Abstract
Wind-driven coastal ocean upwelling supplies nutrients to the euphotic zone near the coast. Nutrients fuel the growth of phytoplankton, the base of a very productive coastal marine ecosystem [Pauly D, Christensen V (1995) Nature 374:255–257]. Because nutrient supply and phytoplankton biomass in shelf waters are highly sensitive to variation in upwelling-driven circulation, shifts in the timing and strength of upwelling may alter basic nutrient and carbon fluxes through marine food webs. We show how a 1-month delay in the 2005 spring transition to upwelling-favorable wind stress in the northern California Current Large Marine Ecosystem resulted in numerous anomalies: warm water, low nutrient levels, low primary productivity, and an unprecedented low recruitment of rocky intertidal organisms. The delay was associated with 20- to 40-day wind oscillations accompanying a southward shift of the jet stream. Early in the upwelling season (May–July) off Oregon, the cumulative upwelling-favorable wind stress was the lowest in 20 years, nearshore surface waters averaged 2°C warmer than normal, surf-zone chlorophyll-a and nutrients were 50% and 30% less than normal, respectively, and densities of recruits of mussels and barnacles were reduced by 83% and 66%, respectively. Delayed early-season upwelling and stronger late-season upwelling are consistent with predictions of the influence of global warming on coastal upwelling regions.
Keywords: climate variability, coastal marine ecosystems, coastal ocean upwelling, marine ecology
Equatorward winds along the eastern boundaries of the world's oceans drive offshore surface Ekman transport and the upwelling of cold, nutrient-rich water into the euphotic zone near the coast. These nutrient pulses stimulate high phytoplankton production, which, in turn, supports a rich coastal marine ecosystem and productive fisheries (1). Examples of such dynamics include the California Current, the Humboldt Current, the Benguela Current, and the Canary Current (2).
The strength and extent of the seasonal cycle in upwelling-favorable winds varies along the U.S. west coast. In the northern California Current Large Marine Ecosystem (CCLME), there is a strong seasonal cycle with upwelling-favorable winds, the appearance of cold, saline, nutrient-rich water near the coast, and equatorward currents over the shelf occurring after a spring transition (3). Alongshore winds in the northern CCLME are more variable than those farther south because they are more frequently influenced by eastward-traveling Gulf of Alaska low-pressure systems. The intermittent cessation of upwelling-favorable winds is called relaxation and plays an important role in coastal circulation and the recruitment of marine organisms††. The timing of the spring transition and the total amount of upwelling-favorable winds during the spring–summer upwelling season have a considerable impact on coastal ecosystem responses. Farther south in the CCLME, winds are more persistently upwelling-favorable and the transition to a more productive spring–summer season is less pronounced. The extent to which the timing of the spring transition and the intensity of upwelling-favorable winds might be influenced by climate variability is an area of active research (5, 6).
Spatial and temporal changes in nutrient availability and phytoplankton biomass propagate up marine food webs and may strongly mediate the structure and dynamics of nearshore ecological communities (7). Decreased upwelling can have two effects: reduced nutrient supply to phytoplankton (8) and reduced offshore transport of phytoplankton and planktonic fish and invertebrate larvae that are crucial for replenishing coastal populations (9). Nutrient and phytoplankton reductions may decrease zooplankton (including larvae) survival, and thus decrease recruitment rates of planktotrophic larvae. Slower offshore transport could have the opposite effect, with greater retention and increased recruitment rates. When intraseasonal variations in upwelling forcing are substantial, changes in the timing of phytoplankton blooms can potentially decouple food availability from consumer demands (10). Such temporal trophic mismatches may have particularly important effects on recruitment class strength in fish and invertebrates (11).
Recruitment patterns of rocky shore barnacles and mussels can provide insight into the dynamics of inner-shelf ecosystems (12). Sessile adult barnacles and mussels release larvae or gametes into the water that spend ≈2–4 weeks in the plankton, feeding on small plankton for their sustenance (13). Larvae develop until they are competent to settle, then they must be transported back to shore if they are to recruit and begin life as tiny sessile barnacles or mussels. Growth rates, settlement sizes, and survival of mussel larvae, and probably barnacle larvae, depend on phytoplankton concentration that, in turn, depends on nutrients provided by upwelling events (14). Previous work has documented that both barnacles and mussels recruit heavily but intermittently throughout summer in Oregon (9). Recruitment events of barnacles in California (15) and Oregon†† often follow upwelling relaxation, i.e., times when larvae are transported shoreward.
Barnacles and mussels create 3D spatial structure (habitat) in rocky intertidal communities and are the primary food for many predators (16). Barnacle and mussel juveniles and adults are abundant and relatively easy to monitor and have similar life histories to numerous other ecologically or economically important pelagic and benthic inner-shelf species. Consequently, they are good surrogates for many other inner-shelf species with respect to the impact of environmental changes in the inner shelf. There are few long-term, ecological time series available for the detection and quantification of ocean ecosystem responses to environmental change. We use a long-term set of physical, chemical, and biological measurements to examine the unusual presence of warm water, low nutrients and low chlorophyll-a (chl-a) near the coast during 2005 that led to unprecedented low recruitment of rocky intertidal organisms in the northern CCLME during the early upwelling season.
Results and Discussion
Perturbations to Upwelling Regime.
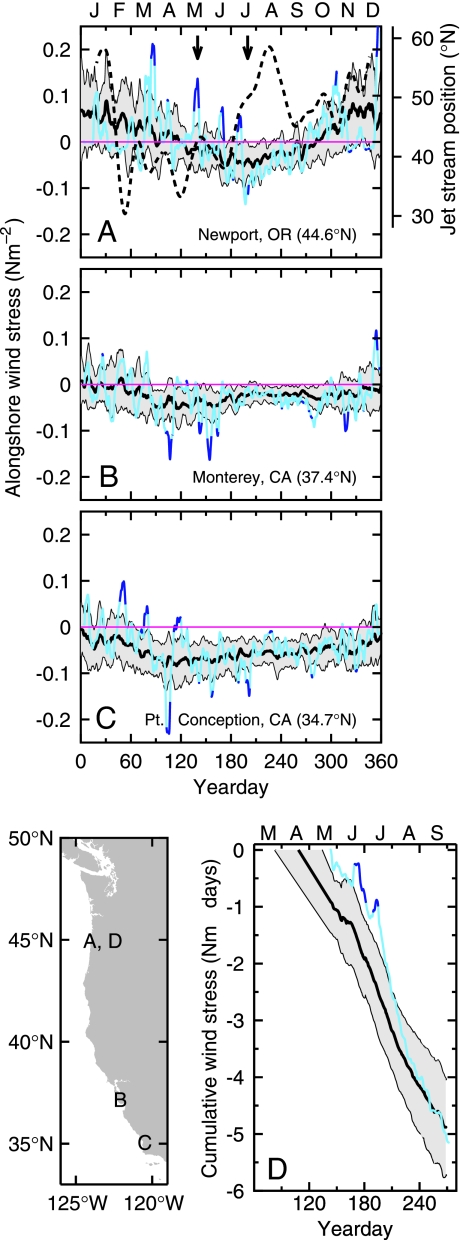
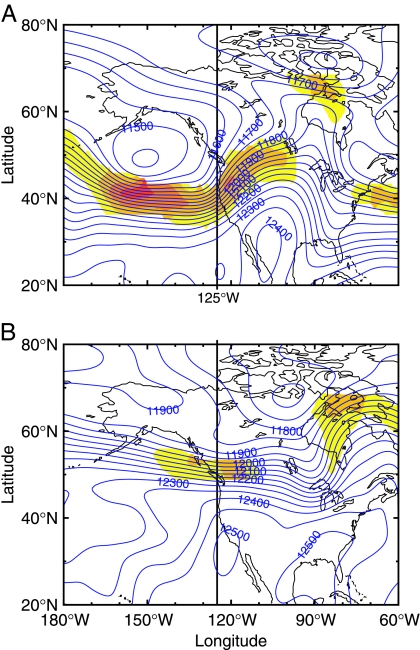
Year-to-year differences in the timing of the spring transition and the total amount of seasonal upwelling-favorable winds may have considerable impact on coastal ecosystem responses. Off central Oregon (44.62°N) where winds have a pronounced seasonal cycle, anomalous winds in 2005 were dominated by five strong, northward wind events, separated by 20–40 days, during March–July (Fig. 1A). The onset of unusually strong, upwelling-favorable winds off central Oregon in early to mid-July coincided with a northward shift of ≈1,000 km in the position of the jet stream (Fig. 1A). A map of 200-hPa surface height during the mid-May northward wind event that occurred before this northward shift shows a jet-stream position, and hence storm track, more typical of wintertime conditions (17) compared with a typical summertime jet-stream location in mid-July after the shift (Fig. 2).
Fig. 1.
Alongshore wind stress off the U.S. west coast. (A–C) Alongshore wind stress from three west-coast buoys: Newport, OR (A), Monterey Bay, CA (B), and Point Conception, CA (C). Values for 2005 (blue) are plotted on top of the climatological mean (black) ± 1 SD (gray shading) for 1985–2005. In A, the dashed curve is the north–south position of the jet stream, and the two arrows indicate the times of maps shown in Fig. 2. (D) Cumulative alongshore (north–south) wind stress from National Oceanic and Atmospheric Administration NDBC Buoy 46050 offshore of Newport, OR, starting from the spring transition. The black curve and shading represent the mean ± 1 SD for 1985–2005, and the blue curve is for 2005. At zero cumulative wind stress, the black curve and shading represent the mean and ±1 SD of the date of the spring transition, and the blue line represents the date of the 2005 spring transition. The 2005 curves are dark blue when absolute values exceed any observed values during the previous 20 years. Data locations are indicated at lower left.
Fig. 2.
Maps of 200-hPa surface height (m, blue contours) and wind speed at 300 hPa (m·s−1, color shading), from six hourly National Centers for Environmental Prediction reanalyses (25) when the jet stream is located anomalously south (May 20, 2005, 1200 Coordinated Universal Time; A) and in its more typical summer position (July 19, 2005, 0000 Coordinated Universal Time; B). Wind speeds >35 m·s−1 are shown, with color increments at 45, 55, and 65 m·s−1.
Off central California (37.36°N), 2005 winds were not unusual except for perhaps some stronger-than-normal southward winds from April to June (Fig. 1B). Off of southern California (34.72°N), but outside the southern California Bight lee region, there were two unusually strong northward wind events in February-March followed by normal or slightly stronger upwelling-favorable winds the remainder of the year (Fig. 1C).
In 2005, the cumulative alongshore wind stress since the spring transition off of Oregon contrasts strongly with the long-term average and reveals the unusual timing of the upwelling-favorable winds (Fig. 1D). By examining wind and sea-level records, we determined that the 2005 spring transition was on May 24, over a month later than average and outside of the 1-SD range of the last 20 years. The first substantial upwelling did not occur until late June, ≈2 months later than average, but was terminated by the last of the northward wind events in July 2005. Stronger-than-average upwelling-favorable winds commenced in early to mid-July and persisted until the seasonal accumulation of upwelling-favorable winds reached the long-term average in September.
Effects on Temperature.
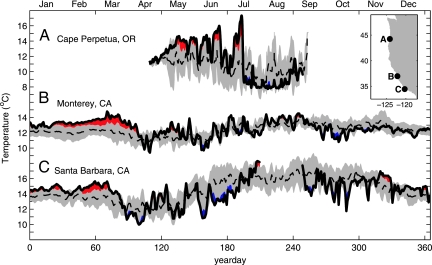
Near-surface temperatures measured close to shore illustrate the coastal ocean response to the unusual winds of 2005 (Fig. 3). Off of central Oregon from May to mid-July 2005, nearshore surface waters averaged 2°C warmer than normal, with a maximum warming of 6.4°C (Fig. 3A). Upwelling-favorable winds in mid-June (weak) and late in June (stronger) cooled upper-ocean temperatures to nearly normal. However, after each of these cooling periods, nearshore temperatures returned to above-average levels, as warmer offshore waters were advected onshore by surface Ekman transport driven by northward wind events. In mid-July, nearshore temperatures cooled to below normal as a result of persistent and vigorous coastal upwelling. Off of central California, nearshore surface temperatures were 2–3°C higher than normal until early to mid-April, in part because of the northward wind event in mid-March, after which temperatures returned to normal (Fig. 3B). Off of southern California, nearshore surface temperatures were near normal, except for perhaps slight above-average temperature in late February–early March in response to two northward wind events (Fig. 3C).
Fig. 3.
Surface (0–2 m) temperatures during 2005 (solid lines) compared with climatological means (dashed lines) with ±1 SD (shaded) from inner-shelf moorings in 15 m of water off of central Oregon (44.25°N, 124.13°W) (1998–2004 mean) (A), 21 m of water off of Monterey Bay, CA (36.97°N, 122.16°W) (1999–2004 mean) (B), and 15 m of water off of Santa Barbara, CA (34.46°N, 120.29°W) (1999–2004) (C). Temperatures during 2005 that are warmer (colder) than the climatological mean ± 1 SD are shaded in red (blue).
Bottom-Up Effects.
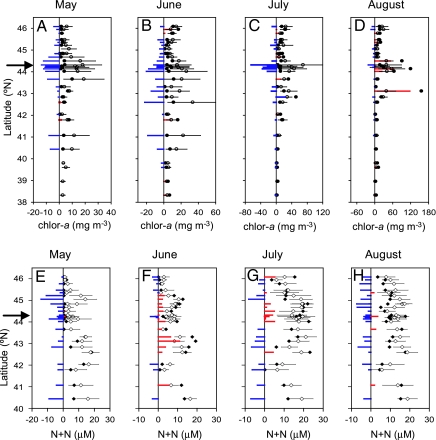
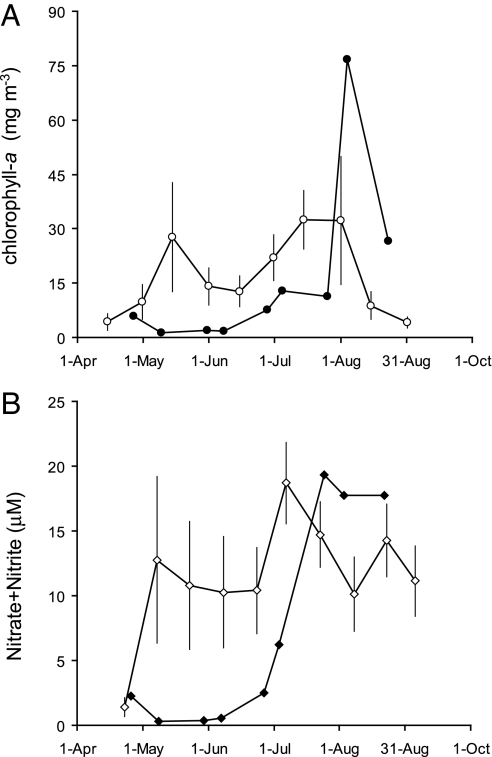
In 2005, coastwide negative chl-a anomalies were evident during May, with the largest anomalies off of the central Oregon coast (Fig. 4A). Depression of surf-zone chl-a continued through July before reverting to neutral or increased levels in August (Figs. 4A and 5). The May 2005 negative chl-a anomalies were accompanied by strong, coastwide decrease in nitrate concentration (Fig. 4B). Negative nitrate anomalies did not accompany chl-a reductions in June and July at the coastwide scale, although persistent negative nitrate anomalies were evident from a number of sites along the central and southern Oregon coasts (Figs. 4 and 5).
Fig. 4.
Surf-zone chlorophyll and nutrients measured along the Oregon and north/central California coasts. (A–D) Chl-a measured along the coast during May-August 2005 (●), the long-term climatological mean (○) with 95% C.I. (bars), and 2005 anomalies from the mean (colored bars). (E–H) As in A–D, but for nitrate plus nitrite (N + N). The arrows at 44.25°N indicate the location of time series shown in Fig. 5.
Fig. 5.
Time series of surf-zone chlorophyll and nutrients off the central Oregon coast. (A) Time series of chl-a (circles) measured at the coast off of central Oregon (44.25°N): 2005 (filled symbols); 1993–2004 climatological mean (open symbols) with 95% C.I. (bars). (B) As in A, but for nitrate plus nitrite (diamonds).
Effects on Recruitment.
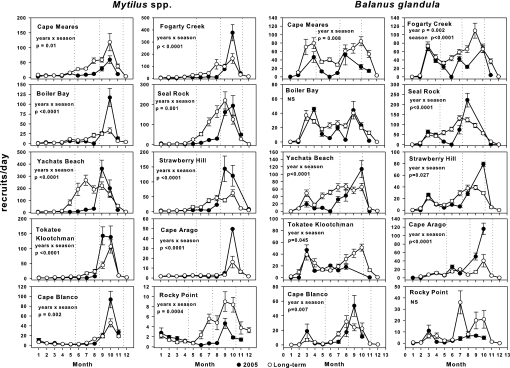
The ≈2-month delay in the onset of substantial upwelling in 2005 had serious consequences for recruitment of mussels and barnacles. Mussel recruitment during May-August in 2005 was the lowest ever recorded for these 4 months at every site (Fig. 6). During the next 2 months, September–October, after unusually intense and continuous upwelling, recruitment rebounded to values higher than average except for the two stations in the extreme south and north of the measurement region.
Fig. 6.
Mussel (Mytilus spp.) (Left) and barnacle (B. glandula) (Right) recruitment at sites along the Oregon coast: 2005 (●) compared with climatological monthly means (○) computed for 8–17 years (including 2005) depending on site. Means and SEM are shown for all data. Dotted vertical lines mark the “early” and “late” recruitment seasons used in data analysis. The absence of dotted lines indicates sites where no differences between years or season occurred.
Barnacles and mussels often show different recruitment patterns (8). Early in the season (May–July), barnacle (Balanus glandula) recruitment was lower than normal at a number of, but not all, sites (Fig. 6; see Methods for details of analysis). During August–October, after strong and persistent upwelling, B. glandula recruitment was higher, indicating a rebound, at many, but not all, sites. For both barnacles and mussels, recruitment in 2005 differed greatly between early (June–August for mussels, May–July for barnacles) versus late (September–November for mussels, August–October for barnacles) recruitment at most sites (Fig. 6).
Summing results across all 10 sites indicates that in 2005 during June–August, mussel recruitment was reduced by 83%. During the next 3 months (September–November), mussel recruitment rebounded for an increase of 53% compared with normal years. Overall, however, total mussel recruitment for the entire 6-month recruitment season (June–November) in 2005 was normal. Barnacle recruitment in 2005 was depressed 66% during May–July and was nearly normal (2.5% increase) during August–October, although this late-season response was variable among sites. Total barnacle recruitment for the 6-month recruitment season (May–October) in 2005 was 38% less than normal.
The influence of a delayed transition to upwelling was most dramatic in the northern CCLME (18) as might be expected because the seasonal cycle in wind stress is most pronounced in this region (Fig. 1A). Because upwelling was abnormally low in the early season, and because weak upwelling should lead to retention of larvae close to shore, the unprecedented low early-season recruitment of mussels and barnacles was probably caused by low food supply (i.e., low phytoplankton, a consequence of low nutrients) rather than by offshore loss of larvae caused by advection. By midsummer, however, upwelling-favorable winds were stronger than normal, resulting in higher phytoplankton biomass (Figs. 4 and 5). During the late summer season, recruitment of both mussels and barnacles rebounded. Because spawning to settlement of these organisms takes 2 weeks to 2 months (13), it is likely that these late-season recruits (August–November) were the result of reproduction that occurred shortly after the resumption of upwelling in mid-July. Spatial differences in recruitment patterns may reflect among-site differences in nearshore circulation.
Consequences for the Marine Food Web.
The ecological consequences of these changes are potentially severe. The low recruitment of the intertidal species reported here is consistent with lower-than-normal concentrations of zooplankton (17) in the northern CCLME. Reproductive failure of a planktivorous seabird, Cassin's auklet (Ptychoramphus aleuticus) in the CCLME during 2005 (17) is also a likely result of the chain of reductions at lower trophic levels in coastal food webs initiated by the reduced nutrients and phytoplankton at a critical time in the life history of coastal seabirds. Reduced phytoplankton is likely to slow growth and suppress reproductive output of sessile invertebrates (19). Reduced recruitment of sessile invertebrates could diminish the food supply for predators such as whelks, sea stars, crabs, and shorebirds, with potentially far-reaching consequences elsewhere in the food web (20).
Intraseasonal Oscillations (ISOs).
Recent studies have shed light on the importance of ISOs of the wind (20- to 40-day periods) on coastal upwelling (21, 22) and coastal ecosystem dynamics (J.M.B., Y. Spitz, R. Letelier, and W. T. Peterson, unpublished work). The five large wind ISOs played a key role in delaying the spring transition in the northern CCLME in 2005. Wind ISOs at midlatitude are formed through interactions of the midlatitude jet stream with large-scale topography (23). During the summer of 2001, ISOs in alongshore wind stress off of Oregon (44.6°N) correlated well with the north–south position of the jet stream (22). The cessation of strong wind ISOs and the onset of unusually strong upwelling-favorable wind in the northern CCLME in early to mid-July 2005 were consistent with a shift of the jet stream from the south to the north of this region (Figs. 1A and 2).
Delayed early-season upwelling and stronger late-season upwelling documented here for the northern CCLME during 2005 are consistent with predictions of the influence of global warming on coastal upwelling regions (5, 6). The global-warming scenario relies on increased land–sea temperature contrasts, created by preferential heating of the land caused by elevated greenhouse gases, driving stronger equatorward winds, and hence stronger upwelling.
In experiments using a regional climate model, albeit in the absence of an active ocean submodel, increased greenhouse gas forcing results in a 1-month delay in the onset of the coastal upwelling season in the northern CCLME and an increase in upwelling intensity later in the season (June–September) (6). The mechanism by which this happens, changes in radiative forcing, is different from the dynamics described above involving wind ISOs and an anomalously southward jet-stream position that led to delayed upwelling. Sorting out the relative roles of these atmospheric processes is key to our ability to predict future changes in coastal ocean ecosystems caused by climate variability and climate change.
Methods
Meteorological Data.
Wind stress was calculated by using measurements from the National Oceanic and Atmospheric Administration National Data Buoy Center (NDBC) buoys and Coastal-Marine Automated Network (C-MAN) stations (24) and then low-pass-filtered with a filter with a 40-h width at half-amplitude to remove short-period (e.g., diurnal) fluctuations. The alongshore wind stress was computed by rotating into a coordinate system aligned with the local coastline (rotation angle indicated for each site below). Winds were measured at NDBC buoy 46050 off of central Oregon (44.62°N, 124.53°W; 3°), buoy 46012 off of Monterey, CA (37.36°N, 122.88°W; 327°), and buoy 46023 off of Point Arguello, CA (34.72°N, 120.97°W; 329°). Gaps in the NDBC buoy records were filled through regression with nearby buoys and/or C-MAN stations: 46050 with Newport, OR, C-MAN (NWPO3) and occasionally the Cape Arago, OR, C-MAN (CARO3); 46012 with buoys 46026 and 46042; 46023 with buoy 46011 and Point Conception, CA, C-MAN (PTGC1). A 5-day running mean was then applied to all time series. To assess the cumulative alongshore wind stress off of central Oregon during the upwelling season, north–south stress was summed starting from the spring transition, defined as when winds turn to predominantly upwelling-favorable (southward) usually during March to April (3).
The latitudinal position of the jet stream in the northern CCLME was taken as the location, along 125°W longitude, of the strongest horizontal gradient in the height of the 200-hPa surface (22), determined from six hourly National Centers for Environmental Prediction reanalysis pressure maps (25). The jet-stream position time series was then filtered with a 35-d cosine low-pass filter.
Mooring-Based Measurements.
Temperature was measured at 1- to 2-min intervals just below the surface (0–3 m) by using StowAway XTI Temperature Loggers (Onset Computer Corp.) on moorings deployed in water depths of 15 m (central Oregon, 44.25°N, 124.13°W; Santa Barbara, CA, 34.46°N, 120.29°W) or 21 m (Monterey, CA, 36.97°N, 122.16°W).
Coastal Transects.
Surf-zone chl-a and inorganic nutrient measurements (nitrate plus nitrite) were made between 2001 and 2005 during May and June and between 1997 and 2005 during July and August, at 30 sites along the Oregon and north/central California coasts (46–38°N). Once a month from May through August, synoptic measurements were all made on the same day along the coast between 3 h before and 3 h after low tide. In central Oregon (44.25°N), additional data were collected more frequently (daily to biweekly) between 1993 and 2005. Anomalies were computed by differencing values from 2005 with a long-term mean for each month: May–June (2001–2005) and July–August (1997–2005). Chl-a and nitrate plus nitrite measurements were made according to standard methods (26, 27).
Mussel and Barnacle Recruitment.
Monthly recruitment (the appearance of new young on rocky shores) of mussels and barnacles has been measured monthly at 10 sites spanning two-thirds of the Oregon coast: for 17 years at Boiler Bay (44.83°N, 124.06°W) and Strawberry Hill (44.25°N, 124.13°W) (since 1989); for 12 years at Fogarty Creek (44.84°N, 124.06°W) and Seal Rock (44.50°N, 124.10°W) (since 1994); for 11 years at Cape Meares (45.47°N, 123.97°W) and Cape Arago (43.31°N, 124.40°W) (since 1995); for 9 years at Yachats Beach (44.32°N, 124.11°W) (since 1997); and for 8 years at Tokatee Klootchman (44.20°N, 124.12°W), Cape Blanco (42.84°N, 124.56°W), and Rocky Point (42.72°N, 124.47°W) (since 1998). Mussel recruitment was quantified by using plastic mesh balls (Tuffys; The Clorox Company, Oakland, CA), and barnacle recruitment was quantified by using settlement plates [0.10 × 0.10 × 0.004-m poly(vinyl chloride) plates with Saf-T-Walk (3M Company, St. Paul, MN), a textured plastic tape, on the top side] (4, 28). Collectors were deployed/recovered monthly and processed in the laboratory where counts of mussels (number per collector) and barnacles (number per 10−2 m2) were made under dissecting microscopes. Barnacle recruits were separated by species with B. glandula and Chthamalus dalli as the most abundant barnacles for which we have long-term recruitment data. Mussel species cannot be reliably identified visually. Judging from the species composition of mussels growing to identifiable sizes on the shore after recruitment, most mussel recruits are Mytilus trossulus, but very small numbers of Mytilus californianus also settle jointly with Mytilus trossulus.
Because we were interested in comparing among-year and within-season differences, recruitment data were analyzed by using two-way ANOVA. Factors tested were year (2005 vs. the full data set) and recruitment season (early and late). Both factors were fixed. To improve the fit of the data to a normal distribution, we transformed the response variable to ln (recruits per day + 1); we used P = 0.05 as our level of significance. Sample size (n) in these analyses varied among sites from 181 to 517 (mussels) and 184 to 571 (barnacles) and is based on five to eight recruit collector samples for each species per month per site for the time periods listed above. Sample size varied because of varying numbers of years in which sampling was done and occasional losses of collectors. We report P values for interactions (year × season), which subsumes main effects when significant, or main effects (year or season) when interactions were not significant (Fig. 6). For barnacles, analysis of data using May–July and August–October provided the clearest contrast between early and late recruitment seasons, respectively (except for Cape Arago and Cape Blanco). For mussels, analysis of data using June–August and September–November provided the clearest contrast between early and late recruitment seasons (except for Rocky Point) The variation in which season was analyzed is shown by the vertical dotted lines in Fig. 6.
Acknowledgments
We thank the many scientists, technicians, and students of the Partnership for Interdisciplinary Studies of Coastal Oceans who were involved with the collection of the long-term data sets and who made this study possible. The Partnership for Interdisciplinary Studies of Coastal Oceans is supported by the David and Lucile Packard Foundation and the Gordon and Betty Moore Foundation. Additional support for J.A.B. was provided by National Science Foundation Grants OCE-9907854, OCE-0435619, OCE-0453071, and OCE-0527168. Additional support for B.A.M. and J.L. was provided by the A. W. Mellon Foundation, the Wayne and Gladys Valley Foundation, and the Robert and Betty Lundeen Marine Biology Fund. This paper is Partnership for Interdisciplinary Studies of Coastal Oceans contribution 250 and contribution number 516 of the U.S. Global Ocean Ecosystem Dynamics program, jointly funded by the National Science Foundation and National Oceanic and Atmospheric Administration.
Abbreviations
- CCLME
California Current Large Marine Ecosystem
- chl-a
chlorophyll-a
- ISO
intraseasonal oscillation
- NDBC
National Data Buoy Center
- C-MAN
Coastal-Marine Automated Network.
Footnotes
The authors declare no conflict of interest.
Grantham, B. A., Menge, B. A., Lubchenco, J. (2002) Eos Trans Am Geophys Union 83(Suppl):OS12K-03 (abstr.).
References
- 1.Pauly D, Christensen V. Nature. 1995;374:255–257. [Google Scholar]
- 2.Wooster WS, Reid JL. In: The Sea. Hill MN, editor. Vol 2. New York: Wiley; 1963. pp. 253–280. [Google Scholar]
- 3.Huyer A, Sobey EJC, Smith RL. J Geophys Res. 1979;84:6995–7011. [Google Scholar]
- 4.Menge BA, Daley BA, Lubchenco J, Sanford E, Dahlhoff E, Halpin PM, Hudson G, Burnaford J. Ecol Monogr. 1999;69:297–330. [Google Scholar]
- 5.Bakun A. Science. 1990;247:198–201. doi: 10.1126/science.247.4939.198. [DOI] [PubMed] [Google Scholar]
- 6.Snyder MA, Sloan LC, Diffenbaugh NS, Bell JL. Geophys Res Lett. 2003;30:1823. [Google Scholar]
- 7.Menge BA, Daley BA, Wheeler PA, Dahlhoff E, Sanford E, Strub PT. Proc Natl Acad Sci USA. 1997;94:14530–14535. doi: 10.1073/pnas.94.26.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIsaac JJ, Dugdale RC, Barber RT, Blasco D, Packard TT. Deep-Sea Res. 1985;32:503–529. [Google Scholar]
- 9.Connolly SR, Menge BA, Roughgarden J. Ecol. 2001;82:1799–1813. [Google Scholar]
- 10.Edwards M, Richardson AJ. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 11.Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC. Nature. 2003;426:661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- 12.Menge BA, Lubchenco J, Bracken MES, Chan F, Foley MM, Freidenburg TL, Gaines SD, Hudson G, Krenz C, Leslie H, et al. Proc Natl Acad Sci USA. 2003;100:12229–12234. doi: 10.1073/pnas.1534875100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strathmann MF. Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast. Seattle: Univ Wash Press; 1987. [Google Scholar]
- 14.Phillips NE. Ecology. 2002;83:2562–2574. [Google Scholar]
- 15.Farrell TM, Bracher D, Roughgarden J. Limnol Oceanogr. 1991;36:279–288. [Google Scholar]
- 16.Menge BA, Branch GM. In: Marine Community Ecology. Bertness MD, Gaines SD, Hay ME, editors. Sunderland, MA: Sinauer; 2001. pp. 221–251. [Google Scholar]
- 17.Sydeman WJ, Bradley RW, Warzybok P, Abraham CL, Jahncke J, Hyrenbach KD, Kousky V, Hipfner JM, Ohman MD. Geophys Res Lett. 2006;33:L22S09. [Google Scholar]
- 18.Thomas AC, Brickley P. Geophys Res Lett. 2006;33:L22S05. [Google Scholar]
- 19.Leslie HM, Breck E, Chan F, Lubchenco J, Menge BA. Proc Natl Acad Sci USA. 2005;102:10534–10539. doi: 10.1073/pnas.0503874102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menge BA. Ecol Mon. 1995;65:21–74. [Google Scholar]
- 21.Breaker LC, Liu PC, Torrence C. Cont Shelf Res. 2001;21:727–750. [Google Scholar]
- 22.Bane JM, Levine MD, Samelson RM, Haines SM, Meaux MF, Perlin N, Kosro PM, Boyd T. J Geophys Res. 2005;110:C10S02. [Google Scholar]
- 23.Lott F, Robertson AW, Ghil M. Geophys Res Lett. 2001;28:1207–1210. [Google Scholar]
- 24.Large WG, Pond S. J Phys Oceanogr. 1981;11:324–336. [Google Scholar]
- 25.Kalnay E, Kanamitsu M, Kistler R, Collins W, Deaven D, Gandin L, Iredell M, Saha S, White G, Woollen J, et al. Bull Am Meteorol Soc. 1996;77:437–471. [Google Scholar]
- 26.Grasshoff K, Kremling K, Ehrhardt M. Methods of Seawater Analysis. Weinheim, Germany: Wiley; 1999. [Google Scholar]
- 27.Welshmeyer NA. Limnol Oceanogr. 1994;39:1985–1992. [Google Scholar]
- 28.Menge BA, Berlow EL, Blanchette CA, Navarrete SA, Yamada SB. Ecol Monogr. 1994;64:249–286. [Google Scholar]