). These results suggest that serum factors affecting HT-H activation are EGF and HB-EGF. We therefore asked whether GWRQ affects binding of EGF and HB-EGF to HT-H cells and found that both bind HT-H cells equally well in the presence and absence of GWRQ (Fig. 4 C and D).
As demonstrated in ref. 18, EGF caused EGF receptor (EGFR) phosphorylation in A431 cells cultured in serum-depleted medium (Fig. 4E, lane 3). Surprisingly, EGF caused no detectable EGFR phosphorylation in HT-H cells under the same conditions (Fig. 4E, lane 7). Whereas the GWRQ had no effect on tyrosine phosphorylation in A431 cells (Fig. 4E, lane 4), in HT-H cells tyrosine phosphorylation occurred only when both EGF and GWRQ were added (Fig. 4E, lane 8). HB-EGF showed a similar effect: HB-EGF activated HT-H cells when GWRQ was present (Fig. 4F, lane 4). These results indicate that trophinin occupancy is critical for phosphorylation of ErbB family receptors in HT-H cells.
Trophinin Occupancy Allows EGFR Activation.
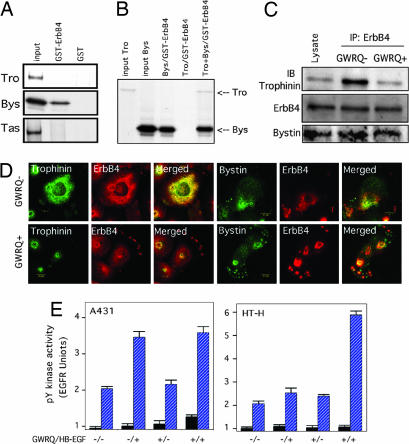
Although HT-H cells express all four members of the ErbB family receptors (SI Fig. 10), we focused on ErbB4 because trophectoderm cells of both mouse and human blastocysts express ErbB4 on apical cell membranes (19, 20). We have previously shown that the cytoplasmic domain of trophinin directly interacts with bystin (14) and that bystin interacts with the proline-rich protein tastin (21). Because the cytoplasmic domain of ErbB4 is proline-rich (22), we examined the binding of bystin to ErbB4. In vitro pull-down assays showed that bystin, but not trophinin or tastin, binds to the cytoplasmic domain of ErbB4 (Fig. 5A). When a mixture of trophinin and bystin was incubated with GST-ErbB4, trophinin also bound to ErbB4, presumably through bystin (Fig. 5B). These results suggest that the active factor in serum is EGF or HB-EGF, but we cannot exclude the possibility that some other mechanism, such as transactivation by G protein coupled receptor ligands (23, 24) could be involved. Also it should be noted that these results do not necessarily demonstrate direct interaction between trophinin, bystin and ErbB4 because we used in vitro translated proteins without purification.
Fig. 5.
Interaction of trophinin, bystin, and ErbB4. (A) Pull-down assay for trophinin, bystin, and tastin by GST-ErbB4. In vitro translated 35S-labeled trophinin, bystin, or tastin was incubated with GST or GST-ErbB4 immobilized on glutathione beads. Bead-bound materials were visualized by autoradiography after gel electrophoresis. (B) Pull-down assay of trophinin and bystin by GST-ErbB4. Bead-bound materials were visualized by autoradiography. Note that trophinin is captured by GST-ErbB4 when bystin is present. (C) Interaction of trophinin, bystin, and ErbB4 in HT-H cells. HT-H cells treated with or without GWRQ peptide were subjected to immunoprecipitation with anti-ErbB4 antibody, followed by Western blot analysis. Note that trophinin and bystin coimmunoprecipitated with ErbB4 from HT-H cells not treated with GWRQ, whereas less trophinin was present in precipitates from GWRQ-treated cells. (D) Confocal immunofluorescence micrographs of HT-H cells cultured with or without GWRQ peptide. (Left) Localization of trophinin and ErbB4. (Right) Localization of bystin and ErbB4. (E) Tyrosine kinase activities of ErbB4 from HB-EGF- and/or GWRQ-treated A431 and HT-H cells. Tyrosine kinase activities in immunoprecipitates with control IgG (black bars) and anti-ErbB4 antibody (blue bars) are shown. Error bars represent the standard deviation.
To determine whether trophinin, bystin, and ErbB4 interact with each other in HT-H cells, HT-H cell lysates were subjected to immunoprecipitation. In the absence of GWRQ, both trophinin and bystin coimmunoprecipitated with ErbB4 (Fig. 5C, top row). Strikingly, when HT-H cells were treated with GWRQ, trophinin levels were reduced in immunoprecipitates, whereas bystin binding was not affected by GWRQ (Fig. 5C, bottom row).
Immunofluorescence microscopy of HT-H cells doubly stained for trophinin and ErbB4 on the cell surface showed overlap of these two proteins in the absence of GWRQ (Fig. 5D). In HT-H cells treated with GWRQ, trophinin localized to the area above the nuclei, and ErbB4 moved to the cell periphery. In the absence of GWRQ, bystin and ErbB4 colocalized, whereas both proteins moved to the cell periphery in GWRQ-treated cells. These results suggest that, in the absence of GWRQ, trophinin, bystin and ErbB4 form a complex, whereas in GWRQ-treated HT-H cells, the ErbB4-bystin complex dissociates from trophinin.
ErbB4 immunoprecipitated from HB-EGF-treated A431 cells exhibited kinase activity both in the presence or absence of GWRQ (Fig. 5E Left). By contrast, ErbB4 from HB-EGF-treated HT-H cells showed no tyrosine kinase activity in the absence of GWRQ but exhibited strong kinase activity in cells treated with both HB-EGF and GWRQ (Fig. 5E Right). These results are consistent with the profile of tyrosine-phosphorylated proteins shown in Fig. 4F and indicate that the presence of GWRQ is needed for EFGR to be active in HT-H cells.
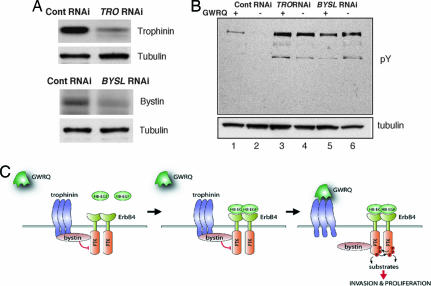
To further ascertain the role of trophinin and bystin in ErbB4 regulation, trophinin and bystin were knocked down in HT-H cells by siRNA (Fig. 6A). HT-H cells transfected with negative control siRNA showed tyrosine-phosphorylated proteins in the presence but not in the absence of GWRQ peptide (Fig. 6B, lanes 1 and 2). By contrast, in HT-H cells in which trophinin and bystin were knocked down, remarkable tyrosine phosphorylation was induced by HB-EGF regardless of the presence or absence of GWRQ (Fig. 6B, lanes 3–6). These results indicate that activation of ErbB4 by HB-EGF is suppressed by trophinin and bystin in HT-H cells.
Fig. 6.
Mechanism of ErbB4 activation by trophinin-mediated cell adhesion. (A) Western blot for trophinin (Upper) and bystin (Lower) to determine the levels of targeted proteins in HT-H cells transfected by each siRNA. (B) Western blot of tyrosine-phosphorylated proteins from trophinin and bystin knocked down HT-H cells in the presence of HB-EGF with or without GWRQ binding. HT-H cells were transfected with siRNA for negative control (lanes 1 and 2), for trophinin (lanes 3 and 4), and for bystin (lanes 5 and 6). Cells were cultured in medium without FCS and then were added with HB-EGF and with (lanes 1, 3, and 5) or without (lanes 2, 4, and 6) GWRQ. (C Left) Trophinin and bystin bound together in a cytoplasmic complex, which further interacted with ErbB4 and suppressed its tyrosine kinase activity. (Center) HB-EGF binds to ErbB4, but protein kinase activity is suppressed by trophinin/bystin. (Right) Trophinin-mediated cell adhesion is mimicked by binding of GWRQ peptide to trophinin on the cell surface, resulting in dissociation of trophinin from bystin and activation of ErbB4.
Discussion
Our results indicate that trophinin-mediated cell adhesion acts as a molecular switch for ErbB4 activation for trophoblast invasion and proliferation. We propose that trophectoderm cells of human blastocyst remain silent as long as trophinin arrests ErbB4. Upon trophinin-mediated cell adhesion, which is presumably mimicked by the binding of the GWRQ peptide to the extracellular domain of trophinin, bystin is released from trophinin. This release allows activation of ErbB4 protein tyrosine kinase, eliciting proliferation and increased motility of the trophoblastic cells that engage in trophinin-mediated cell adhesion to uterine epithelium. A schematic representation of our mechanistic model is shown in Fig. 6C.
Previous studies by others have implicated EGFR in mouse embryo implantation (19, 25–27), where interaction between ErbB4 expressed on the trophectoderm and membrane-bound HB-EGF on the uterine epithelium mediates an initial adhesion. This study, placing ErbB4 downstream of trophinin in humans, links underlying mechanisms of human and mouse embryo implantation. In our studies of trophinin mutant mice, we found that trophinin null blastocysts successfully implanted (28). Given that we saw no trophinin-independent EGFR activation in human trophoblast-derived cells, it is reasonable to assume that trophinin may be less important in mouse than in human (and monkey) implantation. These species differences underscore the importance of fertility studies in human cells and human subjects. This study should facilitate development of strategies to ameliorate fertility-related problems in humans, some of which may be unique to humans.
Here we used the GWRQ peptide as a tool to analyze trophinin-mediated cell adhesion. Trophinin is a 69-kDa protein with a short N-terminal cytoplasmic domain following unique decapeptide repeats (10). The decapeptide repeats are thought to be a key structural element for homophilic cell adhesion, but the structural basis for trophinin–trophinin binding is yet unknown. Because a GWRQ sequence is not present in trophinin, GWRQ is presumably a ligand mimic for the trophinin homophilic interaction site. Interestingly, another homophilic cell adhesion molecule, cadherin, also binds a peptide with a GW N terminus (29), suggesting that trophinin and cadherin may share some structural features.
Because GWRQ peptide stimulates proliferation of monkey embryonic cells at the blastocyst stage (Fig. 3), it could be useful in facilitating the culturing of human embryonic stem cells. Because trophinin is also expressed in neurons (30), spermatozoa (31), and testicular cancer cells (32), the GWRQ peptide may also be a useful tool in analyzing the role of trophinin in these cells. Also, identification of bystin as a main target of MYC (33) suggests a role for the trophinin-bystin axis in the development of trophoblastic cancers. Finally, Because cell adhesion is closely associated with signal transduction pathways activated in proliferation and differentiation (34, 35), this study should help facilitate understanding of the molecular basis of trophoblastic transformation in human cancers and the development of new therapeutic strategies.
Materials and Methods
Antibodies and Reagents.
The monoclonal anti-trophinin antibody and anti-bystin antibody were generated as described in ref. 11. A rabbit anti-ErbB4 antibody directed at the cytoplasmic domain was from Santa Cruz Biotechnology (Santa Cruz, CA), and a rabbit antibody directed to the ErbB4 ectodomain was from Abgent (San Diego, CA). Both GWRQ-MAP, in which eight GWRQ peptides are linked to a branched lysine cluster (MAP), and monomeric GWRQ peptide were synthesized by AnaSpec (San Jose, CA). Control peptides, Ala-MAP, and an irrelevant peptide, FAQLDWH-MAP (36), were also synthesized by AnaSpec.
Antibody Cross-Linking and Stimulation of Phosphorylation.
HT-H cells were cultured as a monolayer in a tissue culture plate or on a glass coverslip. Cells were incubated on ice for 15 min with phosphorylation medium, which is composed of high glucose DMEM supplemented with 25 mmol/liter Hepes (pH7.2), 0.1% BSA, and 0.2 mmol/liter Na3VO4. The cells were then incubated with or without 2 μg/ml anti-trophinin antibody for 30 min, followed by 27 μg/ml goat anti-mouse IgM antibody at 4°C. Cells were then warmed to 37°C, cultured for 0–60 min (15), and subjected to immunocytochemistry, immunoprecipitation, and/or Western blot analysis.
Peptide-Displaying Phage Library Screening.
An M13 phage library (≈1011 clones) for random 10-mer peptides was constructed as in ref. 37. Target HT-H cell monolayers were grown in one 24-well plate and fixed with 1% paraformaldehyde in PBS. After washing with PBS containing 1 mM EDTA, the monolayer was blocked with PBS containing 5% BSA, and the phage solution (containing 1013 colony-forming units) was added to HT-H cells. After incubating at room temperature for 15 min, unbound phage was removed by washing with 1 mM EDTA/PBS, and HT-H-bound phage was recovered by transforming K91 Escherichia coli bacteria. Selected phage was amplified in LB medium containing 20 μg/ml tetracycline and 100 μg/ml kanamycin and was subjected to a second round of screening with HT-H cells as a target. After a third enrichment, individual clones were sequenced.
Monkey Blastocyst Culture.
Animals.
Mature rhesus macaque males and females housed in individual cages were used in this study. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Oregon National Primate Research Center at the Oregon Health & Science University.
Embryo culture and GWRQ peptide treatment.
Controlled ovarian stimulation and oocyte recovery has been described in ref. 38. Cumulus–oocyte complexes were collected from anesthetized animals by laparoscopic follicular aspiration [28–29 h after human chorionic gonadotropin (hCG)] and placed in Hepes-buffered modified Tyrode solution with albumin, lactate, and pyruvate (TALP) medium (39) containing 0.3% BSA (TH3) at 37°C. Oocytes stripped of cumulus cells by mechanical pipetting after brief exposure (<1 min) to hyaluronidase (0.5 mg/ml) were placed in chemically defined, protein-free hamster embryo culture medium (HECM)-9 medium (40) at 37°C in 5% CO2, 5% O2, and 90% N2. Fertilization by intracytoplasmic sperm injection and embryo culture were performed as described in ref. 16. Embryos at the 8-cell stage were transferred to fresh plates of HECM-9 medium supplemented with 5% FBS (HyClone, Logan, UT) and cultured until hatching with a medium change every other day. After hatching, blastocysts were placed into cell culture-treated chamber slides (Nunc, Roskilde, Denmark) and allowed to adhere. After adhesion, half of the embryos were treated with 5 μg/ml GWRQ-MAPS for 2 days, and the rest were cultured as negative controls in HECM-9 plus 10% FBS. After treatment, the embryos were fixed and DAPI-stained, and cell outgrowths were analyzed.
SiRNA for Trophinin and Bystin.
SiRNA duplexes targeting trophinin and bystin were designed and synthesized by Ambion (Austin, TX). Targeted sequences were 5′-GCAGUCAAGGCAGUUAUGGtt-3′ for TRO (siRNA ID no. 290172) and 5′-GGUAUUAUCUAAGUACCGCtt-3′ for BYSL (siRNA ID no. 14156). Silencer negative control no. 1 (Ambion) served as negative control. HT-H cells were transfected with siRNA by using X-treme reagent (Roche, Indianapolis, IN). Briefly, 500 μl of Opti-MEM was mixed with 12 μl of X-treme (Roche), and then 12 μl of SiRNA (50 μM in water) was diluted with 500 μl of Opti-MEM. Diluted siRNA and X-treme were combined and added to HT-H cells cultured on 6-cm plates. After culturing in medium containing 10% FCS for 20 h, cells were cultured an additional 48 h in medium without FCS before being subjected to Western blot by using anti-trophinin antibody and anti-bystin antibody (11) and a phosphorylation stimulation assay using GWRQ peptide and HB-EGF.
Supplementary Material
Acknowledgments
We thank Dr. Elise Lamar for editing the manuscript and Drs. Eileen Adamson and Gen-sheng Feng for comments. This study was supported by National Institutes of Health Grant HD34108 and Department of Defense Grant W81XWH-04-1-0917.
Abbreviations
- EGFR
EGF receptor
- HB-EGF
heparin-binding EGF-like growth factor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611516104/DC1.
References
- 1.Enders AC, Blankenship TN. Adv Drug Deliv Rev. 1999;38:3–15. doi: 10.1016/s0169-409x(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 2.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 3.Graczykowski JW, Mishell DRJ. In: Mishell's Textbook of Infertility, Contraception, and Reproductive Endocrinology. Lobo RA, Mishell DRJ, Paulson RJ, Shoupe D, editors. Cambridge, MA: Blackwell Scientific; 1997. pp. 623–637. [Google Scholar]
- 4.Schlabritz-Loutsevitch NE, Hubbard GB, Frost PA, Cummins LB, Dick EJ, Jr, Nathanielsz PW, McDonald TJ. J Med Primatol. 2004;33:55–59. doi: 10.1046/j.1600-0684.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 5.Paria BC, Reese J, Das SK, Dey SK. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 6.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 7.Hertig AT, Rock J. Gynecol Invest. 1973;4:121–139. doi: 10.1159/000301716. [DOI] [PubMed] [Google Scholar]
- 8.Enders AC. Res Reprod. 1976;8:1–2. [PubMed] [Google Scholar]
- 9.Padykula HA. In: Histology. Weiss L, editor. Amsterdam: Elsevier Biomedical; 1983. pp. 966–999. [Google Scholar]
- 10.Fukuda MN, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, Nozawa S. Genes Dev. 1995;9:1199–1210. doi: 10.1101/gad.9.10.1199. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Nakayama J, Shih IM, Aoki D, Nozawa S, Fukuda MN. Biol Reprod. 1999;60:621–627. doi: 10.1095/biolreprod60.3.621. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda MN, Nozawa S. Semin Reprod Endocrinol. 1999;17:229–234. doi: 10.1055/s-2007-1016230. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama J, Aoki D, Suga T, Akama TO, Ishizone S, Yamaguchi H, Imakawa K, Nadano D, Fazleabas AT, Katsuyama T, et al. Am J Pathol. 2003;163:2211–2219. doi: 10.1016/S0002-9440(10)63579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki N, Zara J, Sato T, Ong E, Bakhiet N, Oshima RG, Watson KL, Fukuda MN. Proc Natl Acad Sci USA. 1998;95:5027–5032. doi: 10.1073/pnas.95.9.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tada J, Omine M, Suda T, Yamaguchi N. Blood. 1999;93:3723–3735. [PubMed] [Google Scholar]
- 16.Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S. Biol Reprod. 2004;71:486–493. doi: 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- 17.Yeoman RR, Gerami-Naini B, Mitalipov S, Nusser KD, Widmann-Browning AA, Wolf DP. Hum Reprod. 2001;16:1965–1969. doi: 10.1093/humrep/16.9.1965. [DOI] [PubMed] [Google Scholar]
- 18.Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 19.Paria BC, Elenius K, Klagsbrun M, Dey SK. Development (Cambridge, UK) 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- 20.Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ, Barlow DH, Sargent IL, Mardon HJ. Mech Dev. 2002;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 21.Nadano D, Nakayama J, Matsuzawa S, Sato TA, Matsuda T, Fukuda MN. Biochem J. 2002;364:669–677. doi: 10.1042/BJ20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komuro A, Nagai M, Navin NE, Sudol M. J Biol Chem. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 23.Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 24.Pierce KL, Luttrell LM, Lefkowitz RJ. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- 25.Das SK, Wang X-N, Paria BC, Damm D, Abraham JA, Klagsburn M, Andrews GK, Dey SK. Development (Cambridge, UK) 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 26.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Development (Cambridge, UK) 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 27.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 28.Nadano D, Sugihara K, Paria BC, Saburi S, Copeland NG, Gilbert DJ, Jenkins NA, Nakayama J, Fukuda MN. Biol Reprod. 2002;66:313–321. doi: 10.1095/biolreprod66.2.313. [DOI] [PubMed] [Google Scholar]
- 29.Patel SD, Chen CP, Bahna F, Honig B, Shapiro L. Curr Opin Struct Biol. 2003;13:690–698. doi: 10.1016/j.sbi.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, Yin M, Wu X, Wu C, Yang S, Sheng J, Ni H, Fukuda MN, Zhou J. Eur J Neurosci. 2006;23:2265–2276. doi: 10.1111/j.1460-9568.2006.04782.x. [DOI] [PubMed] [Google Scholar]
- 31.Saburi S, Nadano D, Akama TO, Hirama K, Yamanouchi K, Naito K, Tojo H, Tachi C, Fukuda MN. J Biol Chem. 2001;276:49378–49389. doi: 10.1074/jbc.M108584200. [DOI] [PubMed] [Google Scholar]
- 32.Hatakeyama S, Ohyama C, Minagawa S, Inoue T, Kakinuma H, Kyan A, Arai Y, Suga T, Nakayama J, Kato T, et al. Cancer Res. 2004;64:4257–4262. doi: 10.1158/0008-5472.CAN-04-0732. [DOI] [PubMed] [Google Scholar]
- 33.Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A. Nat Genet. 2005;37:382–390. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- 34.Ruoslahti E, Reed JC. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 35.Karin M, Hunter T. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda MN, Ohyama C, Lowitz K, Matsuo O, Pasqualini R, Ruoslahti E, Fukuda M. Cancer Res. 2000;60:450–456. [PubMed] [Google Scholar]
- 37.Koivunen E, Wang B, Ruoslahti E. J Cell Biol. 1994;124:373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL. Hum Reprod. 1995;10:1658–1666. doi: 10.1093/oxfordjournals.humrep.a136151. [DOI] [PubMed] [Google Scholar]
- 39.Bavister BD, Yanagimachi Biol Reprod. 1977;16:228–237. doi: 10.1095/biolreprod16.2.228. [DOI] [PubMed] [Google Scholar]
- 40.McKiernan SH, Bavister BD. Hum Reprod. 2000;15:157–164. doi: 10.1093/humrep/15.1.157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.