Abstract
Preimplantation embryo development to the blastocyst stage and uterine differentiation to the receptive state are prerequisites for embryo implantation. Burgeoning evidence suggests that endocannabinoid signaling is critical to early pregnancy events. Anandamide (N-arachidonoylethanolamine) and 2-AG (2-arachidonoylglycerol) are two major endocannabinoids that bind to and activate G-protein coupled cannabinoid receptors CB1 and CB2. We have previously shown that a physiological tone of anandamide is critical to preimplantation events in mice, since either silencing or amplification of anandamide signaling causes retarded development and oviductal retention of embryos via CB1, leading to deferred implantation and compromised pregnancy outcome. Whether 2-AG, which also influences many biological functions, has any effects on early pregnancy remains unknown. Furthermore, mechanisms by which differential uterine endocannabinoid gradients are established under changing pregnancy state is not clearly understood. We show here that 2-AG is present at levels one order of magnitude higher than those of anandamide in the mouse uterus, but with similar patterns as anandamide, i.e. lower levels at implantation sites and higher at interimplantation sites. We also provide evidence that region- and stage-specific uterine expression of N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH), and sn-1-diacylglycerol (DAG) lipase fα (DAGLfα) and monoacylglycerol lipase (MAGL) for synthesis and hydrolysis of anandamide and 2-AG, respectively, creates endocannabinoid gradients conducive to implantation. Our genetic evidence suggests that FAAH is the major degrading enzyme for anandamide, whereas COX-2, MAGL and to some extent COX-1 participate in metabolizing 2-AG in the pregnant uterus. The results suggest that aberrant functioning of these pathways impacting uterine anandamide and/or 2-AG levels would compromise pregnancy outcome.
Introduction
Implantation involves a complex discourse between the embryo and uterus and is a gateway to successful pregnancy [1, 2]. The fundamental feature of this process is the synchronized development of the embryo to the activated state of the blastocyst and the differentiation of the uterus to the receptive state [3, 4] that result from coordinated integration of various signaling pathways, influencing cell-cell and cell-matrix interactions between the embryo and the uterus. Emerging evidence points toward the pathophysiological significance of endocannabinoids, a group of lipid mediators, in embryo-uterine cross-talk during implantation [5, 6]. To date, two arachidonate derivatives, N-arachidonoylethanolamine (coined as anandamide) and 2-arachidonoylglycerol (2-AG) have been characterized as major endocannabinoid ligands [7–9] that bind to and activate two classic G-protein coupled cannabinoid receptors CB1 and CB2 [10, 11]. Our earlier studies in mice have shown functional expression of CB1 in preimplantation embryos [12, 13], synthesis of anandamide in the oviduct and uterus [14–17] and its dose and stage-specific effects on embryo development and implantation [18, 19]. We have also found that uterine anandamide levels and blastocyst CB1 are coordinately downregulated with the attainment of uterine receptivity and blastocyst activation prior to implantation as opposed to their higher levels in delayed implanting uteri and dormant blastocysts [14–16], suggesting that lower, but not higher, levels of anandamide and CB1 are beneficial to implantation. This is consistent with our recent findings that anandamide within a very narrow range regulates blastocyst activation and implantation by differentially modulating MAP kinase signaling and Ca++ channel activity via CB1 [20]. Collectively, these results indicate that anandamide signaling is operative very early during pregnancy in influencing the fate of embryo implantation. However, the potential role of 2-AG in early pregnancy remains largely unknown.
Anandamide is thought to be released from its phospholipid precursor by a recently characterized N-acylphosphatidylethanolamine (NAPE)-specific phospholipase D (NAPE-PLD) [21], whereas its biological activity is terminated by intracellular degradation via a fatty acid amide hydrolase (FAAH) [22, 23]. With respect to 2-AG, early studies have characterized two sn-1-diacylglycerol (DAG) lipases (DAGLα and DAGLβ) that participate in generating 2-AG from DAG [24]. Once accumulated in the cell, 2-AG can be degraded by FAAH [25], but it is not the only enzyme responsible for its metabolism [26]. An enzyme responsible for 2-AG hydrolysis, monoacylglycerol lipase (MAGL), has been cloned and characterized in several species [25, 27, 28]. To explore the physiological significance of endocannabinoids during early pregnancy, it is essential to examine the spatiotemporal expression patterns of the key synthetic and hydrolytic enzymes of anandamide and 2-AG in the uterus.
In addition to hydrolytic pathways of endocannabinoid metabolism, recent evidence suggests a role for cyclooxygenases (COX-1 and COX-2) in oxidative metabolism of anandamide and 2-AG, owing to their structural similarity to polyunsaturated fatty acids. There is evidence that both anandamide and 2-AG can serve as substrates for COX-2 [29–31], and also for COX-1 in the context of cell-types and conditions [32]. Since implantation sites contains lower levels of anandamide conducive to postimplantation embryo-uterine growth [14, 16], and since COX-2 is expressed at these sites [33, 34], it is meaningful to measure uterine endocannabinoid levels in mice missing COX-2, COX-1 or cytosolic phospholipase A2α (cPLA2α), the key enzymes expressed in pregnant uteri for arachidonic acid release and subsequent prostaglandin biosynthesis [33, 35]. Therefore in the present investigation, we first analyzed the levels of 2-AG and anandamide in wild-type periimplantation mouse uteri using reverse-phase positive-ion ESI-HPLC-MS-MS. We also examined uterine cell-specific expression profiles of DAGLα, MAGL, NAPE-PLD, FAAH and COX-2 by employing immunohistochemistry. In addition, we compared uterine levels of anandamide and 2-AG in pregnant wild-type mice with those of cPLA2α−/−, COX-2−/−, COX-1−/− and FAAH−/− mice to explore potential links between endocannabinoid and eicosanoid metabolizing pathways.
2. Materials and Methods
2.1. Mice
All experiments were conducted in accordance with National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Vanderbilt University. Wild-type and FAAH−/− mice were generated from heterozygous matings on 129/C57Bl6 mixed genetic background as previously described [36]. The cPLA2α−/−, COX-1−/−, and COX-2−/− mice were originally generated on 129/C57Bl6 mixed genetic background [37–39], and later these mutations were established on the CD-1 genetic background by backcrossing with CD1 wild-type mice (Charles River Laboratories) for 10 generations [35, 40, 41]. Polymerase chain reaction (PCR) analysis of tail genomic DNA determined the genotypes. Females were mated with fertile males of the same strain to induce pregnancy. The morning (0900 h) of finding a vaginal plug was considered day 1 of pregnancy. Implantation sites were visualized by an intravenous injection of Chicago blue dye solution [4]. Uterine tissues were fixed in neutral buffered formalin solution for immunostaining or flash frozen and stored at −80° for anandamide and 2-AG measurement.
2.2. Immunohistochemistry
Immunostaining in formalin-fixed paraffin embedded sections (5 μm) was performed using antibodies specific to NAPE-PLD (Cayman), FAAH (custom made), DAGLα (a gift from Dr. Watanabe), MAGL (abcam) and COX-2 (custom made). A Histostain-Plus (DAB) kit (Zymed) was used to visualize the antigen. Reddish brown deposits indicate the sites of positive immunostaining.
2.3. Analysis of anandamide and 2-AG
Uterine tissues pooled from 3–5 pregnant mice in each group (N=5~7) were assayed for anandamide and 2-AG as previously described [42]. Briefly, the pre-weighed samples were homogenized in ethyl acetate with 0.5% acetic acid. A small aliquot of internal standard solution containing anandamide-d8 and 2-AG-d8 was added to a mortar immediately prior to homogenization. The homogenate was centrifuged and the supernatant was dried, reconstituted in chloroform and purified on a silica solid phase extraction cartridge. The eluent was dried, reconstituted in 1:8 of aqueous silver acetate to methanolic silver acetate and analyzed via reverse-phase positive-ion ESI-HPLC-MS-MS. Quantitation was performed by stable isotope dilution against the octadeuterated internal standard.
2.4. Statistical Analysis
Anandamide and 2-AG levels in different uterine samples were expressed as mean±SEM, and the significance was analyzed by Student’s t-test.
3. Results and Discussion
3.1. Uterine anandamide and 2-AG levels are associated with changing pregnancy states
In mice, uterine environment with respect to implantation is divided into prereceptive, receptive and nonreceptive (refractory) phases [3, 4, 43, 44]. The “window” of uterine receptivity for implantation occurs only for a limited period during pregnancy. The prereceptive uterus becomes receptive on day 4 of pregnancy (the day of implantation [45]), while by late day 5 the uterus becomes refractory and fails to respond to blastocyst attachment reaction [4, 35]. Although molecular mechanisms by which various uterine phases are achieved are not clearly understood, our studies demonstrate that ligand-receptor signaling with endocannabinoids particularly involving anandamide is closely associated with uterine receptivity [14–16]. Consistent with previous findings, we again observed that uterine anandamide levels fluctuate with the state of pregnancy with higher levels at the interimplantation site, but lower levels at the site of implantation (Fig. 1A). Furthermore, a similar pattern of 2-AG, but at much higher levels, was noted in the uterus during early pregnancy (Fig. 1B). The results suggest that 2-AG in parallel to anandamide also participates in regulating the “window” of implantation by synchronizing embryo development and blastocyst activation with preparation of the uterus to the receptive state. Since 2-AG has been shown to inhibit development of 2-cell embryos into blastocysts in culture [18], it is conceivable that heightened levels of 2-AG disrupt embryo survival and uterine receptivity, and consequently pregnancy establishment. It is to be noted that overall levels of 2-AG in periimplantation uteri are about 200-fold higher than anandamide (Fig. 1A and B), which is similar to 2-AG/anandamide profiles present in the brain and other tissues [46]. This raises the question as to why 2-AG, which is detrimental to early embryo development, is present in the pregnant uterus at such higher levels than anandamide.
Fig. 1.
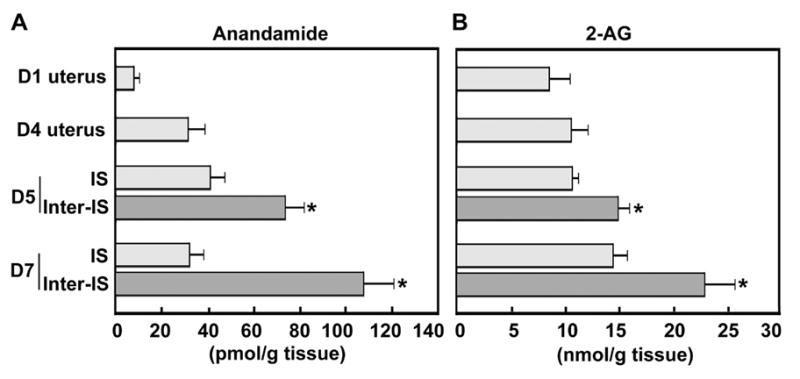
Levels of anandamide (A) and 2-AG (B) in the periimplantation mouse uterus. Anandamide and 2-AG concentrations at interimplantation sites (Inter-IS) were significantly higher (*P<0.05) than those from implantation sites (IS) (unpaired t test). Uterine tissues from
Low basal levels of anandamide are thought to be due to the availability of small amount of sn-1-arachidonoyl phosphoglyceride precursors. Since 2-AG is at the crossroad of several metabolic pathways, and since it is an important precursor and degradation product of phospho-, di- and tri-glycerides, it is suggested that only a minor part of (10–20%) of 2-AG diffuses through the plasma membrane to interact with cannabinoid receptors [47]. Therefore, it is possible that the local concentration of 2-AG at the sites of blastocysts does not reach the levels that affect embryonic functions and implantation under normal conditions. Furthermore, there is evidence that 2-AG exerts higher selectivity for CB2 over CB1 [48, 49], whereas the reverse is true for anandamide [50, 51]. In addition, anandamide has higher binding affinity than 2-AG for CB1 [9, 26, 48, 52]. Our previous findings of absence of CB2 in the oviduct and uterus [17, 53] suggests that 2-AG detected at nmol/g tissue concentration is perhaps physiological to influence the embryo-uterine dialogue during implantation via CB1. Collectively, these results place the uterus and embryo as physiologically relevant targets for ligand-receptor signaling with endocannabinoids. Because the uterus is comprised of heterogeneous cell types, information regarding cell-specific synthesis and degradation of 2-AG and anandamide is required to further explore the significance of these lipid mediators in early pregnancy.
3.2. Spatiotemporal expression of DAGLα, MAGL, NAPE-PLD, FAAH and COX-2 creates appropriate endocannabinoid gradients for normal pregnancy
As described above, endocannabinoid signaling triggered by uterine anandamide and/or 2-AG and primarily acting through uterine and embryonic CB1, directs embryo development and implantation in a stage and dose dependent manner in mice. However, it remains unknown how appropriate endocannabinoid levels are spatiotemporally created for synchronizing embryo development and uterine receptivity for implantation. The objective of this study was to explore the cell-specific uterine expression of the key synthetic and metabolic enzymes of 2-AG (DAGL, MAGL) and anandamide (NAPE-PLD, FAAH) during the periimplantation period. As shown in Fig. 2, DAGLα, the predominant DAGL isoform in adult tissues [24], is highly expressed in both circular and longitudinal muscle layers of uteri, whereas DAGLα expression is undetectable in luminal and glandular epithelia and stroma on day 1 of pregnancy. On day 4 (the day of uterine receptivity), the apical border of the luminal epithelium is decorated with DAGLα immunostaining with persistent high expression in the myometrium (Fig. 2). The results suggest that the myometrium is the major source of uterine 2-AG prior to implantation. With the onset and progression of implantation and decidualization on days 5–7, DAGLα expression is markedly upregulated in the luminal epithelium of the inter-implantation region with modest expression in the myometrium, while its expression in the stroma and deciduoma at the implantation site remains at low levels (Fig. 2). This spatiotemporal expression pattern of DAGLα in the periimplantation uterus is consistent with 2-AG levels (Fig. 1B), indicating that DAGLα is an important player in creating uterine 2-AG gradient during early pregnancy. We next sought to examine uterine expression of MAGL, the enzyme that hydrolyzes 2-AG, to explore its potential contribution toward the stage and region specific 2-AG levels during early pregnancy. As illustrated in Fig. 3, MAGL was primarily localized in the luminal and glandular epithelia with lower levels of accumulation in the stroma and myometrium on days 1–4 of pregnancy. With the initiation of implantation on day 5, MAGL was induced in the subepithelial stromal cells at the site of blastocyst attachment; whereas at the interimplantation site lower levels of expression were restricted to the luminal and glandular epithelia. With the progression of pregnancy on day 7, MAGL was clearly localized in the decidualizing stromal cells and growing embryos, the levels of which exceed those at the interimplantation region with localization primarily in the epithelium (Fig. 3). This dynamic expression of MAGL in the periimplantation uterus suggest that MAGL plays a role in timely degradation of 2-AG, thus protecting embryos from exposure to excessive levels of 2-AG. These findings are also consistent with lower levels of 2-AG at the implantation site. Collectively, the results suggest that coordinated activity of DAGLα and MAGL regulate uterine levels of 2-AG during normal pregnancy. However, it remains to be identified the definitive subcellular sites of 2-AG synthesis and degradation and whether they are transported across the cell by diffusion or specific transporters.
Fig. 2.
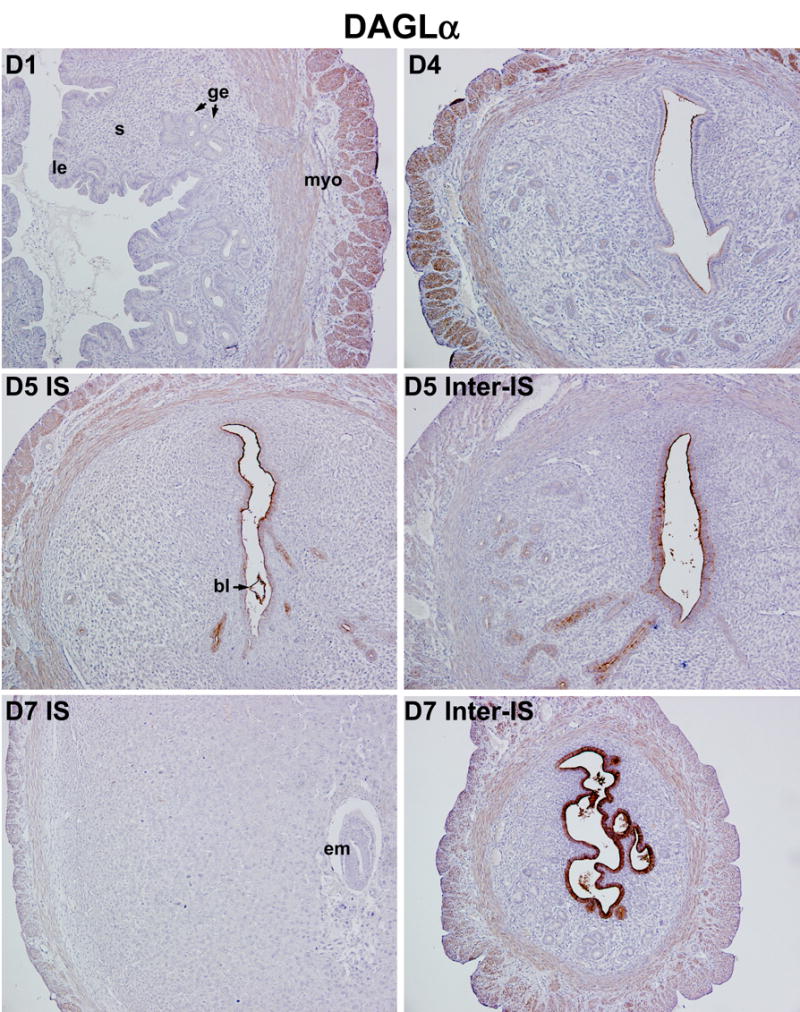
Spatiotemporal expression of DAGLα in the periimplantation mouse uterus. Uterine expression of DAGLα was analyzed by immunohistochemistry. Photomicrographs of representative uterine cross sections are shown (100X). le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; bl, blastocyst; em, embryo; IS, implantation site; Inter-IS, interimplantation site.
Fig. 3.
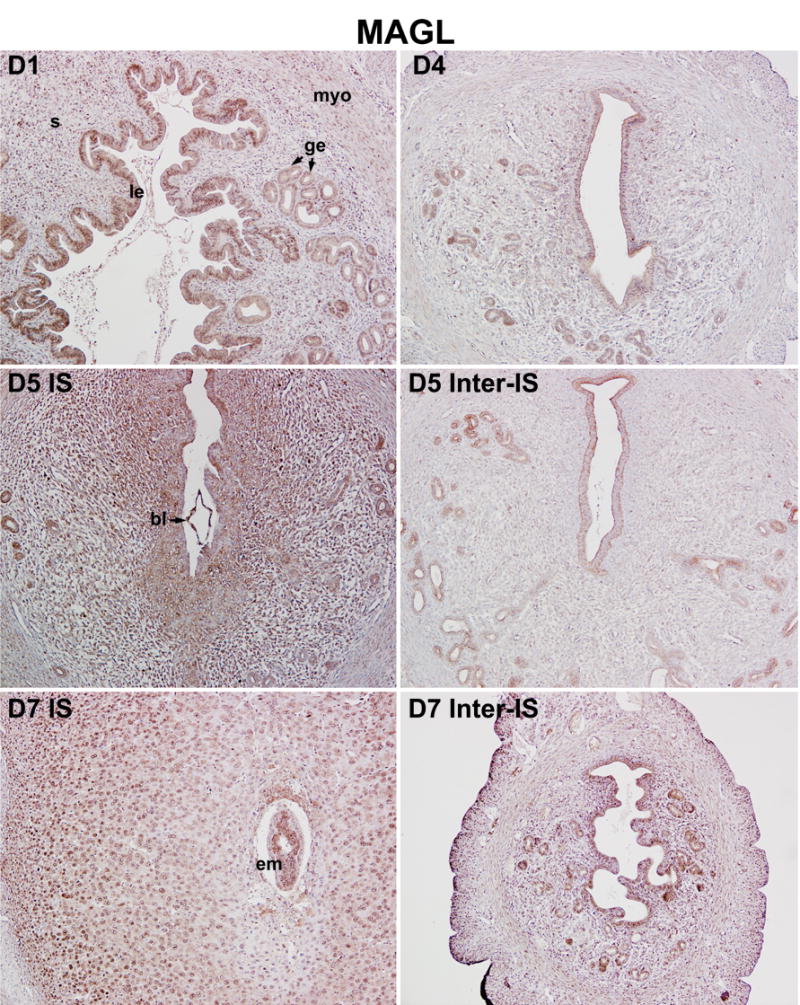
Uterine cell-specific expression of MAGL during early pregnancy in mice. MAGL protein expression was analyzed using immunohistochemistry. Photomicrographs of representative uterine cross sections are shown (100X). le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; bl, blastocyst; em, embryo; IS, implantation site; Inter-IS, interimplantation site.
In parallel to our studies on 2-AG synthesis and hydrolysis, we also examined uterine cell-specific expression patterns of NAPE-PLD and FAAH that are linked to anandamide synthesis and degradation. As illustrated in Fig. 4 and Fig. 5, both NAPE-PLD and FAAH are primarily localized in the luminal and glandular epithelium with lower levels of accumulation in the stroma and myometrium on days 1–4 of pregnancy, indicating that the epithelium is the major site of anandamide synthesis and degradation during the preimplantation period. Observation of co-expression of both enzymes in same uterine cell-types provides evidence for a mechanism for on-site anandamide synthesis and hydrolysis that protects the newly formed blastocyst from exposure to excessive levels of uterine anandamide during normal pregnancy. With the initiation and progression of implantation and decidualization, we observed an interesting inverse relationship between NAPE-PLD and FAAH expression at implantation sites versus inter-implantation sites. For example, while NAPE-PLD accumulation declines in the luminal epithelium close to the implanting blastocyst on day 5, its accumulation in the luminal epithelium at the interimplantation site is upregulated on days 5–7 (Fig. 4). Furthermore, NAPE-PLD accumulation in decidual cells is also relatively low compared with interimplantation stromal cells (Fig. 4). In contrast, while FAAH accumulation in the epithelium and stroma at the interimplantation region is downregulated on days 5–7, accumulation in the luminal and glandular epithelia, stroma and decidualizing stroma at the implantation site is detected at much higher levels (Fig. 5). In addition, the implanting blastocyst also shows high levels of FAAH. This region-specific inverse expression profile of NAPE-PLD and FAAH in the periimplantation uterus is well correlated with the changing pattern of uterine anandamide levels as well as NAPE-PLD and FAAH enzymatic activities as previously observed by us [16, 54]. This suggests that a spatiotemporal coordination of anandamide synthesis and degradation in the uterus and blastocyst ensures normal blastocyst activation and uterine preparation for implantation and subsequent decidualization. This is supported by previous findings that an aberrantly enhanced cannabinoid ligand-receptor signaling impairs on-time implantation [14, 17, 18, 55] and decidualization [56].
Fig. 4.
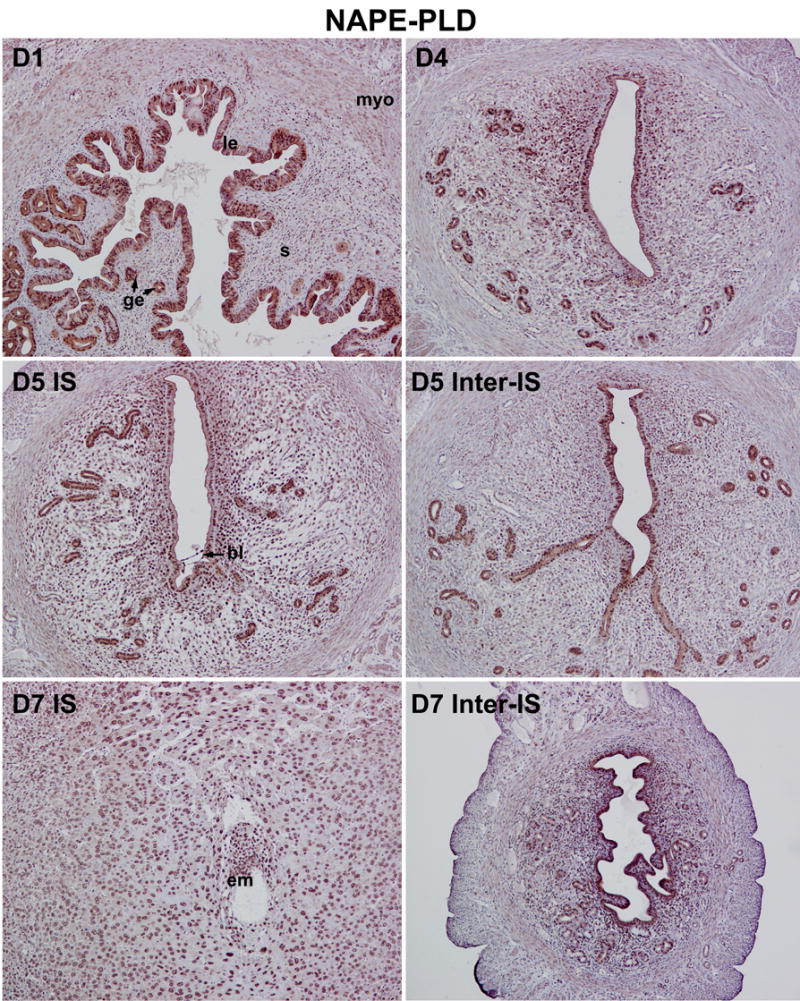
Cell and stage-specific expression of NAPE-PLD in pregnant mouse uteri. The spatiotemporal accumulation of NAPE-PLD in the periimplantation uterus was examined by immunohistochemistry. Photomicrographs of representative uterine cross sections are shown (100X). le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; bl, blastocyst; em, embryo; IS, implantation site; Inter-IS, interimplantation site.
Fig. 5.
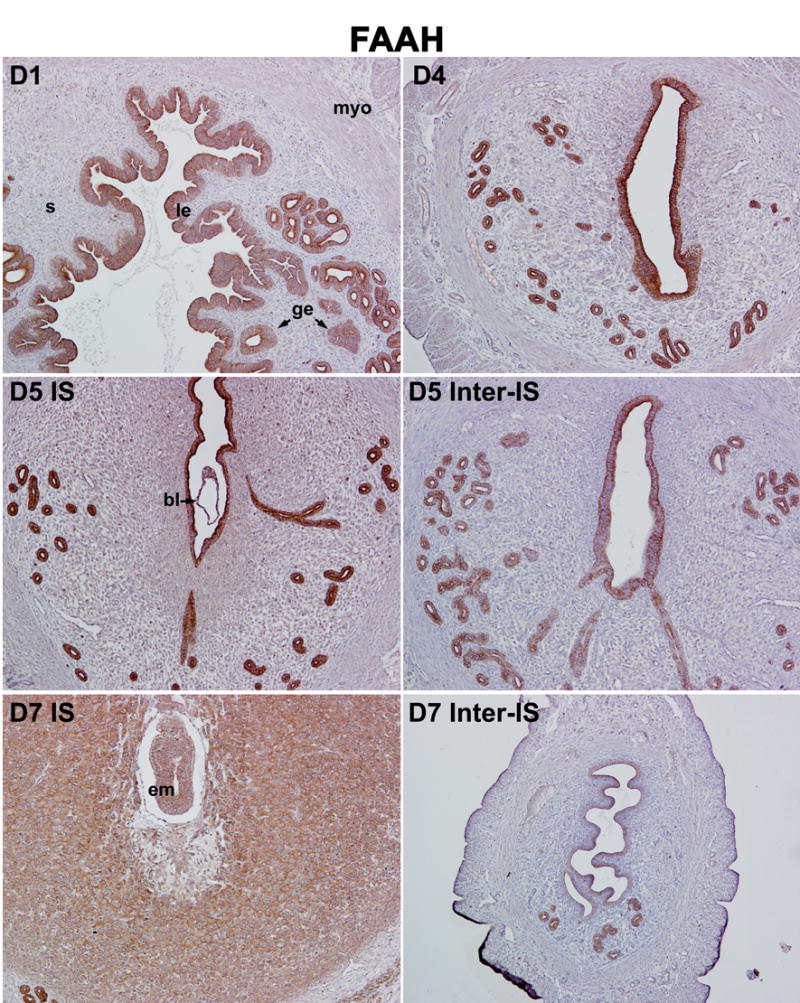
Cell and stage-specific expression of FAAH in mouse uteri during early pregnancy. FAAH localization in uterine cells of various pregnancy stages was examined by immunohistochemistry. Photomicrographs of representative cross uterine sections are shown (100X). le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; bl, blastocyst; em, embryo; IS, implantation site; Inter-IS, interimplantation site.
Recent studies point towards a novel oxidative metabolic pathway of endocannabinoids by a fatty acid cyclooxygenase COX-2, a rate-limiting inducible enzyme, that converts arachidonic acid to prostaglandins [29–31]. Therefore, we analyzed COX-2 protein expression in the periimplantation uterus. Consistent with our previous observations [33, 34], we found that COX-2 protein is present in the luminal epithelium, but not in the glandular epithelium, stroma or myometrium, on day 1 of pregnancy (Fig. 6). The expression declines rapidly after day 1 and is maintained at undetectable levels through day 4. Following implantation on day 5, COX-2 expression is exclusively induced in the luminal epithelium and subepithelial stromal cells at the antimesometrial pole surrounding the implanting blastocyst. With the progression of implantation and decidualization, COX-2 expression becomes undetectable at the antimesometrial pole, but instead is detected in the decidualizing stromal cells at the mesometrial pole of the implantation chamber. Of note, COX-2 expression is barely detected at interimplantation sites (Fig. 6). These results reinforce the essential role of COX-2 derived prostaglandins in embryo implantation and decidualization [34, 57, 58], and at the same time raises the possibility that COX-2 perhaps contributes to lower levels of anandamide and 2-AG at the implantation site. To better understand the relationship of endocannabinoid and prostaglandin synthesis and metabolism in the uterus, we explored the consequences of functional loss of FAAH, COX-2, COX-1 and cPLA2α on uterine anandamide and 2-AG levels during embryo implantation.
Fig. 6.
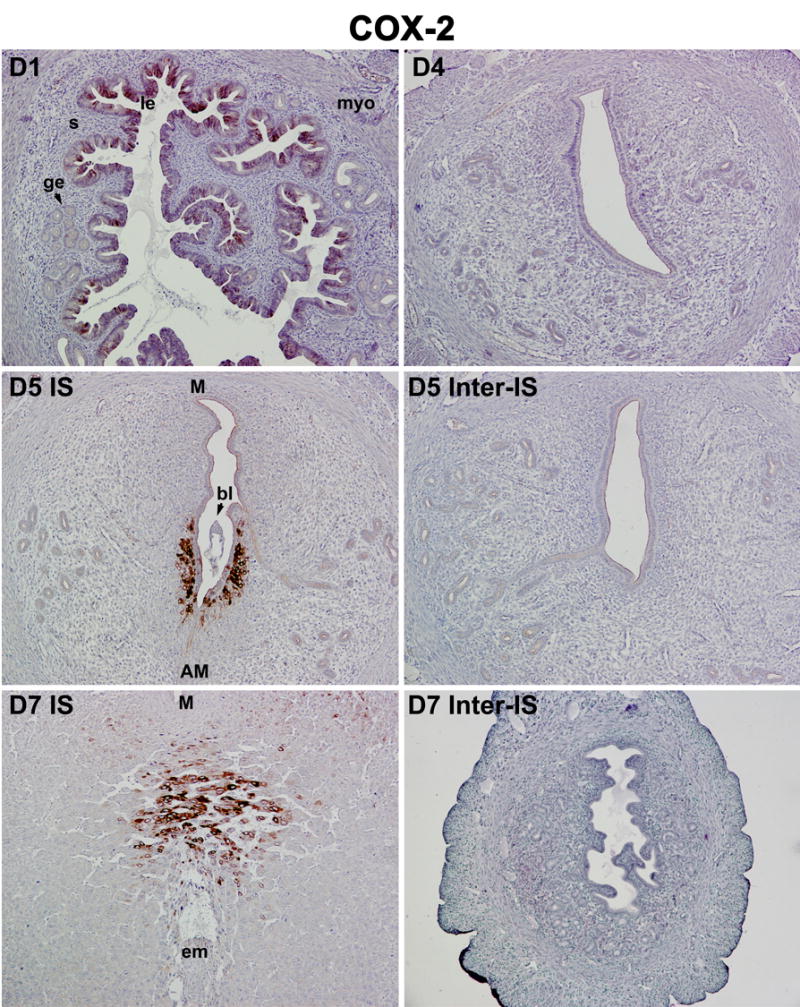
Region-specific uterine induction of COX-2 in mice during the periimplantation period. Immunohistochemistry was employed to determine COX-2 protein localization in the pregnant uterus. Photomicrographs of representative cross uterine sections are shown (100X). le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; bl, blastocyst; em, embryo; IS, implantation site; Inter-IS, interimplantation site; M, mesometrial site; AM, anti-mesometrial site.
3.3. FAAH or COX-2 deficiency modulates uterine anandamide and 2-AG accumulation differentially during implantation
We have recently shown that genetic or pharmacological inactivation of FAAH increases oviductal accumulation of anandamide with constrains on preimplantation embryo development, eventually leading to deferred on-time implantation and poor pregnancy outcome [55]. Since FAAH is expressed at higher levels in both implanting embryos and decidualizing stromal cells within the implantation chamber as compared to those at interimplantation sites (Fig. 5), we suspected that FAAH deficiency will derail the normal region-specific pattern of anandamide gradient with lower levels at implantation sites and higher levels at interimplantation sites. Indeed, we found increased accumulation of anandamide, but not 2-AG, in implantation sites on day 7 of pregnancy in the absence of FAAH (Fig. 7A and B). These results suggest that FAAH is a primary hydrolytic enzyme for anandamide, but not for 2-AG, in the uterus during early pregnancy, reinforcing the idea that enzymes other than FAAH are responsible for catabolic regulation of the MAG family of signaling lipids in vivo [26].
Fig. 7.
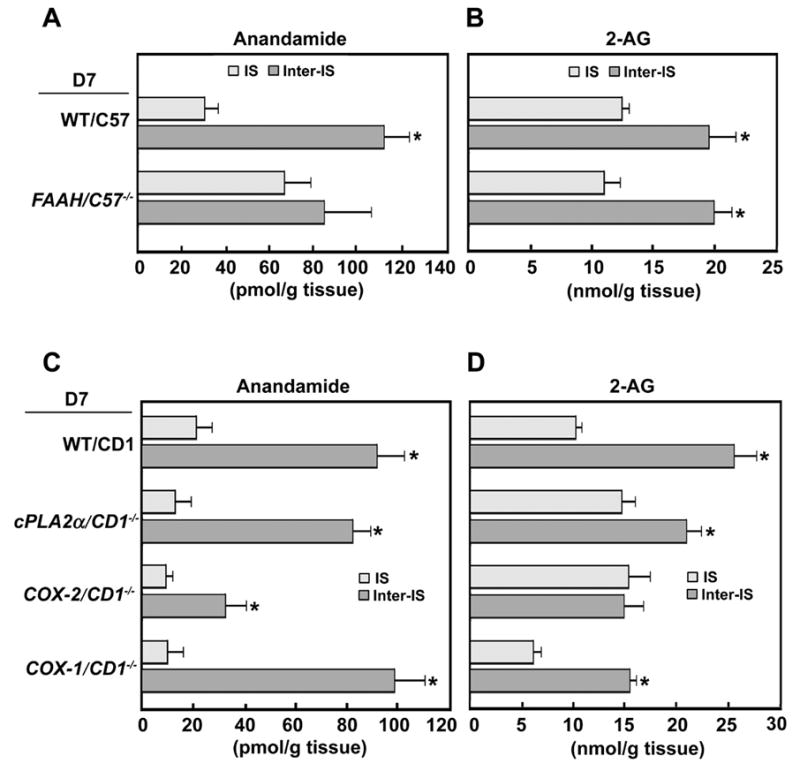
Uterine levels of anandamide (A, C) and 2-AG (B, D) in day 7 pregnant wild-type, FAAH−/−, COX-2−/−, cPLA2α−/− and COX-1−/− mice. We noted that FAAH deficiency increases anandamide levels at the implantation sites, while the absence of COX-2 derails the normal uterine 2-AG accumulation pattern. The loss of cPLA2α or COX-1 shows no obvious changes in uterine patterns anandamide and 2-AG levels. *P<0.05 (unpaired t test).
In contrast to the findings in FAAH−/− mice, we observed an inverse pattern of uterine anandamide vs 2-AG levels in mice missing COX-2. For example, although overall levels of uterine anandamide in COX-2 null mice are lower than wild-type mice, its region-specific pattern showing lower levels at implantation sites and higher levels at inter-implantation sites is still maintained in pregnant COX-2 null uteri similar to that seen in wild-type mice (Fig. 7C). In contrast, this region-specific pattern of 2-AG accumulation which shows no changes in FAAH−/− mice is lost in the absence of COX-2 (Fig. 7D). Levels of 2-AG at implantation sites in COX-2 null are substantially elevated, pointing toward a novel function of COX-2 in metabolizing locally produced 2-AG within the implantation chamber. This is consistent with early findings that COX-2 in addition to its key role in prostaglandin biosynthesis can also metabolize 2-AG into glyceryl prostaglandins [30, 31]. The potential function of these novel metabolic lipids in embryo implantation warrants further investigation. The substrate preference of COX-2 for anandamide over 2-AG in oxidative metabolism by the pregnant uterus remains elusive. The fact that 2-AG levels are 200-fold higher than anandamide (Fig. 1A and B) suggests the possibility of glycerylprostaglandin biosynthesis from 2-AG by locally expressed uterine COX-2.
Since cPLA2α-COX-1-COX-2 signaling axis appears to be critical for normal embryo implantation and decidualization [33–35, 41], we next compared uterine endocannabinoid levels in mice missing cPLA2α or COX-1. As shown in Fig. 7C and D, the uterine patterns of 2-AG and anandamide levels are comparable in wild-type, cPLA2α−/− and COX-1−/− mice, although the levels of 2-AG are lower in uteri of mice lacking COX-1. These results suggest that cPLA2α and COX-1 are not important players in directing region-specific uterine endocannabinoid gradients during early pregnancy. Therefore, FAAH, COX-2 and MAGL appear to be key metabolic enzymes that differentially maintain the optimal levels of anandamide and 2-AG favorable to implantation initiation and its progression. Since uterine 2-AG levels are lower in COX-1 null mice, the participation of COX-1 in metabolizing 2-AG cannot be completely ruled out. Indeed, there is evidence that COX-1 can also metabolize 2-AG to glycerylprostaglandins depending on the substrate availability and cell microenvironment [32]. Further investigations are required to explore the 2-AG hydrolytic metabolism in the uterus using MAGL deficient mice.
Collectively, the present work provides biochemical, physiological and genetic evidence that uterine levels of 2-AG and anandamide fluctuate with the state of normal pregnancy: higher in the interimplantation sites and lower at the site of embryo implantation. The levels of endocannabinoids conducive to implantation are primarily regulated by dynamic expression of NAPE-PLD and FAAH for anandamide, and DAGLα, MAGL and COX-2 for 2-AG. Thus, an aberrant expression and activity of either these key synthetic and hydrolytic enzymes in the uterus may constrain early pregnancy events. This study is clinically relevant, since elevated endocannabinoid levels in peripheral circulation are associated with spontaneous pregnancy loss in women [59, 60].
Acknowledgments
We thank Dr. Masahiko Watanabe (Hokkaido University School of Medicine, Japan) for his generous gift of the DAGLα antibody. This work was supported in part by NIH Grants, DA06668, HD12304 and HD050315. S. K. Dey is recipient of Method to Extend Research in Time (MERIT) Awards from the National Institute on Drug Abuse (NIDA) and the National Institute of Child Health and Human Development (NICHD). H Wang is recipient of Solvay/Mortola Research Award from the Society for Gynecologic Investigation. H.X. is a Lalor Foundation postdoctoral fellow. We also thank the Turner Foundation for generous support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 2.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 3.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paria BC, Dey SK. Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem Phys Lipids. 2000;108:211–220. doi: 10.1016/s0009-3084(00)00197-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: Two Faces of Cannabinoid Signaling in Male and Female Fertility. Endocr Rev. 2006 doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- 7.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 8.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 11.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZM, Paria BC, Dey SK. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biol Reprod. 1996;55:756–761. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]
- 13.Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci U S A. 1995;92:9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, Bonner TI, Zimmer A, Dey SK. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276:20523–20528. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- 15.Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 1997;94:4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Wang H, Okamoto Y, Ueda N, Kingsley PJ, Marnett LJ, Schmid HH, Das SK, Dey SK. N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. J Biol Chem. 2005;280:23429–23432. doi: 10.1074/jbc.C500168200. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- 18.Paria BC, Ma W, Andrenyak DM, Schmid PC, Schmid HH, Moody DE, Deng H, Makriyannis A, Dey SK. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biol Reprod. 1998;58:1490–1495. doi: 10.1095/biolreprod58.6.1490. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Paria BC, Dey SK, Armant DR. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod. 1999;60:839–844. doi: 10.1095/biolreprod60.4.839. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci U S A. 2003;100:14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 22.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 23.Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999;57:417–423. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- 26.Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Ho SY, Delgado L, Storch J. Monoacylglycerol metabolism in human intestinal Caco-2 cells: evidence for metabolic compartmentation and hydrolysis. J Biol Chem. 2002;277:1816–1823. doi: 10.1074/jbc.M108027200. [DOI] [PubMed] [Google Scholar]
- 28.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 30.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 31.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 32.Rouzer CA, Tranguch S, Wang H, Zhang H, Dey SK, Marnett LJ. Zymosan-induced glycerylprostaglandin and prostaglandin synthesis in resident peritoneal macrophages: roles of cyclooxygenases-1 and -2. Biochem J. 2006 doi: 10.1042/BJ20060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- 34.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 35.Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A2alpha is crucial for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 36.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 38.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 39.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 40.Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci U S A. 2000;97:9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Ma WG, Tejada L, Zhang H, Morrow JD, Das SK, Dey SK. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J Biol Chem. 2004;279:10649–10658. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- 42.Kingsley PJ, Marnett LJ. Analysis of endocannabinoids by Ag+ coordination tandem mass spectrometry. Anal Biochem. 2003;314:8–15. doi: 10.1016/s0003-2697(02)00643-7. [DOI] [PubMed] [Google Scholar]
- 43.Psychoyos A. Endocrine control of egg implantation. In: Greep ROAE, Geiger SR, editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1973. pp. 187–215. [Google Scholar]
- 44.Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- 45.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 46.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J. 1997;322 (Pt 2):671–677. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69:169–178. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 50.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- 51.Lin S, Khanolkar AD, Fan P, Goutopoulos A, Qin C, Papahadjis D, Makriyannis A. Novel analogues of arachidonylethanolamide (anandamide): affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J Med Chem. 1998;41:5353–5361. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- 52.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 53.Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci U S A. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paria BC, Deutsch DD, Dey SK. The uterus is a potential site for anandamide synthesis and hydrolysis: differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Mol Reprod Dev. 1996;45:183–192. doi: 10.1002/(SICI)1098-2795(199610)45:2<183::AID-MRD11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Xie H, Guo Y, Zhang H, Takahashi T, Kingsley PJ, Marnett LJ, Das SK, Cravatt BF, Dey SK. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006;116:2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kessler CA, Moghadam KK, Schroeder JK, Buckley AR, Brar AK, Handwerger S. Cannabinoid receptor I activation markedly inhibits human decidualization. Mol Cell Endocrinol. 2005;229:65–74. doi: 10.1016/j.mce.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scherle PA, Ma W, Lim H, Dey SK, Trzaskos JM. Regulation of cyclooxygenase-2 induction in the mouse uterus during decidualization. An event of early pregnancy. J Biol Chem. 2000;275:37086–37092. doi: 10.1074/jbc.M006168200. [DOI] [PubMed] [Google Scholar]
- 59.Maccarrone M, Bisogno T, Valensise H, Lazzarin N, Fezza F, Manna C, Di Marzo V, Finazzi-Agro A. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol Hum Reprod. 2002;8:188–195. doi: 10.1093/molehr/8.2.188. [DOI] [PubMed] [Google Scholar]
- 60.Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet. 2000;355:1326–1329. doi: 10.1016/S0140-6736(00)02115-2. [DOI] [PubMed] [Google Scholar]


