Abstract
An existing attentional-associative model of classical conditioning (Schmajuk, Lam, and Gray, 1996) is applied to the description of reinstatement in animals and humans. According to the model, inhibitory associations between the context (CX) and unconditioned stimulus (US) are formed during extinction, which help preserve the association between the conditioned stimulus (CS) and the US. However, summation and retardation tests fail to reveal these associations because (a) the CX is not attended or (b) a CX-CS configural stimulus formed during extinction is both poorly attended and weakly active during testing. When US presentations and testing occur in the same context, reinstatement is the consequence of a decreased CX inhibition and the increased attention to the CS, which activates the remaining CS-US association. When US presentations occur in the context of extinction but the CS is tested in a different context, reinstatement results from an increased attention to the CS and the combination of CS-CX and CX-US excitatory associations. The assumption that associations between CSs are impaired following neurotoxic hippocampal lesions or in amnesia, is sufficient to describe absence of reinstatement in those cases.
Keywords: classical conditioning, amygdala, medial temporal lobe, implicit memory, reinstatement, emotion
Fear is a rudimentary emotion that promotes survival by engaging defensive responses to threatening cues in the environment. Organisms have evolved robust brain mechanisms to evaluate the threat value of sensory stimuli and engage fight-or-flight reactions accordingly. To facilitate adaptive behavior, it is important to learn the predictive relationship among stimuli and reinforcers and to remember their sensory features and spatial locations. However, because the recruitment of defensive reflexes is metabolically expensive, it is also important to learn when environmental contingencies change so that aversive outcomes are no longer likely. Such knowledge safeguards an organism from an unnecessarily prolonged state of anxiety. Research on Pavlovian (classical) fear conditioning has revealed ways in which the signal value of external stimuli are related to each other and to the environmental context in order to flexibly engage protective stress responses. Although the brain is wired to readily acquire conditioned fear, extinguishing prior threat associations is a more fragile process that requires new learning and is susceptible to contextual influences (reviewed in Bouton, 1993; 2002; Sotres-Boyen, Bush, & LeDoux, 2004). Given their importance in the treatment of anxiety disorders, the factors that contribute to the re-emergence of latent conditioned fear associations following extinction training have come under intense investigation in recent years.
Bouton (1993) has described several experimental approaches that illustrate the recovery of extinguished fears. One of these, called reinstatement, was originally described by Pavlov (1927), further developed by Rescorla and Heth (1975), and later elaborated by Bouton and Bolles (1979a). In a reinstatement procedure, extinguished responding to a conditioned stimulus (CS) is recovered by giving a few presentations of the unconditioned stimulus (US) in a particular environmental context (CX). If the US is presented in the CX of extinction, the conditioned response (CR) to the CS in that CX returns to levels previously seen during the original acquisition phase of learning. The fear response then re-extinguishes with subsequent unreinforced repetitions of the CS. In contrast, if the US is presented in a CX different from that of extinction, the CR does not recover in the extinction CX. Bouton (1993) has argued that following extinction, the animal has two memory traces that compete to guide behavior – the reinforced (CS-US) ‘fear’ associations from acquisition training and the unreinforced (CS-alone) ‘safety’ associations from extinction training. Although the extinction procedure lays down a retroactive inhibition of the original CS-US association, if the CS is subsequently encountered in a fearful CX, retrieval of the original fear association is favored. In this way, current contextual cues help to disambiguate the meaning of the CS, which has been associated with two predictive outcomes.
Like Bouton and Bolles (1979a), Westbrook et al. (2002, Experiment 2) also reported reinstatement by presenting the US and testing the CS in the same contexts. However, they also reported reinstatement by presenting the US in the context of extinction and testing the CS in a different context, and explained this result in terms of the combination of CS-CX and CX-US excitatory associations.
Lesion studies in rodents have shown that the CX-dependent reinstatement of fear in this procedure depends on the integrity of the hippocampus. Wilson, Brooks, and Bouton (1995) found that fornix transection selectively reduced fear reinstatement but did not impact the original acquisition or extinction training. A similar result was found in rats with neurotoxic lesions to the hippocampus by Frohardt, Guarraci, and Bouton (2000). In contrast, Fox and Holland (1998) showed no effect of hippocampal neurotoxic lesions on reinstatement when animals were trained with an appetitive US. This latter result may reflect a selective role of the hippocampus in reinstatement of fear responses, although other differences (e.g., duration of the inter-trial interval, food provided both in the training cage and home cage) in experimental procedures could account for this discrepancy.
Recently, contextual fear reinstatement procedures have been adapted for use in humans. Hermans et al. (2005) examined fear reinstatement in healthy adults using a differential conditioning protocol and verbal reports of US expectancy, fear ratings and reaction times as dependent measures. After extinction training, four US-alone presentations yielded subsequent recovery of fear to the CS as indexed by the verbal reports but not by reaction time measures. A control group that did not receive the US-alone presentations exhibited no fear reinstatement, which ruled out spontaneous recovery (i.e., re-emergence of the CR when the CS is tested some time after extinction [Pavlov, 1927]) as an alternate account for the results. This study, however, did not measure fear responses physiologically and did not determine whether the fear reinstatement was context-specific.
In a series of experiments, LaBar and Phelps (2005) demonstrated the context-specificity and hippocampal dependence of fear reinstatement in humans using skin conductance response (SCR) as a dependent measure. In Experiment 1, participants underwent acquisition and extinction of a visual CS- auditory US association. Following extinction, four presentations of the US were given. Subsequent presentations of the CS resulted in fear recovery but only for participants who experienced all experimental phases in the same environmental CX. Participants who experienced the post-extinction US presentations in an irrelevant CX did not show fear recovery to the CS. Experiment 2 used a differential conditioning procedure to show that fear reinstatement was specific to the CS paired with the US (CS+) and not to an unreinforced control stimulus (CS−). Experiment 3 replicated Experiment 1 but used a shock US. In addition, two amnesic patients with hypoxic damage to the hippocampus showed normal fear acquisition and extinction but did not exhibit fear recovery, despite being tested in the same CX. These initial findings in humans suggest that the CX- and hippocampal-dependency of fear reinstatement are conserved across species.
Despite the importance of this behavioral approach to the study of fear recovery following extinction, most existing theories of classical conditioning can describe only some aspects the phenomenon. The goal of the present study was to provide a mechanistic account of fear reinstatement through application of the neural network model of classical conditioning introduced by Schmajuk, Lam, and Gray (SLG, 1996), which also describes challenging experimental results that alternative models fail to explain (Schmajuk and Larrauri, 2006), as well as the results of behavioral manipulations following extinction (Larrauri and Schmajuk, submitted). Furthermore, by introducing changes in the computation of some of its variables, Buhusi, Schmajuk, and Gray (1998) showed that the SLG model can describe many of the effects of hippocampal dysfunction on classical conditioning.
In the present study, we simulated behavioral and lesion data from rats and humans to determine if the CX- and hippocampal-dependency of fear reinstatement could be understood in terms of attentional-associative principles, and their alteration after hippocampal lesions. We applied the model to the simulation of Bouton and Bolles’ (1979a; Experiments 1 and 2) data using normal rats, Westbrook et al.’s (2002; Experiment 2) data using normal rats, Frohardt et al.’s (2000; Experiment 1) experimental results using rats with hippocampal lesions, and LaBar and Phelps’ (2005; Experiment 3) data using normal humans and amnesic patients. In addition, we simulated results reported by Rescorla and Cunningham (1977) and Bouton and King (1983) regarding the associative properties of the participating stimuli in different phases of reinstatement. Through this modeling effort, new insights can be gained concerning the mechanisms underlying the return of fear following extinction training, which could have important implications for understanding relapse in anxiety disorders.
Method
The Schmajuk, Lam and Gray (1996) model of storage and retrieval
Schmajuk, Lam, and Gray (SLG, 1996; Schmajuk and Larrauri, 2006) proposed a model of classical conditioning that incorporates and extends the properties of several previous models. The SLG model includes (1) a recurrent system that stores between-CS and CS-US associations and permits the generation of inferences, (2) a real-time attentional variable regulated by the novelty of the US, the CSs, and the CX, and (3) a competitive rule that describes CS-US and between-CS (i.e., CS-Other CS, CX-CS and CS-CX) associations. A formal description of the model as a set of differential equations is presented in Schmajuk et al. (1996). The model can describe numerous classical conditioning paradigms (Schmajuk et al., 1996; Schmajuk, 1997; Schmajuk and Larrauri, 2006).
As shown in Figure 1, the SLG network includes:
Figure 1. Block diagram of the Schmajuk-Lam-Gray (SLG; 1996) network.
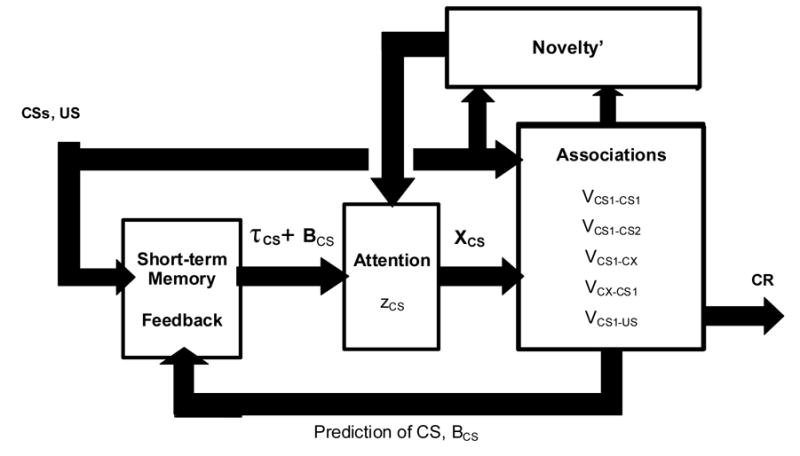
CS = conditioned stimulus; US = unconditioned stimulus; τCS = short-term memory trace of the CS; BCS = prediction of the CS; zCS = attentional memory; XCS = internal representation of the CS; VCS1-CS1, V CS1-CS2, …,VCS1-US = associations CS1-CS1, CS1-CS2, …, CS1-US; CR = conditioned response.
1. Short-term Memory and Feedback
In order to allow a CS to establish associations with other CSs or the US even when separated by a temporal gap (e.g., trace conditioning), a CS activates a short-term memory trace, τCS, which increases over time to a maximum when the CS is present and then gradually decays back to its initial value when the CS is absent.
The output of the Short-term Memory and Feedback Block in Figure 1 is proportional to τCS + BCS, where BCS is the prediction of the CS by itself, other CSs, and the CX. This output is active either in the presence of a CS or when an absent CS is predicted by other CSs or the CX.
2. Attention
The model assumes that organisms respond to novelty by increasing attention to environmental stimuli (Gray, 1971). In order to increase attention to the CSs when novelty is sensed, the output of the feedback system (τCS + BCS) becomes associated with the normalized value of total novelty detected in the environment, Novelty’. Attentional memory, zCS, reflects the association between the output of the feedback system and Novelty’. The initial value of zCS is zero. When Novelty’ is relatively large, zCS increases; and when Novelty’ is small, zCS decreases. The output of the Attentional Block in Figure 1 is proportional to XCS = zCS (τCS + BCS). It is assumed that attention zCS always lags behind Novelty’.
Notice that because the output of the feedback system is proportional to τCS + BCS, even when CS is absent but predicted by other CSs through BCS, zCS becomes modified by the value of Novelty’.
3. Associations
The model assumes that organisms build an internal model of their environment (Sokolov, 1960). In the network, environmental regularities are stored in the associative system as (1) associations of each XCS with its corresponding CS, VCS1-CS1, (2) associations between XCS with other CSs, VCS1-CS2, (3) associations of XCS with the context (CX), VCS1-CX, (4) associations of XCX of the CX with the CS, VCX-CS1, and (5) associations of XCS with the US, VCS1-US. Presentation of a CS or a CX will increase the activity of the representations of their associated CS, CX, or US if the associations are excitatory. If the associations are inhibitory, those presentations decrease the activity of the representations of their associated CS, CX, or US. The activity of the representations of CS, CX, and US are regarded as aggregate predictions of the CSs (BCS), the CX (BCX), and the US (BUS) by all CSs and CX active at a given time. The prediction of the US, BUS, controls the CR.
Note that because the τCS + BCS output is active when the absent CS is predicted by other CSs or the CX, associations VCS1-CS2, VCS1-CX, and VCS1-US can change even in its absence.
Storage
As in the Rescorla-Wagner (R-W) (1972) model, changes in associations are proportional to the difference between predicted and real values of the CS or US, (CS - BCS) or (US - BUS). However, in our model BCS and BUS do not assume negative values and, therefore, conditioned inhibitors are not extinguished by non-reinforced CS presentations. Because the rate of change of every association is directly proportional to XCS, XCS controls the storage (formation or read-in) of the associations.
Retrieval
Since the magnitude of the aggregate predictions BUS and BCS is proportional to XCS, XCS also controls the retrieval of those associations. Because attentional memory zCS controls the magnitude of the internal representation XCS, attention controls storage and retrieval of between-CS and CS-US associations.
Simultaneous control of memory storage and retrieval is a most important feature of the SLG model. This property makes the SLG model different from most other models of classical conditioning in which attention controls only the storage of associations.
4. Novelty’
The novelty system computes the total novelty, Novelty’, present in the environment at a given time. The novelty of a CS, CX or US is computed as the absolute value of the difference between the average observed value of the CS, CX or US, and the average of the sum of all predictions of that CS, CX or US, by all active CSs and CXs. Total novelty, Novelty’, is given by the sum of the novelty of all stimuli present or predicted at a given time, normalized between 0 and 1.
5.CR strength
As mentioned, the CR is proportional to the prediction of the US, BUS. Because the model assumes a threshold for responding, the CR can be zero even if the CS-US is greater than zero. In purely appetitive or aversive behaviors the model assumes that because the subject orients towards the novel stimulus, this orienting response (OR) decreases the strength of the CR. For simplicity, simulations presented in this paper do not include inhibition by the OR.
How the model explains reinstatement
The model explains reinstatement in the following terms. During acquisition, the target CS and the CX compete to gain association with the US. Because the CS is active for a limited period of time, but the CX is assumed to be active for the whole intertrial interval (ITI), CS-US associations are strong and CX-US associations weak by the end of conditioning. During extinction, the initially strong CS-US association decreases and the initially weak CX-US association becomes inhibitory, which results in the attenuation of the CR. Because CX-US associations are inhibitory, the remaining excitatory CS-US associations are protected from extinction (Chorazyna, 1962). Attention to both the CS and the CX is small at the end of extinction. Presentations of the US in the CX (in the absence of the CS) increase attention to the CX and yield an excitatory CX-US association and the consequent reinstatement of the CR. Also, because the image of the CS is evoked by the CX, attention to the CS also increases. During testing, elimination of the CX-US inhibitory association and an increased attention to the CS translate into a relative large CR. In certain cases, CS-CX associations formed during extinction combine with CX-US associations formed during US presentations in the CX, to contribute to the generation of the CR.
Suppression ratios
Some of the data analyzed in the present study were collected using a conditioned emotional response paradigm (Bouton & Bolles, 1979a; Frohardt et al., 2000). In order to compare experimental and simulated results, we transformed CR strengths provided by the model into suppression ratios. Suppression ratios were calculated with the equation A / (A + B), were A represents the appetitive responding (e.g., bar pressing for food, water licking) when the CS is present and B represents the appetitive responding during the preceding non-CS period of equal duration. We assume that responding during the CS period is given by β − CR(CS), and responding during the preceding non-CS period is given by β − CR(CX), where β is proportional to the intensity of the appetitive behavior. Therefore, the suppression ratio was calculated by (β − CR(CS)) / ((β − CR(CX)) + β− CR(CS)) = (β − CR(CS)) / (2 β − CR(CS)−CR(CX)). In order to avoid unrealistic negative suppression ratios, the value of β was arbitrarily set to the maximum value of the CR(CS). Notice that when V(CX,US) ~ 0 or < 0, then the suppression ratio is well approximated by (β − CR(CS)) / (2 β − CR(CS)).
Effects of neurotoxic hippocampal lesions
A number of studies seem to indicate that selective excitotoxic hippocampal lesions (Talk, Gandhi, and Matzel, 2005; but see Ward-Robinson et al., 2001), fimbrial lesions (Port and Patterson, 1984), and kainic lesions of hippocampal CA1 (Port, Beggs, and Patterson, 1987) impair the acquisition of between-CS associations. In the framework of the SLG model, Buhusi et al. (1998) described the effect of these lesions by assuming that between-CS associations, presumably stored in cortical areas, remain zero. They also assumed that associations of the CS with itself, which produce habituation to the CS, are modified when the CS is perceived. Under these assumptions, the model is able to explain the apparently contradictory effects (i.e., impairment, preservation, and facilitation) of hippocampal lesions on latent inhibition. Furthermore, Pothuizen et al. (2006) recently confirmed the predictions generated by the model regarding the facilitation of latent inhibition of conditioned taste aversion by selective lesions of a brain region that receives hippocampal input, i.e., the shell of the nucleus accumbens.
Also, in support for these assumptions, the marked deficits shown by amnesic patients in learning unrelated word pairs (Shimamura and Squire, 1984) can be represented by impairments in between-CS associations.
Parameters
Parameter values used in our simulations were identical to those used by Schmajuk and Larrauri (2006) and Larrauri and Schmajuk (submitted). These values are similar to those used in Schmajuk et al. (1998), Schmajuk et al. (1996), Buhusi et al. (1998), and Schmajuk et al. (2001), with exception of the parameter that controls an inhibitory effect of the OR on the CR, which was set to zero in order to avoid incorrect results when suppression ratios are used (see Schmajuk and Larrauri, 2006, for a complete discussion).
In our simulations, several tonic contextual stimuli were used to represent the contexts in which the animals are placed: (a) CXh represents the home cage where animals are housed during the interval between different daily sessions, (b) different CXc’s represent the features of the different operant chambers, and (c) CXg is used to represent the common features to both chambers and the home cage, that is, the generalization between all contexts. We used CXh=.5 and CXg=.4 in all simulations, but CXc varied according to the training apparatus used in the different experiments.
Results
Rat data from two experimental papers were simulated. First, data from Bouton and Bolles (1979a; Experiments 1 and 2) were simulated to account for fear reinstatement when the CS is tested in the context in which the US was presented after extinction. Second, data from Westbrook et al. (2002; Experiment 2) were simulated to account for reinstatement when the CS is tested in a context different from the one in which the US was presented. Third, lesion data were simulated from Frohardt et al. (2000; Experiment 1) to account for the consequences of hippocampal damage on fear reinstatement. Finally, data from LaBar and Phelps (2005; Experiment 3) were simulated to account for consequences of context shifts and hippocampal lesions in humans.
Fear reinstatement when the CS is tested in the context in which the US was presented
Experimental data
In Bouton and Bolles (1979a, Experiment 1), conditioning took place in CXB, extinction in CXA, US exposure in CXA or CXB, and testing in CXA. Conditioning took place in conditioning boxes, CXB, and consisted of 15 CS-US presentations in one day, with a 60-sec tone CS followed by a .5 sec footshock in the Test Context (TC) group, or preceded by the footshock in the Backward Conditioning (BC) group. Extinction took place in the Skinner box, CXA, for 4 days. Whereas Groups TC and BC received 4 US presentations in CXA, Group CC received 4 US presentation in CXB. Finally, testing took place in CXA for 3 trials. As shown in Figure 2 (Top Panel), whereas rats in the BC and CC groups show large suppression ratios (no responding), rats in the TC group showed smaller suppression ratios (strong responding) to the CS. Bouton and Bolles (1979a, Experiment 1) concluded that reinstatement was obtained when the US was presented in the context in which testing occurs (AAA sequence) but not when the US was presented in a different context (ABA sequence).
Figure 2. Reinstatement of conditioned fear in rats.
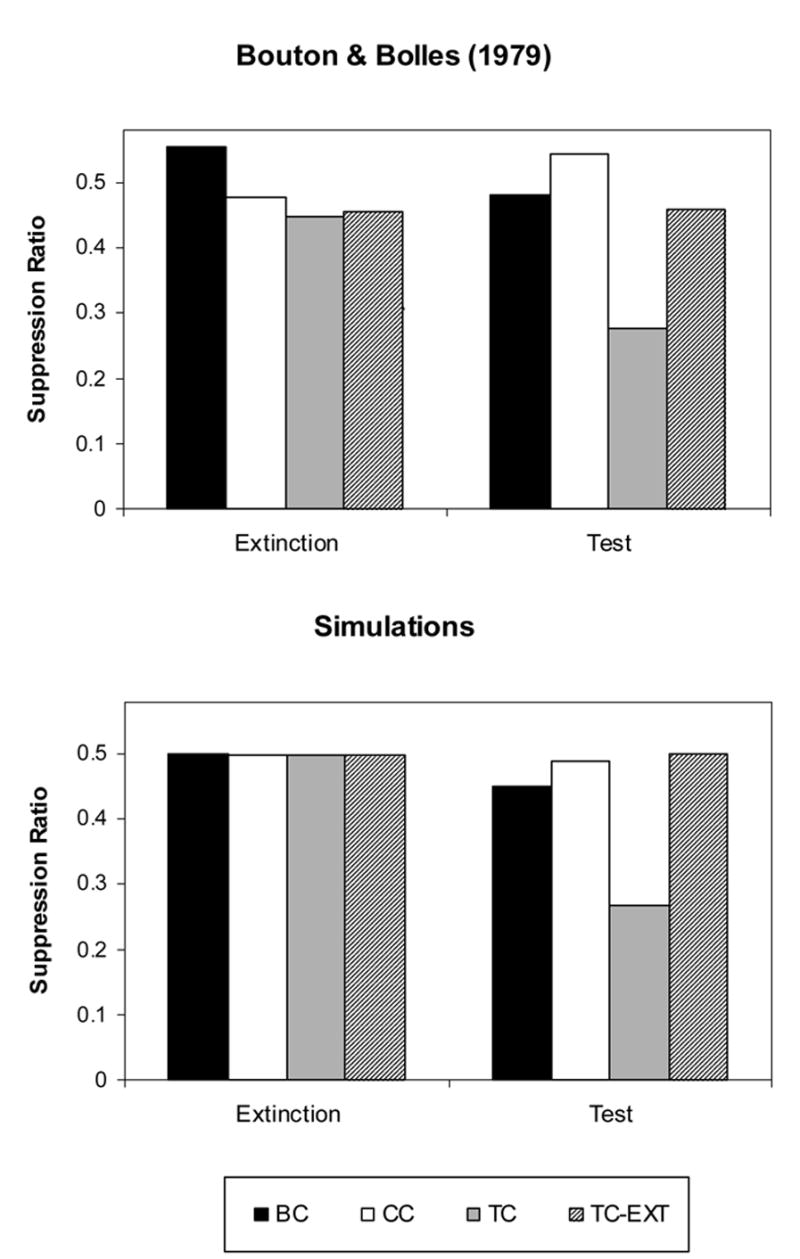
Data indicate suppression ratios in different conditions. BC = backward conditioning, TC = reinstatement, CC = control group, TC-Ext = reinstatement and extinction group. Top Panel: Data from Bolles and Bouton (1979a, Experiments 1 and 2). Bottom Panel: Computer simulations. Average Suppression Ratio over 10 test trials.
Bouton and Bolles (1979a, Experiment 2) also reported that the interpolation of non-reinforced exposure to the context between reinstatement and testing resulted in the elimination of reinstatement. In their experiment, rats in the TC-Extinction group received the same treatment described above for the TC group, but extinction trials in the Skinner box (Context A) were interspersed between reinstatement and testing stages. Testing consisted of 4 trials. As shown in Figure 2 (Top Panel), the TC-Extinction group shows weak responding to the CS. As explained below, reinstatement can be also eliminated by presentation of another reinforced CS following reinstatement trials (Rescorla and Cunningham, 1977).
Some experiments have explored the associations formed during the different phases of the reinstatement experiment. Following extinction, Bouton and King (1983, page 253) found that responding to an excitatory CS in the extinction context was not significantly different from responding to that CS in a neutral context. Based on the results of a summation test, they concluded that the context of extinction had not become inhibitory. In addition, Richards and Sargent (1983) showed that the rate of extinction to a given CS was not significantly affected by the prior extinction of other CSs, a result that suggests that the context does not become inhibitory during extinction. Following the reinstatement phase, Bouton and Bolles (1979a, page 376) reported that contextual associations with the US were not detected by bar-pressing rates in the absence of the CS. However, such excitatory contextual associations were discovered using a context-preference test (Bouton and King, 1983).
Simulation results
Our simulations included 7 CS-US acquisition trials (Groups CC, TC, and TC-EXT) or 7 US-CS trials (Group BC) in CXB (conditioning box), 25 CS extinction trials in CXA (Skinner box), and 5 US trials in CXB (Group CC) or in CXA (Groups TC, TC-EXT and BC). Group TC-EXT received 20 trials of exposure to CXA, whereas Groups CC and TC spent an equivalent amount of trials in the home cage. The salience of CXA (Skinner box) was set to CXc=.5 and CXg=.4. The salience of CXB (Conditioning box) was set to CXc=.1 and CXg=.4. The CS had a 10 t.u. duration and salience .8, the US was 5 t.u. long with salience 2, the interstimulus interval (ISI) was 5 t.u., and the ITI was 100 t.u. Simulations of 20 home-cage trials (CXh=.5, CXg=.4) were included between the different sessions.
As shown in Figure 2 (Bottom Panel), in agreement with Bouton and Bolles (1979a, Experiment 1), whereas simulations for the BC and CC groups show large suppression ratios (no conditioning), simulations for the TC group showed relatively small suppression ratios (strong responding) to the CS. In addition, in agreement with Bouton and Bolles (1979a, Experiment 2), the TC-EXT group shows absence of reinstatement.
According to the model, extinction in CXA results in a decreased CS-US and increased inhibitory CXA-US associations and, consequently, in the absence of a CR. As mentioned, because CXA-US associations become inhibitory, the remaining excitatory CS-US associations are protected from extinction. Whereas subsequent presentations of the US in CXA yield an excitatory CXA-US association and the consequent generation of a CR, US presentations in CXB leave intact those inhibitory associations, which continue to hinder the generation of the CR in CXA. In addition to these associational mechanisms, attentional mechanisms also play a role. During US presentations in CXA, attention to CXA increases due to the absence of the CS, which makes possible the formation of excitatory CXA-US associations. During testing, Novelty’ and attention to the CS increase in the TC group because the US, presented in CXA during the previous phase of the experiment, is now expected but not present in that context. Instead, Novelty’ decreases in the CC group because the US presented during reinstatement in CXB is not expected to be present in CXA. Therefore, a larger CXA-US association combined with an increased attention to the CS in CXA translates into larger CR in the TC than in the CC group.
Absence of reinstatement in the group TC-EXT is explained as follows. Nonreinforced presentations in CXA decrease the CXA-US excitatory associations. However, in the absence of the excitatory CS-US association, the CXA-US association cannot become inhibitory (as it had in extinction in the presence of the CS) and, therefore, cannot counterbalance the excitatory CS-US association during testing. According to the model, responding to the CS is weak during testing because attention to the CS decreases when CXA is presented by itself. Attention to the absent CS decreases when the animal is placed in CXA because (a) CXA activates the CS representation through CX-CS associations, and (b) Novelty’ is small due to the small difference between the weakly predicted US (the CXA-US association is weak) and the absent US. In brief, extinction of the CXA-US is not enough to bring back the situation encountered at the end of extinction, when the CX-US association was inhibitory and counterbalanced the excitatory CS-US association. Therefore, attention to the CS decreases and eliminates responding by lack of activation of the CS-US association. Also, because the CXA-US associations and attention to CXA take a number of trials to decrease, the model correctly shows that reinstatement is not affected with few extinction trials (see Bouton and Bolles, 1979a, Figure 2).
Also, as reported by Rescorla and Cunningham (1977, Experiment 1), simulations show that presentation of another, previously reinforced CS, following presentation of the US in the context, also eliminates reinstatement. According to the model, when the previously reinforced CS is presented in the reinforced CX, the aggregate prediction of the US increases and, therefore, context-US associations decrease faster than when the context is extinguished by itself.
Contextual associations
In addition to correctly describing the results reported by Bouton and Bolles (1979a, Experiments 1 and 2) and Rescorla and Cunningham (1977), the model explains results of experiments that evaluated the associations formed during the different phases of the experiment. For instance, additional simulations (Larrauri and Schmajuk, submitted) reveal why Bouton and King (1983, page 253) reported that responding to an excitatory CS in the extinction context was not significantly different from responding to that CS in a neutral context (summation test). According to the model, during extinction, Novelty’ and attention to both the CS and the context decrease. Because of the reduced attention to the context, the inhibitory association accrued by the CX does not inhibit the excitation produced by another CS associated with the US. Therefore, in agreement with Bouton and King’s results, even if the CX becomes inhibitory, it cannot inhibit responding to an excitatory CS. Computer simulations show that the CR to that CS in the context of extinction is similar to the CR to that CS even when extinction occurred in a different context. The model also reproduces Richards and Sargent’s (1983) results showing that the rate of extinction to a given CS is not significantly affected when preceded by the extinction of other CSs. In sum, a decreased attention the CX might be sufficient to explain the apparent absence of CX-US inhibitory associations.
Another possible reason why the CX-US inhibitory association is not manifested in the above experiments is that the CX forms a configural stimulus with the CS. During extinction, this configural CX-CS stimulus is fully active, inhibits the generation of the CR, and protects the CS-US association from decreasing. However, the configural CX-CS stimulus is only partially active in the absence of the extinguished CS, and therefore its inhibitory abilities are decreased. The configural CX-CS assumption is similar, but not identical, to Bouton’s (1993, 1994) notion that the extinguished CS and the CX activate an AND gate whose output becomes inhibitory during extinction but is inactive when the CX is tested alone. The advantage of the configural assumption over the AND assumption is that only the configural one allows for the formation of configural-US associations during US presentations in the CX alone and, therefore, is able to describe reinstatement.
Even though computer simulations confirm that the CX-CS configural assumption is able to describe reinstatement and the absence of contextual inhibition when the CX of extinction is tested in the absence of the extinguished CS, it is not sufficient to explain the elimination of reinstatement when presentations of the CX alone follow US presentations in that CX (Group TC-EXT). As mentioned, this result is explained in terms of both (a) the extinction of the CX-US association, and (b) a mediated (through CX-CS associations) decrease in attention to the CS.
Because the attentional assumption is sufficient to explain all the results shown above, but the addition of a configural assumption is needed to guarantee that no CX-alone inhibition exists, Larrauri and Schmajuk (submitted) suggested an experiment to decide between these alternatives. They propose to test an excitatory CS in the extinction CX after increasing attention to the CX by presenting a novel, surprising CS. In that case, the CX will show inhibition if the attentional assumption is correct, but no inhibitory power if the configural assumption is right.
Finally, Bouton and Bolles (1979a, page 376) reported that, following the reinstatement phase, contextual associations with the US were not detected by bar-pressing rates in the absence of the CS. However, Bouton and King (1983) found an aversion to the shocked context using a context-preference test. In agreement with these results, simulations show almost no suppression to the context, even when the context-US association is a relative large percent of the value of the CS-US association and would be avoided given a preference test.
Fear reinstatement when the CS is tested in a context different from the one in which the US was presented
Experimental data
Westbrook et al. (2002) extended Bouton and Bolles’ (1979a, Experiment 1) demonstration that reinstatement is present when extinction, US presentations and testing occur in the same context (AAA procedure); but not when US exposure occurs in a different context (ABA procedure). As shown in Figure 3 (Top Panel), Westbrook et al. (2002, Experiment 2) also reported reinstatement when US presentations and testing occur in the same contexts (e.g., AAA and ABB procedures), and absence of reinstatement when those contexts are different (e.g., ABA sequence). In addition, they also reported reinstatement when the US is presented in the context of extinction and tested in a different context (e.g., AAB sequence). Westbrook et al. (2002, Experiment 3) reported that Group AAB show stronger freezing than Group A–B, which did not receive US presentations, a result that demonstrates that responding in the AAB group is reinstatement (the effect of the US received in CXA) and not renewal (the effect of changing from the extinction CXA to CXB).
Figure 3. Reinstatement of conditioned fear in rats.
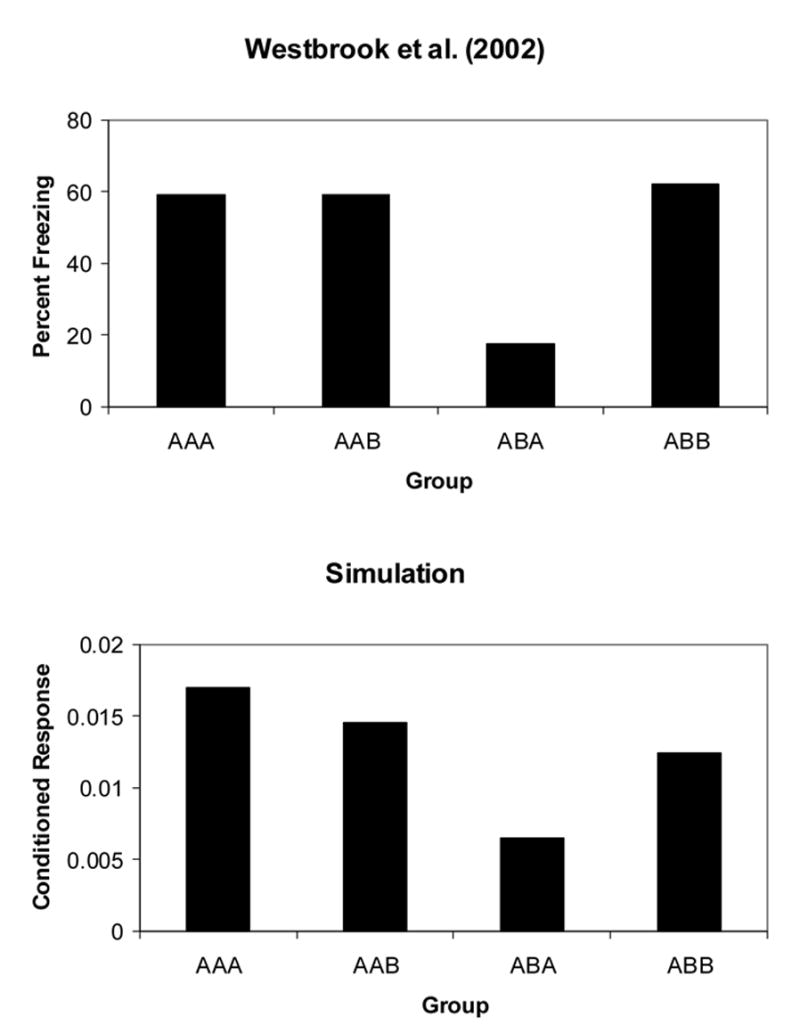
Data indicate Percent Freezing in groups conditioned and extinguished in Context A, presented with the US and tested in Contexts A or B. Top Panel: Data from Westbrook et al. (2002, Experiment 2). Bottom Panel: Computer simulations. Amplitude of the conditioned response on the first test trial.
Simulation results
Our simulations included 7 CS-US acquisition trials, 25 CS extinction trials, and 5 US presentation trials. The salience of all CXs was set to CXc=.1 and CXg=.1. The CS had a 30 t.u. duration and salience 1 the US was 5 t.u. long with salience 2, the ISI was 25 t.u., and the ITI was 100 t.u. Simulations of 20 home-cage trials (CXh=.5, CXg=.1) were included between the different sessions.
As shown in Figure 3 (Bottom Panel), in agreement with Westbrook et al (2002, Experiment 2a), whereas simulations for the AAA, AAB, and ABB groups show large freezing, simulations for the ABA group showed relatively small freezing. In addition, in agreement with Westbrook et al. (2002, Experiment 3) simulations show that whereas a group conditioned, extinguished, and presented with the US in CXA, and tested in CXB (Group AAB) shows stronger freezing than another group trained with a similar procedure with exception of the US presentation (Group AA–B). As mentioned, this result demonstrates that the strong responding in Group AAAB is reinstatement and not renewal.
Computer simulations describe two different ways to achieve reinstatement. One, when US presentations and testing occur in the same context (AAA and ABB groups), reinstatement seems to be the consequence of the elimination of CX-US inhibitory associations and the increased attention to the CS, which activates the remaining CS-US association. Two, when US presentations occur in the context of extinction but the CS is tested in a different context (AAB group) reinstatement seems to be the result of an increased attention to the CS and the combination of CS-CX and CX-US associations. In this case, the model solution is similar to Westbrook et al.’s (2002) suggestion that a combination of CS-CX associations (acquired during extinction) and CX-US associations (acquired during US presentations in the CX) mediate reinstatement.
Effect of hippocampal lesions on reinstatement after aversive conditioning
Experimental data
Frohardt et al. (2000) reported that neurotoxic hippocampal lesions eliminated reinstatement. In their experiment, rats received: (1) alternated training to criterion in two Skinner boxes (Contexts A and B) in a VI 90 schedule, (2) conditioning in CXA consisting of 8 conditioning trials during 2 days, with a 60-sec light offset CS followed by a .5 sec footshock, alternated with exposure to CXB, (3) extinction in CXA for 4 days alternated with exposure to CXB, (4) reinstatement consisting of 8 US presentations in CXA (Same group) alternated with exposure to CXB, and (5) testing in CXA for 4 trials. A control group (Different group) received 8 US presentations in CXB. As shown in Figure 4 (Top Panel), whereas sham lesioned rats in the Same group (shocks delivered in the extinction context) showed small suppression ratios (strong responding) to the CS, HP lesioned rats and sham lesioned rats in the Different group (shocks delivered in another CX) show large suppression ratios (no responding).
Figure 4. Effect of neurotoxic lesions on reinstatement in rats.
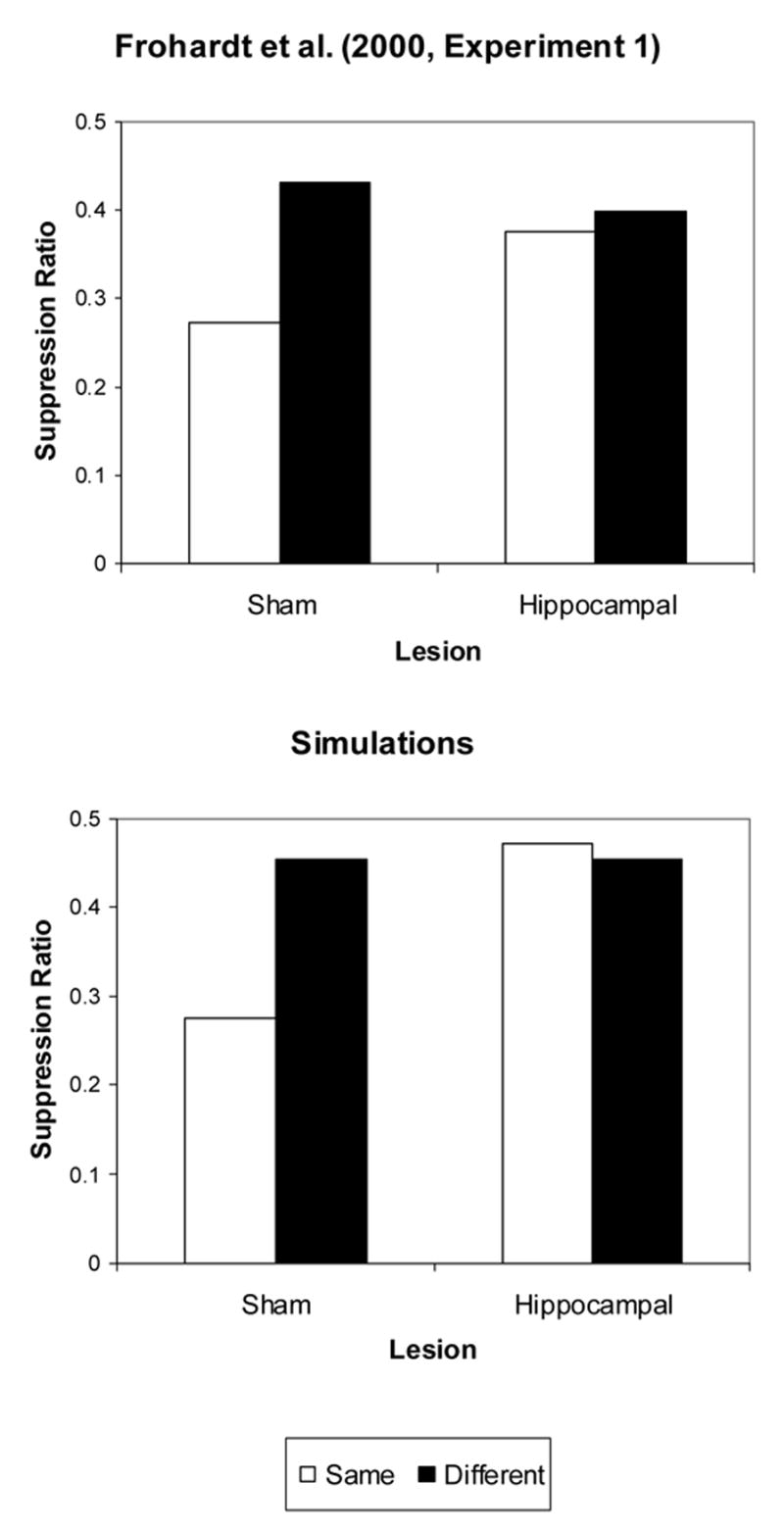
Top Panel: Data from Frohardt et al. (2000, Experiment 1) indicate suppression ratios following US presentations in the Same (TC) or Different (CC) context in animals receiving Sham or Hippocampal lesions. Bottom Panel: Computer simulations. Average Suppression Ratio over 7 test trials.
Simulation results
We applied the same simulation parameters used to simulate Bouton and Bolles (1979a, Experiment 1) under the assumption that between-CS associations are impaired by the excitoxic hippocampal lesions. As shown in Figure 4 (Bottom Panel), simulations for the Sham Same group showed a smaller suppression ratio (stronger responding) than the Sham Different and both Hippocampal groups (weak conditioning),.
According to the model, in both groups, extinction in CXA results in a decreased CS-US association and an inhibitory CXA-US association and, consequently, in the absence of a CR. However, the groups differ during the US administration in CXA. Because normal animals expect to find the CS in CXA, when the CS is absent, Novelty’ and attention to CXA increase. Consequently, the CXA-US association becomes excitatory. Instead, in the absence of CXA-CS associations, hippocampal lesion animals are insensitive to the absence of CS in CXA. Therefore, Novelty’ and attention to CXA do not increase. As a result, the CXA-US association remains inhibitory. Finally, during testing in CXA, because of the remaining CXA-US inhibitory association, hippocampal lesioned animals do not show reinstatement. In sum, because they are insensitive to the absence of the CS in CXA, hippocampal lesioned animals do not increase attention to CXA and the CXA-US association responsible for reinstatement remains inhibitory.
In addition to impairment of reinstatement, Frohardt et al. (2000, Experiment 2) also reported that neurotoxic hippocampal lesions spare renewal, i.e., the re-emergence of the CR following extinction in a different context. The model correctly describes this result. According to the model, renewal is the consequence of the formation of inhibitory associations between the context of extinction and the US, which are not active when the animal is removed from that context. Because neurotoxic lesions do not affect CX-US inhibitory associations, renewal is not expected to be impaired.
In sum, under the assumption that between-CS associations are absent after neurotoxic hippocampal lesions, the model correctly describes impaired reinstatement and preserved renewal. Interestingly, Wilson et al. (1995) reported that fornix lesions, in addition to having similar consequences on reinstatement and renewal, also preserve spontaneous recovery. This additional result is also described by model under the assumption that between-CS associations are absent. In the normal case, the model describes spontaneous recovery because Novelty’ increases due to the unpredicted re-appearance of the CS, which had been absent for some time and therefore unpredicted by the context. This increase in Novelty’ increases attention to the CS, thereby allowing for the expression of the remaining CS-US association, and the generation of a CR. In the lesioned case, spontaneous recovery is also present because Novelty’ increases when the CS is presented even in the absence of CX-CS associations. Attention increases because attention to the CX decreases during the absence of the CS (as CX-CX associations increase), making the CX unable to activate the CX-US inhibitory association and counterbalance the CS-US excitatory association. Again, this increase in Novelty’ increases attention to the CS and results in the generation of a CR.
Comparison with alternative assumptions
In contrast to our assumption that excitoxic hippocampal lesions impair the formation of between-CS associations, Frohardt et al. (2000) suggested that neurotoxic hippocampal lesions impede the formation of CX-US excitatory associations. This suggestion has received recent support from Otto and Poon’s (2006) report showing that excitotoxic lesions of the dorsal hippocampus impair contextual fear conditioning, presumably mediated by CX-US associations. Lack of a CS-US excitatory association would impair reinstatement by eliminating the effect of reinforced CX exposures, but not affect renewal because it does not depend on those associations. In agreement with these notions, computer simulations with the SLG model also show that, under the assumption that CX-US excitatory associations are absent after selective hippocampal lesions, reinstatement is abolished.
Even if the simulations generated assuming an (a) impairment of between-CS associations, and (b) CX-US excitatory associations are similar for the spontaneous recovery, reinstatement, and renewal cases, they differ in their predictions for fast reacquisition with few extinction trials and slow reacquisition with many extinction trials (Ricker and Bouton, 1996). Whereas impairment of CX-US association should not have a major effect on either fast or slow reacquisition, impairment of between-CS associations will have an impact on slow acquisition because attention to the CS and the CX will not change during the prolonged extinction. According to the model, presentation of the US during reacquisition causes the initially inhibitory CX-US association to become excitatory, thereby exposing the remaining CS-US excitatory association. When attention to the CX is small, the CX-US association will change slowly and reacquisition will be slow.
Finally, it is worth observing that, in contrast to the effect of neurotoxic hippocampal lesions and fornix lesions, muscimol infusions in the dorsal hippocampus (Corcoran and Maren, 2004), muscimol infusions in the ventral hippocampal (Hobin, Ji, and Maren, 2005) or electrolytic lesions of the dorsal hippocampus (Ji and Maren, 2005) impair (at least some types of) renewal. Such results would call for assuming both the impairment of excitatory and inhibitory CX-US associations, an assumption that might simply imply the absence of context representations following hippocampal lesions (O’Keefe and Nadel, 1978). In the absence of inhibitory CX-US associations, CS-US associations are necessarily eliminated and any form of recovery from extinction is impossible. To the extent that inhibitory CS-US associations are intact following neurotoxic hippocampal lesions (Chan, Jarrard, and Davidson, 2003), this impairment would be limited to inhibitory CX-US associations.
In sum, it is possible that a combination of impaired (a) between-CS associations and (b) excitatory and inhibitory CX-US associations is needed to correctly describe the effect of excitotoxic hippocampal lesions on both latent inhibition and post-extinction effects.
Reinstatement in humans
Experimental data
LaBar and Phelps (2005; Experiment 3) reported that reinstatement of skin conductance response was absent in two amnesic patients with hypoxic damage to the hippocampus. In this experiment, participants received: (1) 4 habituation trials of a visual CS presented alone (blue square, 4-sec duration), (2) 4 acquisition trials of the CS paired with a shock US delivered to the wrist (200-msec duration, co-terminating with the CS, 100% reinforcement), (3) 8 extinction trials of the CS alone, (4) 4 trials of re-exposure to the US alone, (5) 8 CS-alone recovery test trials. Five-minute waiting periods separated the extinction and US re-exposure phases, as well as the US re-exposure and CS recovery test phases. The ITI duration was 16 (± 2) sec throughout all experimental phases, except for the US re-exposure phase, which was 50 (± 1) sec in duration. Amnesic patients and one group of matched healthy control subjects (N = 8) were placed in the same environmental context for all phases of the study. Another healthy control group (N = 8) underwent US re-exposure in a novel (irrelevant) context.
Recovery of extinguished fear (between vertical solid lines in Figure 5, Top Panel) occurred only for control participants who underwent reinstatement in the same environmental context. Amnesics showed intact acquisition and extinction of fear during the initial training session but they did not show fear recovery following reinstatement in the same environmental context.
Figure 5. Reinstatement of conditioned fear in normal human participants and amnesic patients.
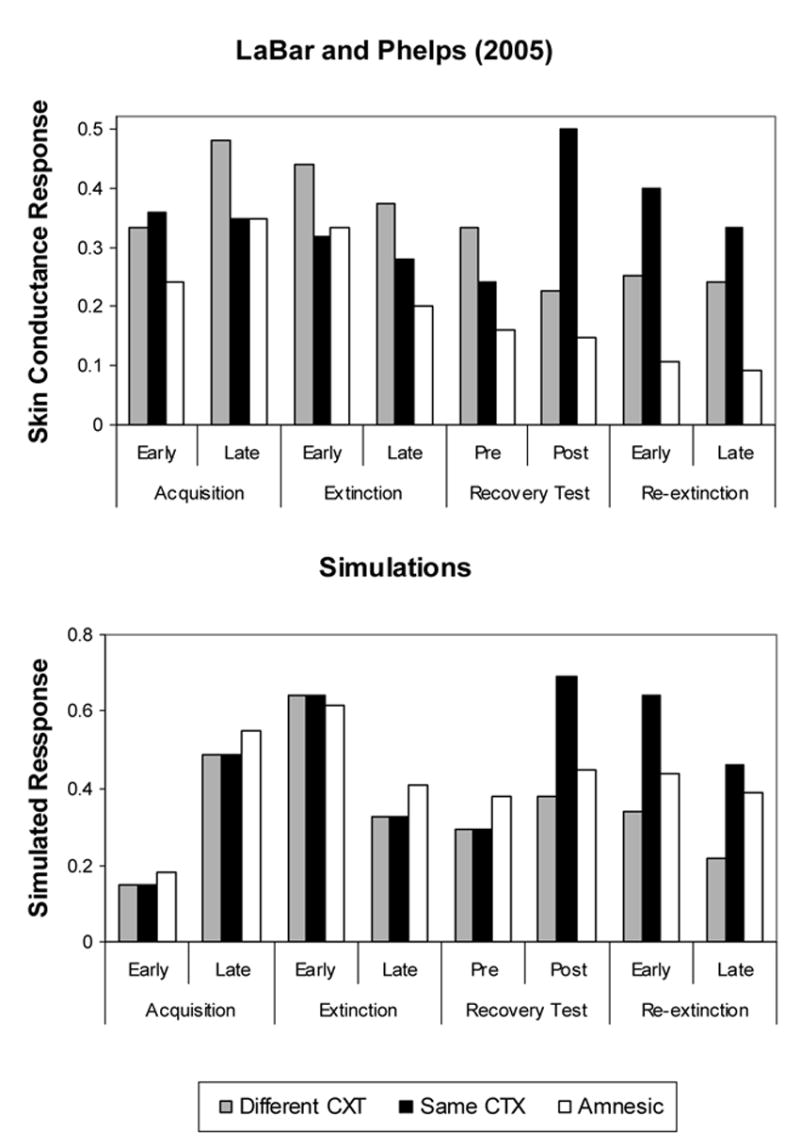
Top Panel: Normalized skin conductance conditioned responses by group and experimental phase. Data from LaBar and Phelps (2005, Experiment 3). Bottom Panel: Computer simulations. Average conditioned responses over 2 trials.
Although renewal has been demonstrated recently in humans (Milad, Orr, Pitman & Rauch, 2005; Vansteenwegen et al., 2005), its presence has not been yet assessed in amnesic patients.
Simulation results
We approximated the skin conductance response used in the experiment with the CR provided by the model. Therefore, habituation trials were not included in the simulations because, unlike the skin response, the CR starts at zero. We applied the same simulation parameters used to simulate Bouton and Bolles (1979a, Experiment 1), with exception of the number of extinction trials, which was reduced to only 8 in order to capture the incomplete extinction achieved in the LaBar and Phelps (2005) experiment.
According to the model, amnesic patients do not show reinstatement for the same reasons animals with neurotoxic hippocampal lesions fail to do it. Patients are insensitive to the absence of the CS during reinstatement trials and, therefore, do not increase attention to the CX when the US is presented in the absence of the CS, which is needed to make positive the CX-US association that is responsible for reinstatement.
Discussion
The present study shows that an existing neural network model of classical conditioning (Schmajuk et al., 1996) provides a clear mechanistic account for the different properties of reinstatement and how the phenomenon is impaired by hippocampal dysfunction. The model correctly describes that reinstatement (a) follows US presentations in the testing context (Bouton and Bolles, 1979a, Experiment 1; Bouton and King, 1983), (b) follows US presentations in the context of extinction with testing conducted in a different context (Westbrook et al., 2002), (c) is related to CX-US excitatory associations formed after reinforcement trials (Bouton and King, 1983), (d) is eliminated by exposure to the context (Bouton and Bolles, 1979a, Experiment 2), and (e) is eliminated by exposure to an independently reinforced CS (Rescorla and Cunningham, 1977). In addition, according to the model (f) the inhibitory CX-US associations formed during extinction are difficult to detect in summation tests (Bouton and King, 1983) either because the (a) CX is not attended or (b) because CX and CS form a configural stimulus which is only partially activated and poorly attended, when the inhibitory power of the context is evaluated in the absence of the CS.
Associational and attentional mechanisms involved in reinstatement
According to the SLG model, conditioned responding is determined by (a) the magnitude of CS-US and CX-US associations, and (b) the attention directed to the CS and the CX. Attention to a CS, zCS, or the CX, zCX, is proportional to the novelty detected by the subject in the environment, Novelty’. Attention can increase even when that CS (or that CX) is absent, if that CS (or CX) is predicted by other CSs or the CX. Novelty’ is proportional to the sum of the absolute values of the difference between expected and actual events. Like the Rescorla-Wagner algorithm, changes in associations are proportional to the difference between actual values of the CS and their predicted values (BCS, BUS). However, because in our model BCS and BUS do not assume negative values, conditioned inhibitors are not extinguished by CS presentations.
In a reinstatement protocol, the CX-US associations become inhibitory during extinction, which protects the excitatory CS-US associations from extinction and precludes the generation of the CR. When the US is presented in the CX following extinction, the CX-US association becomes excitatory (Bouton and King, 1983) and releases the CR during testing. Whereas the basic phenomenon can be explained in terms of associational processes, attentional mechanisms are needed to describe the results. For instance, the inhibitory context-US associations would be difficult to detect because attention to the context is very small. Also, the absence of the expected CS during US presentations in the context of testing increases Novelty’ and attention to the CX, which makes possible the formation of a CX-US excitatory association. Finally, reinstatement is eliminated by exposure to the context (or by exposure to an independently reinforced CS) because it results in a decreased CX-US association and a decreased attention to the CS. It is important to notice that, because CX-US associations cannot become inhibitory when the CX is extinguished in the absence of the CS (as it had occurred during extinction), an attentional mechanism is needed to decrease responding to the CS, in the absence of contextual inhibition.
Other simulated results also related to extinction
Larrauri and Schmajuk (submitted) have shown that the model is capable of describing, in great detail, many experimental results also related to extinction: (1) Spontaneous recovery, the re-emergence of the CR when the CS is tested some time after extinction mentioned above (Pavlov, 1927; Robbins, 1990; Rescorla, 2004; Thomas et al., 2003), (2) Renewal, the re-emergence of the CR when the CS is tested in a context different from that of extinction (Bouton and Bolles, 1979b; Bouton and Brooks, 1994; Thomas et al., 2003; Denniston et al., 2003), and (3) Reacquisition, that can be slower or faster than the original acquisition (Bouton, 1986; Bouton and Swartzentruber, 1989; Bouton et al., 2004; Napier et al., 1992; Ricker and Bouton, 1986). In addition, Larrauri and Schmajuk (submitted) reported that the model correctly describes external desinhibition (Bottjer, 1982), backward conditioning (Siegel and Domjan, 1971; Napier et al., 1992), and contextual discrimination (Bouton and Swartzentruber, 1986).
As mentioned above, according to the model, the inhibitory CX-US associations formed during extinction are difficult to detect in summation tests due to the reduced attention to the context. An alternative explanation was proposed by Bouton (1993, 1994) who suggested that the inhibitory associations are activated by the combined presentation of the CX and the CS at an AND gate. Therefore, in the absence of the CS, the CX does not appear inhibitory. Larrauri and Schmajuk (submitted) simulated an experiment that decides between the attentional and the AND gate hypotheses. In the proposed study, the experimental group would receive presentations of a novel CS, preceding the summation trials in the test context. This manipulation would increase the internal representation of context through the Novelty’ of the unexpected CS, and possibly allow for the expression of the CX-US inhibitory association. If no inhibition is detected in such summation test, the AND gate hypothesis should be favored. Larrauri and Schmajuk (submitted) pointed out that the applicability of the model is independent of this experimental result, which only decides the way the variable representing the CX is interpreted.
Besides the extinction-related phenomena, the network correctly describes (1) acquisition of delay and trace conditioning, (2) acquisition with different CS and US durations and intensities, (3) external inhibition, (4) extinction, (5) partial reinforcement with different percentages of reinforced trials, (6) conditioned inhibition, (7) nonextinction of a conditioned inhibitor by CS-alone presentations, (8) blocking, (9) the "reinforcer nonspecific" aspects of unblocking, (10) overshadowing, (11) discrimination acquisition and reversal, (12) second-order conditioning, (13) sensory preconditioning, and (14) a very large number of the different properties of latent inhibition (Schmajuk at al., 1996).
Importantly, Schmajuk and Larrauri (2006) have shown that the model describes a number of challenging experimental results showing that (a) extinction of the blocking or overshadowing stimulus results in the recovery of the response to the blocked or overshadowed stimulus, (b) backward blocking is possible, (c) backward blocking shows spontaneous recovery, (d) extinction of the training context results in the recovery from latent inhibition, (e) interposing a delay between conditioning and testing in latent inhibition results in super latent inhibition, and (f) latent inhibition antagonizes overshadowing.
Theories of reinstatement
Some of the above-described theories can account for reinstatement. For Rescorla’s (1974) theory of decreased US processing, spontaneous recovery and reinstatement are the result of the recovery of the US processing. For inhibitory theories of extinction (Konorski, 1948), reinstatement might be the result of inhibitory connections being more recent and, therefore, more changeable than the excitatory ones. For the competing memories theories (Gleitman, 1971; Spear, 1971), reinstatement is the result of the older memories of acquisition interfering with the more recent ones of extinction. For generalization decrement models (Capaldi, 1971), reinstatement is the consequence of making the situation similar to that experienced during acquisition.
The Rescorla and Wagner (1972) model explains extinction in terms of a CX-US inhibitory association and reinstatement as the result of the inhibitory CX-US association being neutralized or made excitatory during US presentations in the context of testing. Also, reinstatement is decreased, but not eliminated by nonreinforced CX presentations, because excitatory CX-US associations can be reduced to zero but not made back inhibitory. Because our model incorporates a modified, real-time competitive rule, it also addresses these data in ways similar to the Rescorla and Wagner (1972) rule. But in addition, other experimental results are explained as the consequence of attentional mechanisms. These results include the fact that reinstatement can be completely eliminated by nonreinforced presentations of the reinforced context.
Bouton (1993, 1994) suggested that (a) excitatory CS-US associations increase during acquisition, (b) CS-US associations do not decrease during extinction, (c) the CS and the spatial or temporal CX are combined into an AND gate, (d) inhibitory AND gate-US associations are formed during extinction, and (e) AND gate-US associations do not change unless both the CS and the CX are simultaneously present to activate the gate. According to Bouton (1993, 1994), reinstatement is explained because CX-US associations are part of the conditioning context, and presenting the US in the context is equivalent to returning the animal to the context of conditioning (Bouton et al., 1993). Unfortunately, in contrast with experimental data (Westbrook et al., 2002, Experiment 3), Bouton’s (personal communication) assumptions make reinstatement identical to an ABA renewal.
Westbrook et al. (2002) suggested that context plays two roles in reinstatement depending on the CX where extinction, US presentation, and testing take place. When US presentations and testing occur in the same context (Groups AAAA, AABB, ABBB, and ABCC), Westbrook et al. agree with Bouton’s view that reinstatement is the consequence of CX-US associations retrieving a CS-US memory. Instead, when the US is presented in the context of extinction but tested in a different context (Groups AAAB and ABBC), Westbrook et al. agree with Holland’s (1990) suggestion that CS-CX and CX-US associations mediate reinstatement. Interestingly, our model also describes AAAB and ABBC reinstatement through sensory preconditioning that combines CS-CX with CX-US associations.
Hippocampal dysfunction
By assuming that a deficit in the formation of CS-CX and CX-CS associations, the SLG model describes the effect of hippocampal neurotoxic lesions in animals (Frohardt et al., 2000, Experiment 1) and in human amnesics (LaBar and Phelps, 2005). According to the model, amnesic patients and animals with hippocampal lesions fail to show reinstatement because they are insensitive to the absence of the CS during US presentation trials and, therefore, do not increase attention to CX and the CX-US association responsible for reinstatement.
Conclusion
The present paper shows how an attentional-associative model of classical conditioning can describe reinstatement. According to the model, two factors are critical for the reinstatement of fear following extinction: (a) decrements of the inhibitory CX-US associations established during extinction by subsequent presentations of the US in the CX, and (b) increased attention to the CS during testing. Importantly, in the model attention is controlled by the novelty that the subjects detect in the environment.
We also show that the effect of selective lesions of the hippocampus on reinstatement is well described by the model under the assumption that between-CS associations are impaired. This assumption has been successful at describing the effect of hippocampal, and predicting the effect of accumbal, lesions on latent inhibition. In addition to impaired reinstatement, the assumption describes preservation of spontaneous recovery and renewal. Although some data seem to support these predictions, other experimental results suggest that renewal might be impaired. Therefore, it is possible that a combination of impaired (a) between-CS associations and (b) excitatory and inhibitory CX-US associations is needed to correctly describe the effect of excitotoxic hippocampal lesions on both latent inhibition and post-extinction effects.
Footnotes
Nestor Schmajuk, José Larrauri, and Kevin S. LaBar, Department of Psychology and Neuroscience, Duke University.
Correspondence concerning this article should be addressed to Nestor Schmajuk, Department of Psychology and Neuroscience, Duke University, Box 90086, Durham, NC 27708-0086. E-mail: nestor@duke.edu.
This work was supported by National Institutes of Health grant R01 DA14094 and National Science Foundation CAREER award 0236914. We thank Mark Bouton for his helpful comments on his theories and Blake London for assistance with the simulations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bottjer SW. Conditioned Approach and Withdrawal Behavior in Pigeons: Effects of a Novel Extraneous Stimulus during Acquisition and Extinction. Learning and Motivation. 1982;13:44–67. [Google Scholar]
- Bouton ME. Slow reacquisition following the extinction of conditioned suppression. Learning and Motivation. 1986;17:1–15. [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: Implications for exposure therapy. Behaviour Research and Therapy. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and classical conditioning. Current Directions in Psychological Science. 1994;3:49–53. [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of Conditioned Contextual Stimuli in Reinstatement of Extinguished Fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979a;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual Control of the Extinction of Conditioned Fear. Learning and Motivation. 1979b;10:445–466. [Google Scholar]
- Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behaviour Research and Therapy. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes, 9. 1983;24:8–265. [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Behavioral Processes. 1986;12:333–350. [Google Scholar]
- Bouton ME, Swartzentruber D. Slow reacquisition following extinction: Context, encoding, and retrieval mechanisms. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:43–53. [Google Scholar]
- Bouton ME, Woods AM, Pineño O. Occasional reinforced trials during extinction can slow the rate of rapid reacquisition. Learning and Motivation. 2004;35:371–390. doi: 10.1016/j.lmot.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:366–379. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Gray JA, Schmajuk NA. The perplexing effects of hippocampal lesions on latent inhibition: A neural network solution. Behavioral Neuroscience. 1998;112:316–351. doi: 10.1037//0735-7044.112.2.316. [DOI] [PubMed] [Google Scholar]
- Capaldi EJ. A sequential theory of instrumental training. In: Spence KW, Spence JT, editors. The psychology of learning and motivation. New York: Academic Press; 1967. pp. 67–156. [Google Scholar]
- Chorazyna H. Some properties of conditioned inhibition. Acta Biologiae Experimentalis. 1962;22:5–13. [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learning & Memory. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denniston JC, Chang RC, Miller RR. Massive extinction treatment attenuates the renewal effect. Learning and Motivation. 2003;34:68–86. [Google Scholar]
- Devenport LD. Spontaneous recovery without interference: Why remembering is adaptive. Animal Learning and Behavior. 1998;26:172–181. [Google Scholar]
- Fox GD, Holland PC. Neurotoxic hippocampal lesions fail to impair reinstatement of an appetitively conditioned response. Behavioral Neuroscience. 1998;112:255–260. [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behavioral Neuroscience. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez A, Rosas JM. Context change as the mechanism of reinstatement in causal learning. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:292–310. doi: 10.1037/0097-7403.29.4.292. [DOI] [PubMed] [Google Scholar]
- Gleitman H. Forgetting of long-term memories in animals. In: Honig WK, James PHR, editors. Animal memory. New York: Academic Press; 1971. pp. 1–44. [Google Scholar]
- Gray JA, Buhusi CV, Schmajuk NA. The Transition from Automatic to Controlled Processing. Neural Networks. 1997;10:1257–1268. doi: 10.1016/s0893-6080(97)00058-0. [DOI] [PubMed] [Google Scholar]
- Hermans D, Dirikx T, Vansteenwegenin D, Baeyens F, Van den Bergh O, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behavioural Research and Therapy. 2005;43:533–551. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2005;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning and Memory. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Conditioned reflexes and neuron organization. New York: Cambridge University Press; 1948. [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Miller R, Matzel L. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Orlando, FL: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Miller RR, Schachtman T. Conditioning context as an associative baseline: Implications for response generation and the nature of conditioned inhibition. In: Miller RR, Spear NE, editors. Information processing in animals: Conditioned inhibition. Hillsdale, NJ: Erlbaum; 1985. pp. 51–88. [Google Scholar]
- Napier RM, Macrae M, Kehoe EJ. Rapid reacquisition in conditioning of the rabbit’s nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:182–192. doi: 10.1037//0097-7403.18.2.182. [DOI] [PubMed] [Google Scholar]
- Otto T, Poon PJ. Dorsal hippocampal contributions to unimodal contextual conditioning. Journal of Neuroscience. 2006;26:6603–6609. doi: 10.1523/JNEUROSCI.1056-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Port RL, Patterson MM. Fimbrial lesions and sensory preconditioning. Behavioral Neuroscience. 1984;98:584–589. doi: 10.1037//0735-7044.98.4.584. [DOI] [PubMed] [Google Scholar]
- Port RL, Beggs AL, Patterson MM. Hippocampal substrate of sensory associations. Physiology and Behavior. 1987;39:643–647. doi: 10.1016/0031-9384(87)90167-3. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Latent inhibition of conditioned taste aversion is not disrupted, but can be enhanced, by selective nucleus accumbens shell lesions in rats. Neuroscience. 2006;137:1119–1130. doi: 10.1016/j.neuroscience.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Effect of inflation of the unconditioned stimulus value following conditioning. Journal of Comparative and Physiological Psychology. 1974;86:101–106. [Google Scholar]
- Rescorla RA. Spontaneous recovery varies inversely with the training-extinction interval. Learning and Behavior. 2004;32:401–408. doi: 10.3758/bf03196037. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Cunningham CL. The erasure of reinstated fear. Animal Learning and Behavior. 1977;5:386–394. [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;104:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner A. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Richards RW, Sargent DM. The order of presentation of conditioned stimuli during extinction. Animal Learning and Behavior. 1983;11:229–236. [Google Scholar]
- Ricker ST, Bouton ME. Reacquisition following extinction in appetitive conditioning. Animal Learning and Behavior. 1996;24:423–436. [Google Scholar]
- Robbins SJ. Mechanisms underlying spontaneous recovery in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:235–249. [Google Scholar]
- Schmajuk NA, Buhusi CV, Gray JA. The pharmacology of latent inhibition: A neural network approach. Behavioural Pharmacology. 1998;9:711–730. doi: 10.1097/00008877-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Cox L, Gray JA. Nucleus accumbens, entorhinal cortex and latent inhibition: A neural network approach. Behavioral Brain Research. 2001;118:123–141. doi: 10.1016/s0166-4328(00)00319-3. [DOI] [PubMed] [Google Scholar]
- Schmajuk N, Lam Y, Gray JA. Latent inhibition: A neural network approach. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:321–349. doi: 10.1037//0097-7403.22.3.321. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Larrauri JA. Experimental challenges to theories of classical conditioning: Application of a computational model of storage and retrieval. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:1–20. doi: 10.1037/0097-7403.32.1.1. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Paired-associate learning and priming effects in amnesia: a neuropsychological study. Journal of Experimental Psychology: General. 1984;113:556–570. doi: 10.1037//0096-3445.113.4.556. [DOI] [PubMed] [Google Scholar]
- Siegel S, Domjan M. Backward conditioning as an inhibitory procedure. Learning and Motivation. 1971;2:1–11. [Google Scholar]
- Sotres-Boyen F, Bush DE, LeDoux JE. Emotional perseveration: An update on prefrontal-amygdala interactions in fear extinction. Learning & Memory. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Spear NE. Forgetting as retrieval failure. In: Honig WK, James PHR, editors. Animal memory. New York: Academic Press; 1971. pp. 45–109. [Google Scholar]
- Talk AC, Gandhi CC, Matzel LD. Hippocampal function during behaviorally silent associative learning: dissociation of memory storage and expression. Hippocampus. 2002;12:648–656. doi: 10.1002/hipo.10098. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Larsen N, Ayres JJB. Role of context similarity in ABA, ABC, and AAB renewal paradigms: Implications for theories of renewal and for treating human phobias. Learning and Motivation. 2003;34:410–436. [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P. Return of fear in a human differential conditioning paradigm caused by a return to the original acquisition context. Behaviour Research and Therapy. 2005;43:323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wagner A. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Ward-Robinson J, Coutureau E, Good M, Honey RC, Killcross AS, Oswald CJ. Excitotoxic lesions of the hippocampus leave sensory preconditioning intact: Implications for models of hippocampal function. Behavioral Neuroscience. 2001;115:1357–1362. doi: 10.1037//0735-7044.115.6.1357. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, Iordanova M, McNally G, Richardson R, Harris JA. Reinstatement of Fear to an Extinguished Conditioned Stimulus: Two Roles for Context. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:97–110. [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behavioral Neuroscience. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]


