Abstract
The ectodomain of LIN-12/Notch proteins is cleaved and shed upon ligand binding. In C. elegans, genetic evidence has implicated SUP-17, the ortholog of Drosophila Kuzbanian and mammalian ADAM10, as the protease that mediates this event. In mammals, however, biochemical evidence has implicated TACE, a different ADAM protein. We have investigated potential functional redundancy of sup-17 and the C. elegans ortholog of TACE, adm-4, by exploring their roles in cell fate decisions mediated by lin-12/Notch genes. We found that reduced adm-4 activity, like reduced sup-17 activity, suppresses an allele of glp-1 that encodes a constitutively active receptor. Furthermore, concomitant reduction of adm-4 and sup-17 activity causes the production of two anchor cells in the hermaphrodite gonad, instead of one--a phenotype associated with loss of lin-12 activity. We also identified a novel fertility defect in the somatic gonad when both sup-17 and adm-4 are depleted. Expression of a truncated form of LIN-12 that mimics the product of ectodomain shedding rescues this fertility defect, suggesting that sup-17 and adm-4 may mediate ectodomain shedding of LIN-12 and/or GLP-1. Our results are consistent with the possibility that sup-17 and adm-4 are functionally redundant for at least a subset of LIN-12/Notch mediated decisions in C. elegans.
Keywords: ADAM, metalloprotease, LIN-12, Notch, C. elegans, ADM-4, TACE, SUP-17, Kuzbanian, Reprolysin
INTRODUCTION
LIN-12/Notch proteins are receptors that mediate cell-cell interactions that specify cell fates. LIN-12/Notch proteins undergo three proteolytic processing events at three distinct sites (reviewed in Kopan and Goate, 2000). Cleavage at “site 1” in the extracellular domain occurs upon transit to the surface, and generates a heterodimer between the N- and C-terminal fragments. Cleavage at “site 2” in the extracellular domain occurs upon ligand binding. The site 2 cleavage leads to ectodomain shedding, and creates a substrate for cleavage by the presenilin protease complex at “site 3” in the transmembrane domain. The site 3 cleavage releases the intracellular domain, which translocates to the nucleus and promotes transcription of target genes.
Two different proteases of the ADAM family have been implicated in mediating the site 2 cleavage event. The ADAM family comprises transmembrane proteins with three hallmark motifs in their extracellular domains: a Distintegrin domain, a Cysteine-rich domain, and a Zinc Metalloprotease domain. In C. elegans, genetic evidence implicated the ADAM protein SUP-17 in site 2 cleavage (Tax et al., 1997; Wen et al., 1997). However, sup-17 null hermaphrodites do not display highly penetrant defects associated with the absence of lin-12/Notch signaling (Tax et al., 1997), raising the possibility of functional redundancy with another protease. In Drosophila, genetic and biochemical evidence has implicated the ADAM protein Kuzbanian (Rooke et al., 1996; Pan and Rubin, 1997; Sotillos et al., 1997; Kidd et al., 2002; Klein, 2002). The loss of Kuzbanian activity causes phenotypes that are equivalent to removing other core Notch signal transduction pathway components (Rooke et al., 1996), suggesting that it is the main mediator of site 2 cleavage in Drosophila.
SUP-17 and Kuzbanian are orthologs, and their mammalian ortholog is called ADAM10. ADAM10-deficient mice die with defects that can be attributed to reduced Notch signaling (Hartmann et al., 2002). However, site 2 cleavage still occurs in ADAM10 null cells (Mumm et al., 2000), and biochemical evidence has implicated a different ADAM protein, TACE (also known as ADAM17), in site 2 cleavage (Brou et al., 2000). TACE null mice do not display strong Notch-like phenotypes (Peschon et al., 1998). Thus, in mammals as in C. elegans, the genetic evidence raises the possibility that there is more than one protease that mediates site 2 cleavage.
Mammalian ADAM10 and TACE have been extensively studied with respect to substrate specificity; these studies have implicated many different transmembrane proteins as substrates for ADAM10 and TACE, and there is evidence both for and against functional redundancy, suggesting that the roles of these proteases may depend on context. There are cases in which ADAM10 and TACE have been found to cleave the same substrates, e.g. collagen XVII in keratinocytes (Franzke et al., 2002) and the IL-6R under conditions of cholesterol depletion (Matthews et al., 2003). There are also cases in which ADAM10 and TACE have been found to cleave the same substrate, but appear to be activated by different conditions or pathways. One example is CD44 (Nagano et al., 2004), for which ADAM10-mediated cleavage is activated by calcium influx and TACE-mediated cleavage is activated by phorbol ester stimulation. Another is EGF precursor, which appears to be cleaved by ADAM10 in response to G-protein coupled receptor activation but by TACE constitutively (Sahin et al., 2004). However, there are other cases in which ADAM10 and TACE appear to have different substrates, including other ligands for the EGF receptor (Sahin et al., 2004) and the CXC-chemokine ligand 16 (CXCL16) (Abel et al., 2004). Thus, there is no a priori reason to suppose that ADAM10 and TACE are interchangeable in LIN-12/Notch signaling, or not.
The C. elegans TACE ortholog of TACE is named ADM-4, and as described herein, it is closely related to SUP-17. Together with the broad and often overlapping substrate specificity defined in biochemical studies and the lack of a strong lin-12/Notch phenotype of single mutants in both C. elegans and mammals, the sequence similarity raises the possibility that these two proteases are functionally redundant. Here, we have characterized the C. elegans gene adm-4 with respect to potential roles in LIN-12/Notch signaling and explored potential functional redundancy of adm-4 and the Kuzbanian/ADAM10 ortholog sup-17 in development. Our results suggest that sup-17 and adm-4 are functionally redundant for at least a subset of LIN-12/Notch-mediated cell fate decisions as well as a novel fertility-promoting function in the somatic gonad, probably the spermatheca, the specialized region of the hermaphrodite gonad in which fertilization takes place.
MATERIALS AND METHODS
Genetic materials
The wild-type parent for the strains used in this study is Caenorhabditis elegans var. Bristol strain N2. Relevant mutations are:
LG I: sup-17(n1258ts)(Tax et al., 1997; Wen et al. 1997).
LG III: lin-12(n302, n379, n950) (Greenwald et al. 1983), glp-1(ar202ts) (Pepper et al., 2003).
LG X: adm-4(ok265) (The C. elegans Gene Knockout Consortium, http://celeganskoconsortium.omrf.org/).
arIs53 expresses an activated form of lin-12, lin-12(ΔE)::gfp, using the sel-12 promoter (D. Shaye and I. Greenwald, unpublished); the site of integration is not known.
Additional information about these alleles, as well as about markers used for facilitating genetic analysis mentioned in the text, can be found via Wormbase (http://www.wormbase.org/).
Mutant analysis and scoring
Strains were grown at 20°C unless otherwise noted and scored at 15°C, 20°C or 25°C as specified. When a temperature shift was required, L1 larvae (unless otherwise specified) were picked to individual plates, shifted to the desired temperature and assessed for phenotypes at the relevant stage.
Criteria used for scoring different phenotypes are indicated here. Egg-laying ability: animals were scored for 2 consecutive days as they became gravid adults. An animal was scored as Egl+ if it showed the ability to lay eggs (irrespective of the brood size) and did not “bag”, and Egl if it laid none or only a few eggs and “bagged” during this period of time. Anchor cells: the number of anchor cells was assessed in L3 larvae using Nomarski microscopy based on their distinctive morphology. Sterility: animals were scored for 3 consecutive days as they became adults. An animal was scored as sterile if no oocytes, eggs or live progeny were observed on the plate and no eggs could be seen in the uterus, and scored as fertile otherwise. Endoreplicating oocytes: gravid adults were examined using Nomarski microscopy or were fixed and stained with DAPI (McCarter et al., 1999). DAPI-stained worms were mounted in a drop of antifade (Molecular Probes) and analyzed with a fluorescence microscope (Zeiss Axioplan 2). Vulval development: the number of pseudovulval protrusions was scored under the dissecting microscope. Rescue by wild type sperm: 47 individual sup-17;adm-4 L4 hermaphrodites were picked to single plates along with 7-10 wild type N2 males, while 58 sup-17;adm-4 L4 were put on single plates at 15°C without males. The number of progeny produced by each sup-17;adm-4 hermaphrodite was scored, as well as the presence of males in the progeny of the crosses as an indicator of successful mating. No difference in the brood sizes was observed between the sup-17;adm-4 hermaphrodites plated alone or with N2 males. We also determined that the fertile sup-17;adm-4 mutants were Egl+ (40/40 at 20°C and 44/44 at 15°C fertile animals were Egl+), indicating that the vulva of these hermaphrodite should allow efficient mating.
Genomic characterization of adm-4(ok265)
adm-4(ok265) is a deletion allele provided by the C. elegans Gene Knockout consortium. To determine the exact ends of the deletion, the genomic adm-4 region was amplified from the mutant and sequenced. The 847 bp deletion starts 2 nucleotides after the end of exon 8 and ends in exon 13, with the addition of one extra C, as has also been reported by Huang et al. (2003). Unmarked adm-4(ok265) chromosomes used in this study have been backcrossed three times, and homozygosity of the mutation in animals was determined by amplifying the adm-4 genomic region around the deletion using the following primers: 5’ el1 tgtgtcaccttttcggtgaa, 3′ZK154-25220 5’ctgactctcattacaactcg and 5′ZK154-23904 5’cttacatctcggcaagatcc which is located inside the deleted region.
RNA-mediated interference (RNAi)
Each RNA strand was synthesized according to the manufacturer (Stratagene) using as templates adm-4 cDNA amplified from yk187d12, or sup-17 cDNA amplified from yk389a8, using T7 and T3 primers. yk187d12 and yk389a8, a gift from Y. Kohara, were sequenced and encode full length ADM-4 and SUP-17 respectively. Each RNA strand was purified using QIAGEN RNeasy columns and annealed in 0.5X injection buffer (Mello and Fire 1995). RNAi was performed as in Fire et al. (Fire et al. 1998). dsRNA or annealing buffer was microinjected into the pseudocoelomic space and the gonad of young adults. Injected hermaphrodites were cultured individually and their progeny were scored for the relevant phenotype. BLAST analyses using the adm-4 or sup-17 cDNA sequences were performed to ensure that no mRNA other than adm-4 or sup-17 respectively could be targeted when this dsRNA is used.
Rescue experiments and transgenic lines
PCR amplified genomic DNA spanning the adm-4 region (using oligos 5′ZK154-21668 5’ctgagtatttgttgtcgagcgg and 3′R03G5-1944 5’ggtccgtcgacaaacccg) was injected at 20 μg/ml together with pmyo3::gfp (20 μg/ml) and pBluescript (up to 100 μg/ml) into recipient strain sup-17(n1258ts); adm-4(ok265) at 15°C. Two transgenic lines, containing the transgenes arEx399 and arEx400, were obtained; both produced progeny at 20°C, indicative of adm-4(+) activity.
Sequence analysis and phylogenetic studies
The sequences of the metalloprotease domains of all the members of the Astacin, Matrixin and Reprolysin families, and of a few members of the more distant Neprolysin family, were retrieved using BLAST analysis, and information in the Wormbase (www.wormbase.org), SMART (smart.embl-heidelberg.de) and Proteome (www.proteome.com/proteome/Retriever/index.html) databases. These sequences were then used to construct a phylogenetic tree using the ClustalX and njplot programs (Higgins et al., 1996). The standard ClustalX parameters for multiple alignment and the BLOSUM series as protein weight matrix were used. Clustal format was used as output format.
RESULTS
Phylogenetic analysis of C. elegans Metzincin Zinc Metalloproteases
Zinc metalloproteases are classified in families and subfamilies based on the sequence around their Zn binding residues (Hooper, 1994). For example, the “Zincin” family is composed of two subfamilies, the “Gluzincins” and the “Metzincins”. The latter is further divided into the “Astacin”, “Matrixin” and “Reprolysin” families. The Reprolysin family includes the ADAM and ADAM-TS proteases (which additionally have at least one thrombospondin domain). Using a combination of BLAST analysis and information contained in the Wormbase, SMART and Proteome databases (see Materials and Methods), we undertook the phylogenetic analysis of the Metzincin metalloproteases in C. elegans (Fig. 1). Among the 158 C. elegans metalloproteases (Möhrlen et al., 2003), 55 belong to the Metzincin family, and 11 to the Reprolysin family (Fig. 1B). Of these 11 Reprolysins, six are ADAM proteins (C34H3.1, F27D9.7, ADM-1/UNC-71, ADM-2, SUP-17 and ADM-4; see Fig. 1A) while five are ADAM-TS (Gon-1, ADT-2, ADT-1, MIG-17 and T19D2.1). Our sequence analysis suggests that SUP-17 and ADM-4 are the most closely related members of the Reprolysin family in worms (Fig. 1B), and furthermore that ADM-4 is the sole ortholog of mammalian TACE in C. elegans (data not shown).
Fig. 1.
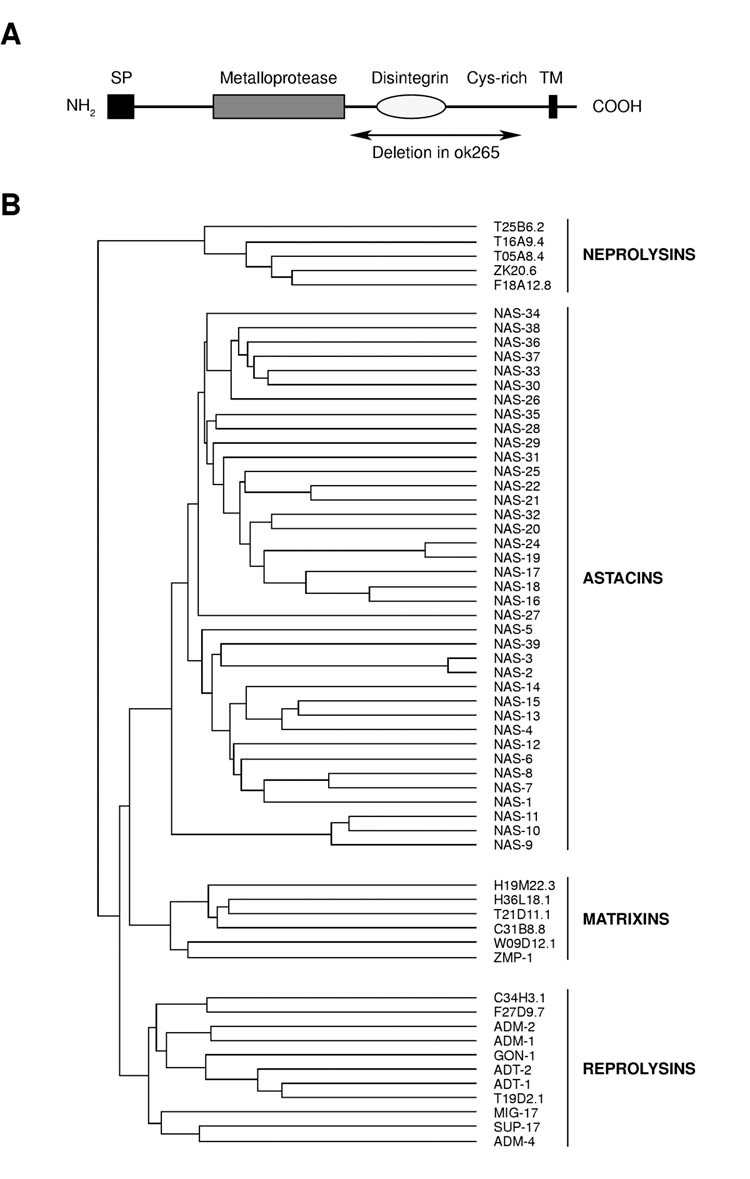
Phylogenetic analysis of C. elegans Metzincin Zinc Metalloproteases. (A) Schematic view of ADM-4 protein structure. SP, signal peptide ; DIS, Disintegrin domain ; Cys-rich, cysteine-rich domain; TM, transmembrane domain. Also shown is the extent of the deletion found in adm-4(ok265). (B) Phylogenetic tree of the Metzincins Zn metalloproteases in C. elegans.
Reduction of sup-17 or adm-4 activity does not cause canonical phenotypes associated with reducing lin-12 or glp-1 activity
lin-12 and glp-1 play unique roles in many different cell fate decisions. lin-12 activity specifies the anchor cell (AC)/ventral uterine precursor cell (VU) decision during hermaphrodite gonadogenesis; normally, a pair of gonadal cells interact so that only one becomes the AC, but reduced lin-12 activity can cause both to become ACs (Greenwald et al., 1983; Sundaram and Greenwald, 1993). lin-12 also specifies two of the six vulval precursor cells (VPCs) to adopt the “2°” fate; reduced lin-12 activity can cause these two VPCs to adopt the “3°” fate (Greenwald et al., 1983; Sundaram and Greenwald, 1993). glp-1 activity promotes germline mitosis; reduced zygotic glp-1 activity causes germ cells to cease mitosis and enter meiosis prematurely, resulting in a sterile, oocyte-deficient phenotype (Austin and Kimble, 1987; Priess et al., 1987). lin-12 and glp-1 are also functionally redundant for other cell fate decisions during embryogenesis (Lambie and Kimble, 1991).
The role of sup-17 has been examined in these decisions (Tax et al., 1997; Wen et al., 1997). sup-17(null) hermaphrodites are maternal effect lethal; sup-17(null) hermaphrodites segregating from heterozygous parents do not display canonical phenotypes associated with loss of lin-12 or glp-1 activity. Similarly, sup-17(n1258ts), which behaves like a null allele at the restrictive temperature, does not display the canonical lin-12 gonadal or glp-1 germline phenotypes when shifted postembryonically, although it does display a low-penetrance defect in VPC fate specification that suggests that lin-12 activity has been partially reduced. In conclusion, the analysis of sup-17 mutants does not argue for an absolute requirement for SUP-17 in S2 cleavage of LIN-12 or GLP-1.
We obtained the adm-4(ok265) mutation, kindly provided by the Caenorhabditis Knockout Consortium, and examined it for canonical phenotypes associated with loss of lin-12 or glp-1 activity (Table 1). We sequenced adm-4(ok265) and found that it is predicted to encode a truncated product that lacks the disintegrin and cysteine-rich domains (Fig. 1A). Various truncated forms of mammalian TACE have activity under different conditions, but there is no strict correlation between disintegrin or cysteine-rich domain, or membrane association, and function (Reddy et al., 2000); therefore, we cannot predict whether adm-4(ok265) is null for TACE function.
Table 1.
adm-4(ok265)does not display highly penetrant phenotypes associated with loss of lin-12 or glp-1activity.
| Mutant phenotype | temperature | #mutant/tot (%) |
|---|---|---|
| Sterile | 15°C | 0/76 (0%) |
| 20°C | 0/83 (0%) | |
| 25°C | 0/43 (0%) | |
| Egl | 15°C | 0/76 (0%) |
| 20°C | 0/83 (0%) | |
| 25°C | 0/43 (0%) | |
| 2 AC | 20°C | 0/26 (0%) |
Scoring criteria are described in Materials and Methods.
adm-4(ok265) is fully recessive and behaves like adm-4(RNAi) in suppression of glp-1(ar202) or in producing a sterile phenotype in conjunction with sup-17 mutant, suggesting that it reduces adm-4 activity (Fig. 2, Table 3; see also below). adm-4(ok265) hermaphrodites, and adm-4(ok265) hermaphrodites that have also been subjected to adm-4(RNAi) to deplete potential residual activity (see below), do not display the canonical phenotypes associated with loss of lin-12 or glp-1 activity and are fully viable, indicating that they do not profoundly affect other cell fate decisions mediated redundantly by lin-12 and glp-1 (Table 1; Fig. 3; data not shown). Thus, the analysis of adm-4(ok265) suggests that if ADM-4 mediates lin-12 or glp-1 activity, it is not likely to be uniquely required.
Fig. 2.
Reduction of adm-4 or sup-17 activity suppresses glp-1(ar202). The first three bars indicate that adm-4(ok265) suppresses the sterility defect associated with glp-1(ar202). L1 hermaphrodites were transferred from 20°C to 25°C and scored as adults for production of oocytes and embryos. We note that 9% of the suppressed, fertile glp-1; adm-4 double mutants produced only embryos that arrested before hatching. This suggests that glp-1(ar202) mutation may also affect another process in the embryo, a phenotype that is normally masked by the sterility of glp-1(ar202) animals. This second defect is also suppressed by adm-4(ok265). The last four bars show that reduction of adm-4 or sup-17 activity by RNAi also suppresses the sterility defect associated with glp-1(ar202). L1 progeny of injected animals were transferred to 25°C and assessed as adults for production of oocytes and embryos. The total number of animals scored is indicated above each bar. Asterisks indicate significant differences at the 99% level in chi-square tests for comparisons shown.
Table 3.
sup-17; adm-4 double mutants display a novel sterile phenotype
| sterile/tot (%) 25°C | sterile/tot (%) 20°C | sterile/tot (%) 15°C | |
|---|---|---|---|
| sup-17 | 0/54 (0%) | 0/52 (0%) | 0/76 (0%) |
| adm-4 | 1/64 (1.5%) | 0/83 (0%) | 0/81 (0%) |
| sup-17 ;adm-4 | 67/68 (98.5%) | 65/115 (56.5%) | 16/115 (13.9%) |
| sup-17 ; mock RNAi | 22/151 (14.6%)* | nd | nd |
| sup-17 ; adm-4 RNAi | 35/100 (35%)* | nd | nd |
Worms were grown at 15°C and transferred as L1s at the experimental temperature. Asterisks indicates differences significant at the 99% level, as asssessed by performing a chi-square test.
Fig. 3.
A synthetic 2AC phenotype is observed in sup-17; adm-4 double mutants injected with adm-4 ds RNA. Each bar indicates the percentage of hermaphrodites with 2 anchor cells. The number of animals scored is indicated above each bar. Asterisks indicate significant differences at the 99% level in chi-square tests for comparisons shown. “from het”, the non-Unc progeny of a sup-17/unc-29 or sup-17/unc-29; adm-4 mother were scored. “25C”, animals grown continuously at 25°C were scored. “L1 shift”, animals shifted in the L1 stage from 15°C to 25°C were scored. For the last two genotypes, we first checked the AC phenotype of individual non-Unc L3s segregating from a sup-17/unc-29 ; adm-4 mother injected with adm-4 ds RNA or the buffer control, and included data only for those individuals that proved to be homozygous for sup-17 and adm-4.
We have observed a subtle phenotype in adm-4(ok265) hermaphrodites grown at 25°C: 25/58 hermaphrodites had occasional oocytes with nuclear morphology suggesting that they have undergone endoreduplication, as opposed to 0/46 N2 wild-type hermaphrodites. This phenotype resembles and appears to be a milder version of the phenotype observed in the sup-17; adm-4 double mutant, as described below.
Assessment of roles for sup-17 and adm-4 in canonical lin-12 or glp-1 mediated cell fate decisions
Certain missense mutations that alter the extracellular domain of LIN-12 or GLP-1 have been associated with constitutive (ligand-independent) activation of the receptor, and confer phenotypes opposite to those caused by reduced activity (Greenwald and Seydoux, 1990; Berry et al., 1997; Pepper et al., 2003). Suppression of phenotypes caused by such mutations of lin-12 [lin-12(d)] has identified many positive factors for lin-12/Notch signaling, including sup-17 (Tax et al., 1997).
We therefore examined the effects of reducing adm-4 activity on lin-12(d) alleles and on glp-1(ar202), a missense mutation that constitutively activates GLP-1 (Pepper et al., 2003). lin-12(d) alleles result in the absence of an anchor cell and ectopic induction of vulval 2° fates, and sup-17 hypomorphic and null alleles suppress these phenotypes (Tax et al., 1997). In contrast, we found that adm-4(ok265) does not suppress lin-12(d) defects (Table 2). In addition, analysis of reporter genes suggests that, while sup-17 is expressed in the VPCs at the time their decision is made, adm-4 is not (data not shown). These observations suggest that adm-4 does not play a major role in modulating lin-12 activity during these cell fate decisions.
Table 2.
adm-4(ok265) does not suppress the defects associated with lin-12(d) alleles.
| A- Egg-laying ability | n | #Egl+ (%) | |
|---|---|---|---|
| lin-12(n302) | 28 | 0 | 0% |
| lin-12(n302); adm-4(ok265) | 77 | 0 | 0% |
| lin-12(n379) | 27 | 4 | 14.8% |
| lin-12(n379); adm-4(ok265) | 28 | 3 | 10.7% |
| B- Multivulva phenotype | n | average number of protrusions | |
| lin-12(n950) | 96 | 4.8 | |
| lin-12(n950) ; adm-4(ok265) | 162 | 4.7 | |
Animals were scored at 20°C. For the Multivulva phenotype, the number of pseudovulval protrusions on individual animals was scored and the average number of protrusions per animal is indicated.
glp-1(ar202) promotes germline mitosis and causes sterility, as germ cells fail to enter meiosis and produce gametes (Pepper et al., 2002). We examined the ability of loss of sup-17 or adm-4 to suppress the sterility associated with glp-1(ar202), and found that reduction of adm-4 activity efficiently suppresses the sterility caused by glp-1(ar202), as does reducing sup-17 activity (Fig. 2). Thus adm-4 displays genetic interactions with at least one of the C. elegans LIN-12/Notch genes that is consistent with function as a positive regulator of this pathway.
Assessment of functional redundancy by examining the phenotype of the sup-17; adm-4 double mutant
If sup-17 and adm-4 are redundant for function in LIN-12/Notch signaling, we might expect that concomitant reduction of their activity would result in phenotypes associated with a reduction in lin-12 and/or glp-1 activity. As described in the next section, when we constructed the double mutant sup-17(n1258ts); adm-4(ok265), we observed a novel, highly penetrant sterile phenotype that would mask many canonical lin-12 or glp-1 phenotypes. Therefore, to overcome this sterility so that we could address potential functional redundancy in canonical lin-12 or glp-1-mediated cell fate decisions, we examined the progeny of sup-17(n1258ts)/unc-29; adm-4(ok265) hermaphrodites. We did not observe highly penetrant defects in the AC/VU decision, VPC specification or germline proliferation (Fig. 3 and data not shown). Nor is any AC/VU defect observed at 25°C in adm-4 mutants (not shown), sup-17(n1258) mutants (Tax) or in sup-17(n1258) animals segregating from a sup-17/+ mother (Fig. 3).
To further deplete any residual activity of adm-4(ok265), we also performed adm-4(RNAi) in various backgrounds. adm-4(RNAi) performed in an adm-4(ok265) mutant background did not affect the number of ACs: the progeny of hermaphrodites injected with adm-4 double stranded RNA or buffer all had one AC, as in wild type (Fig. 3). However, the progeny of sup-17(n1258ts)/unc-29; adm-4(ok265) mutants were affected when adm-4 double-stranded RNA, but not buffer, was injected: we saw a substantial number of individuals that had 2 anchor cells (Fig. 3), as in lin-12 hypomorphic or null mutants (Greenwald et al., 1983; Sundaram and Greenwald, 1993), suggesting that sup-17 and adm-4 are functionally redundant for LIN-12 signaling in the AC/VU decision, and that adm-4(ok265) indeed has some residual activity.
The novel synthetic sterile phenotype of the sup-17; adm-4 double mutant appears to reflect a defect in the function of the somatic gonad
The sup-17(n1258ts); adm-4(ok265) double mutant hermaphrodites displayed a highly penetrant defect in fertility (Table 3) that is rescued by a transgene encompassing the adm-4(+) genomic region (Fig. 5; see below). We examined the anatomy of the somatic gonad and germ line of sup-17(n1258ts); adm-4(ok265) double mutant hermaphrodites. The somatic gonad anatomy appears normal, and most aspects of germline development are normal: the mitotic distal region of the germ line, the maturation of the oocytes and sperm production or accumulation (data not shown). However, we did observe one abnormality in germline development: abnormal oocytes with endoreduplicated DNA, which sometimes appear to rupture, were observed in the uterus (Fig. 4), and in the most extreme cases, no eggs were evident. This defect would be masked by other phenotypic abnormalities in either lin-12 or glp-1 single mutants, or the lin-12 glp-1 double mutant, so we cannot say a priori whether this defect is likely to reflect reduced lin-12/Notch activity. However, additional data described in the next section provides such a link (see below).
Fig. 5.
Rescue of sup-17;adm-4 sterility by ADM-4(+) or LIN-12(ΔE) expression. L1 animals grown at 15°C were transfered to 20°C and their ability to produce progeny was assessed later. (A) arEx399 and arEx400 transgenes each encode ADM-4(+) and rescue the sterility defect of sup-17; adm-4. (B) arIs53, which encodes LIN-12(ΔE)::GFP, a constitutively active form of LIN-12, suppresses the sterility of sup-17; adm-4 double mutants. Percentage of fertile animals (A) or sterile animals (B) is indicated on the y axis. Above each bar is indicated the total number of animals scored. Asterisks indicate significant differences at the 99% level in chi-square tests for comparisons shown.
Fig. 4.
sup-17 ;adm-4 double mutants exhibit abnormal oocytes in the uterus. (A) Nomarski and (B) corresponding DAPI staining pictures of abnormal oocytes found in the uterus of fixed sup-17; adm-4 mutant adults grown at 15°C. Note the presence of big masses of DNA in the abnormal oocytes found in sup-17;adm-4 double mutants (arrowhead), indicative of endoreplicating DNA. (C) Nomarski and (D) corresponding DAPI staining pictures of wild type embryos (arrow) found in the uterus of fixed N2 adults grown at 15°C. The spermatheca and vulva are indicated, anterior is to the bottom.
Abnormal oocytes with this appearance may be caused by abnormal ovulation or fertilization. Abnormal ovulation may result from either a defect in the germ cells, in the sheath cells surrounding them or in the spermatheca (McCarter et al. 1997, 1999; Kariya et al., 2004). Abnormal fertilization may result from absence or abnormal sperm or malfunction of the spermatheca (Kariya et al., 2004). Our results point to abnormal function of the somatic gonad, and most likely the spermatheca, as the cause of the synthetic sterile phenotype of the sup-17(n1258ts); adm-4(ok265) double mutant. First, the synthetic sterile phenotype is efficiently rescued by simple arrays carrying adm-4(+) genomic DNA (Fig. 5A); such simple arrays are generally silenced in the germ line (Kelly et al., 1997). Second, the sterility defect is not rescued by mating with wild-type males, as would be expected if it were due to a problem with sperm (see Materials and Methods; Singson, 2001). Third, the problem appears to be functional as opposed to developmental, as temperature-shift experiments establish that the temperature-sensitive period includes young adulthood (Table 4), after the gonad has been formed and germ cells produced. Within the somatic gonad, defective spermathecal function appears to be the most likely origin of the phenotype, as transcriptional reporters for sup-17 and adm-4 are both expressed strongly in the spermatheca (data not shown), as is a lin-12 transcriptional reporter (Wilkinson and Greenwald, 1995).
Table 4.
Sterility in the sup-17;adm-4 double mutants is not rescued by late shifting of the animals to 20°C
| sup-17 ;adm-4 transfered from 15°C to 20°C as | sterile/tot (%) |
|---|---|
| L1 | 11/21 (52.3%) |
| L4 | 15/23 (65.2%) |
| young adult | 16/30 (53.3%) |
Animals were grown at 15°C and L1, L4 larvae or young adults were transferred at 20°C where their ability to produce progeny was assessed.
The synthetic sterility of the sup-17; adm-4 double mutant is rescued by expression of a constitutively active form of LIN-12 that mimics site 2 cleavage
Engineered forms of Notch that lack much of the extracellular domain are constitutively active, and are believed to mimic the site 2 cleavage (Jarriault et al., 1995; Struhl and Adachi, 1998). The corresponding form of LIN-12, LIN-12(ΔE), causes phenotypes associated with constitutive LIN-12 activity (D. Shaye and I.G., unpublished observations). If the synthetic sterility of the sup-17(n1258ts); adm-4(ok265) double mutant results from loss of ADAM function in the site 2 cleavage of LIN-12 (and/or GLP-1), we would expect that LIN-12(ΔE) might suppress this defect. We found significant suppression of the sterile phenotype of sup-17(n1258ts); adm-4(ok265) mutants (Fig. 5B). Although it is possible that ectopic activation of lin-12 (or glp-1) target gene expression is responsible for rescue, the simplest explanation is that the defect observed sup-17(n1258ts); adm-4(ok265) reflects lower lin-12/Notch signaling activity in the somatic gonad.
DISCUSSION
We have provided evidence that two ADAM family proteases, SUP-17 and ADM-4, are functionally redundant in C. elegans to facilitate LIN-12/Notch signaling. Depletion of either sup-17 or adm-4 suppresses the excessive mitotic proliferation of the germ line caused by an allele of glp-1 that encodes a constitutively active receptor. Concomitant reduction in the activity of sup-17 and adm-4 causes the production of two anchor cells in the hermaphrodite gonad instead of one, a defect that is characteristic of loss of lin-12 activity. Furthermore, a sterile phenotype observed by concomitant depletion of sup-17 and adm-4, which could reflect aberrant spermathecal function, can be suppressed by expression of an activated form of LIN-12 that mimics the product resulting from site 2 cleavage.
In Drosophila, the loss of Kuzbanian activity causes phenotypes that are equivalent to removing other Notch pathway components such as Presenilin (Rooke et al., 1996), suggesting that other proteases, including the Drosophila TACE ortholog, are not likely to play a significant role in most cell fate decisions mediated by Notch. In contrast, neither sup-17 nor adm-4 single mutants display highly penetrant defects associated with eliminating lin-12 or glp-1 activity (Tax et al., 1997; this work). Concomitant depletion of sup-17 and adm-4 activities causes a synthetic abnormality in the specification of the number of anchor cells, suggesting that SUP-17 and ADM-4 are functionally redundant in the anchor cell/ventral uterine precursor cell fate decision during gonadogenesis. However, there does not appear to be a catastrophic and general failure of LIN-12 and GLP-1-mediated signaling even when both sup-17 and adm-4 are depleted. It is possible that residual activity--either of the truncated ADM-4 product or maternal SUP-17--can account for these observations, although if that were the case, then we might have expected to observe a broad spectrum of phenotypes associated with loss of lin-12 and or glp-1 signaling at low penetrance. It is therefore possible that in C. elegans, there are redundant proteases that can mediate the extracellular cleavage. SUP-17 and ADM-4 are the closest ADAM family members in C. elegans, as it is the case for their mammalian orthologs, ADAM10 and TACE (Glassey and Civetta, 2004). However, C. elegans has many additional proteins of the Reprolysin family (see Fig. 1), and it is conceivable that other reprolysins provide redundant activity; furthermore, it is conceivable that proteases of another family provide the redundant activity.
We note that the genetic behavior of sup-17 and adm-4 is reminiscent of the genetic behavior of the two presenilin genes, sel-12 and hop-1. For example, sel-12 null mutants do not display most lin-12 or glp-1 single mutant phenotypes, and hop-1 null mutants display none (Levitan and Greenwald, 1995; Li and Greenwald, 1997; Westlund et al., 1999). Furthermore, sel-12 alleles are efficient suppressors of lin-12(d) alleles (Levitan and Greenwald, 1995; 1998), but hop-1 alleles are not (Westlund et al., 1999). sel-12 is strongly and generally expressed, whereas hop-1 is expressed at such a low level as to be essentially undetectable (Li and Greenwald, 1997). The main difference is that a combination of null alleles in a hop-1; sel-12 double mutant does result in highly penetrant defects suggestive of a general failure of LIN-12 and GLP-1-mediated signaling (Westlund et al., 1999), indicating that these two presenilins can account for all presenilin activity at site 3. In the context of the issue raised above, however, it is notable that partial depletion of hop-1 in a sel-12 background results in a variety of partially-penetrant phenotypes associated with reduced lin-12 and/or glp-1 activity (Li and Greenwald, 1997).
The evidence for functional redundancy of SUP-17 and ADM-4 in C. elegans may account for the relatively mild phenotypes of ADAM10 or TACE null mutant mice: ADAM10 and TACE may also be functionally redundant. Our results further suggest the possibility that functional redundancy may not be limited to just these two ADAM family members. It is indeed important to identify the proteases that may mediate ectodomain shedding of constitutively active LIN-12/Notch proteins, as missense mutations in mammalian Notch 1 that are analogous to missense mutations in lin-12 and glp-1 are highly associated with T-cell acute lymphoblastic leukemia (Chiaramonte et al., 2005), and perhaps other cancers in which Notch activation has been implicated, making these proteases potential therapeutic targets.
ACKNOWLEDGEMENTS
We would like to thank the C. elegans KO consortium and Yuji Kohara for providing useful nematode strains or plasmids. We are grateful to Tim Schedl, Darrell Killian and Jane Hubbard for valuable discussions, to Iskra Katic and Oliver Hobert for a critical reading of the manuscript, to Bernardo Reina San Martin for help with the phylogenetic analysis and figures, to Daniel Shaye for arIs53, and to Xinlan Zhou, Joanna Sesti and Richard Ruiz for technical assistance. This work was supported by NIH grant NS35556 awarded to I.G. and by the Howard Hughes Medical Institute. S.J. is a Postdoctoral Associate and I.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- Berry LW, Westlund B, Schedl T. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development. 1997;124:925–936. doi: 10.1242/dev.124.4.925. [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Chiaramonte R, Basile A, Tassi E, Calzavara E, Cecchinato V, Rossi V, Biondi A, Comi P. A wide role for NOTCH1 signaling in acute leukemia. Cancer Lett. 2005;219:113–120. doi: 10.1016/j.canlet.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Franzke CW, Tasanen K, Schacke H, Zhou Z, Tryggvason K, Mauch C, Zigrino P, Sunnarborg S, Lee DC, Fahrenholz F, Bruckner-Tuderman L. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. Embo J. 2002;21:5026–5035. doi: 10.1093/emboj/cdf532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Higgins DG, Thompson JD, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- Hooper NM. Families of zinc metalloproteases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- Huang X, Huang P, Robinson MK, Stern MJ, Jin Y. UNC-71, a disintegrin and metalloprotease (ADAM) protein, regulates motor axon guidance and sex myoblast migration in C. elegans. Development. 2003;130:3147–3161. doi: 10.1242/dev.00518. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Kariya K, Kim Bui Y, Gao X, Sternberg PW, Kataoka T. Phospholipase Cepsilon regulates ovulation in Caenorhabditis elegans. Dev Biol. 2004;274:201–210. doi: 10.1016/j.ydbio.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. kuzbanian is required cell autonomously during Notch signalling in the Drosophila wing. Dev Genes Evol. 2002;212:251–255. doi: 10.1007/s00427-002-0233-4. [DOI] [PubMed] [Google Scholar]
- Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer's disease. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development. 1998;125:3599–3606. doi: 10.1242/dev.125.18.3599. [DOI] [PubMed] [Google Scholar]
- Li X, Greenwald I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc Natl Acad Sci U S A. 1997;94:12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T, Kidd S, Young MW. kuzbanian-mediated cleavage of Drosophila Notch. Genes Dev. 2002;16:209–221. doi: 10.1101/gad.942302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, Rose-John S. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J Biol Chem. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- Mohrlen F, Hutter H, Zwilling R. The astacin protein family in Caenorhabditis elegans. Eur J Biochem. 2003;270:4909–4920. doi: 10.1046/j.1432-1033.2003.03891.x. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Pepper AS, Killian DJ, Hubbard EJ. Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics. 2003;163:115–132. doi: 10.1093/genetics/163.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000;275:14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- Rooke J, Pan D, Xu T, Rubin GM. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273:1227–1231. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singson A. Every sperm is sacred: fertilization in Caenorhabditis elegans. Dev Biol. 2001;230:101–109. doi: 10.1006/dbio.2000.0118. [DOI] [PubMed] [Google Scholar]
- Sotillos S, Roch F, Campuzano S. The metalloprotease-disintegrin Kuzbanian participates in Notch activation during growth and patterning of Drosophila imaginal discs. Development. 1997;124:4769–4779. doi: 10.1242/dev.124.23.4769. [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Sundaram M, Greenwald I. Genetic and phenotypic studies of hypomorphic lin-12 mutants in Caenorhabditis elegans. Genetics. 1993;135:755–763. doi: 10.1093/genetics/135.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax FE, Thomas JH, Ferguson EL, Horvitz HR. Identification and characterization of genes that interact with lin-12 in Caenorhabditis elegans. Genetics. 1997;147:1675–1695. doi: 10.1093/genetics/147.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C, Metzstein MM, Greenwald I. SUP-17, a Caenorhabditis elegans ADAM protein related to Drosophila KUZBANIAN, and its role in LIN-12/NOTCH signalling. Development. 1997;124:4759–4767. doi: 10.1242/dev.124.23.4759. [DOI] [PubMed] [Google Scholar]
- Westlund B, Parry D, Clover R, Basson M, Johnson CD. Reverse genetic analysis of Caenorhabditis elegans presenilins reveals redundant but unequal roles for sel-12 and hop-1 in Notch-pathway signaling. Proc Natl Acad Sci U S A. 1999;96:2497–2502. doi: 10.1073/pnas.96.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson HA, Greenwald I. Spatial and temporal patterns of lin-12 expression during C. elegans hermaphrodite development. Genetics. 1995;141:513–526. doi: 10.1093/genetics/141.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]






