Abstract
The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway was recently shown to negatively regulate LPS-induced acute inflammatory responses. We previously observed that the metabolic thiol antioxidant α-lipoic acid (LA) inhibits LPS-induced expression of cellular adhesion molecules and adherence of monocytes to human aortic endothelial cells. Here we investigated the mechanism by which LA attenuates LPS-induced monocyte activation in vitro and acute inflammatory responses in vivo. Incubation of human monocytic THP-1 cells with LA induced phosphorylation of Akt in a time- and dose-dependent manner. In cells pretreated with LA followed by LPS, Akt phosphorylation was elevated initially and further increased during incubation with LPS. This LA-dependent increase in Akt phosphorylation was accompanied by inhibition of LPS-induced NF-κB DNA binding activity and up-regulation of TNFα and monocyte chemoattractant protein 1. Lipoic acid-dependent Akt phosphorylation and inhibition of NF-κB activity were abolished by the PI3K inhibitors LY294002 and wortmannin. Furthermore, LA treatment of LPS-exposed C57BL/6N mice strongly enhanced phosphorylation of Akt and glycogen synthase kinase 3β in blood cells; inhibited the LPS-induced increase in serum concentrations and/or tissue expression of adhesion molecules, monocyte chemoattractant protein 1, and TNFα; and attenuated NF-κB activation in lung, heart, and aorta. Lipoic acid also improved survival of endotoxemic mice. All of these antiinflammatory effects of LA were abolished by treatment of the animals with wortmannin. We conclude that LA inhibits LPS-induced monocyte activation and acute inflammatory responses in vitro and in vivo by activating the PI3K/Akt pathway. Lipoic acid may be useful in the prevention of sepsis and inflammatory vascular diseases.
Keywords: inflammation, NF-κB, sepsis, endothelial activation, monocytes
A central feature of the pathophysiology of acute inflammation and septic shock triggered by the bacterial endotoxin LPS is the production of multiple proinflammatory mediators such as cellular adhesion molecules (CAMs), cytokines, and chemokines by vascular endothelial cells and monocyte–macrophages (1, 2). Although these proinflammatory mediators are required for an adequate host-defense response, dysregulation of their production can lead to refractory hypotension, cardiovascular hyporeactivity, intravascular coagulation, multiple organ failure, and death (3–5).
Endothelial cells are a primary target of immunological attack in sepsis, and their injury can lead to vasculopathy and organ dysfunction (2). LPS directly elicits several acute inflammatory responses in endothelial cells, including production of the proinflammatory cytokines and chemokines TNFα, IL-1β, and monocyte chemoattractant protein 1 (MCP-1); increased surface expression of the CAMs, E-selectin, vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1), which elicits leukocyte adhesion to the vasculature and transmigration into the underlying tissue; and increased expression of tissue factor, which causes intravascular coagulation and endothelial injury and dysfunction associated with sepsis (2).
Regulation of endothelial CAM and cytokine expression has been related to oxidative stress through specific reduction–oxidation (redox)-sensitive signaling pathways and transcription factors such as NF-κB and AP-1 (6–8). Intracellular antioxidants may affect cellular redox environment and thus attenuate the response of endothelial cells to oxidative stress and inflammatory cytokines. For example, the antioxidants pyrrolidine dithiocarbamate, N-acetyl-l-cysteine, and α-lipoic acid (LA; 1,2-dithiolane-3-pentanoic acid) inhibit cytokine- and LPS-induced CAM expression in various cell types (9–11) and animal models (12–14).
The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway has been shown to play an important role in negatively regulating LPS-induced acute inflammatory responses in vitro and in vivo (15–21). Inhibition of PI3K/Akt signaling enhances LPS-induced activation of NF-κB, AP-1, and Egr-1 transcription factors and gene expression of TNFα and tissue factor in cultured human monocytic cells (15). Similar effects were observed in vivo, where inhibition of PI3K/Akt enhanced LPS-induced coagulation and inflammation in endotoxemic mice (16). Conversely, activation of the PI3K/Akt pathway by glucan phosphate (17) or angiopoietin-1 (18) inhibited TNFα-induced tissue factor expression in endothelial cells (18) and was correlated with increased survival in a cecal ligation and puncture (CLP) septic mouse model (17). Furthermore, transgenic overexpression of Akt has been shown to protect against septic death in the CLP mouse model (19) as well as mice infected with the Gram-negative bacterium Francisella (20). Recent studies have shown that Akt dampens LPS-induced NF-κB activation by phosphorylating and, hence, inactivating glycogen synthase kinase (GSK) 3β, which negatively regulates its downstream target, NF-κB, and potently suppresses LPS-induced proinflammatory responses and endotoxic shock (15, 22). These studies strongly suggest that the PI3K/Akt pathway limits inflammation in endotoxemia and sepsis.
LA, which is found in the human diet and is also available as a dietary supplement in its natural R-form or racemic R,S-mixture, is rapidly absorbed and reduced intracellularly by NAD(P)H-dependent enzymes to the dithiol compound dihydrolipoic acid (23, 24). In addition to playing an important role in mitochondrial energy metabolism, LA and dihydrolipoic acid can scavenge reactive oxygen species, regenerate physiological antioxidants, chelate metal ions, and stimulate insulin signaling (23–25). Lipoic acid significantly improves diabetic neurovascular and metabolic abnormalities (26) and may play a role in cardiovascular protection and as an antiinflammatory agent (27–30). Using cultured human aortic endothelial cells, we have shown that LA inhibits TNFα- or LPS-induced expression of CAMs and MCP-1 and subsequent monocyte–endothelial interactions (11). However, little is known about the effect of LA on endothelial and monocyte activation in vivo and the underlying mechanisms of LA's antiinflammatory effects. Here we investigated the potential protective role of LA in LPS-induced monocyte activation in vitro and acute inflammatory responses in vivo and found that LA strongly attenuated LPS-induced acute inflammatory responses by activating the PI3K/Akt signaling pathway.
Results
Lipoic Acid Activates the PI3K/Akt Signaling Pathway in Human Monocytic THP-1 Cells.
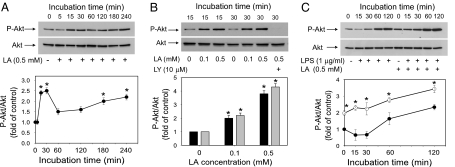
Inhibition of the PI3K/Akt signaling pathway in cultured human monocytic THP-1 cells has been shown to increase LPS-induced expression of TNFα and tissue factor because of increased activation of NF-κB and AP-1 (15). Using the same experimental model, we investigated whether LA could activate the PI3K/Akt pathway and affect LPS-induced Akt phosphorylation. Incubation of THP-1 cells with 0.5 mM LA induced phosphorylation of serine residue 473 of Akt in a time-dependent manner (Fig. 1A). Interestingly, LA elicited a biphasic response, with peak levels of phospho-Akt observed after 30 min of incubation (2.5-fold increase compared with the 0 time point; P < 0.05), followed by a drop at 60 min and then a steady increase up to 240 min of incubation (2.2-fold; P < 0.05). A similar time course of Akt phosphorylation was observed in cells treated with 0.1 mM LA. Furthermore, LA dose-dependently increased Akt phosphorylation (Fig. 1B). For example, the phospho-Akt levels increased ≈2.2- and 4.3-fold, respectively, in cells treated for 30 min with 0.1 or 0.5 mM LA compared with untreated control cells (P < 0.05). Importantly, LA-induced Akt phosphorylation was blocked by the PI3K inhibitor LY294002 [Fig. 1B and supporting information (SI) Fig. 8]. These data strongly suggest that LA causes Akt phosphorylation by activating the PI3K pathway.
Fig. 1.
Lipoic acid induces Akt phosphorylation in human monocytic THP-1 cells. (A) Cells were serum-starved for 16 h and then incubated with 0.5 mM LA for the indicated times. (B) Cells were serum-starved for 16 h and then incubated without or with 0.1 or 0.5 mM LA for 15 min (black bars) or 30 min (gray bars); where indicated, cells were incubated with 10 μM LY294002 (LY) for 30 min before adding 0.5 mM LA. (C) Cells were serum-starved for 2 h and then incubated with 0.5 mM LA or vehicle for 30 min. Subsequently, the cells were washed once and incubated for the indicated times with LPS (1 μg/ml) without (filled circles) or with (open circles) 0.5 mM LA in culture medium containing 2% FCS. For all panels, whole-cell lysates were prepared and phospho-Akt was determined by Western blotting. The intensity of the phospho-Akt bands was quantitated by densitometry and, after normalization to total Akt protein, expressed as fold of untreated control cells. Data shown are means ± SEM of three independent experiments. *, P < 0.05 compared with the 0 time point (A), untreated control cells (B), or cells treated with LPS only (C).
We also examined whether LA affects Akt phosphorylation induced by LPS. Incubation of THP-1 cells with LPS (1.0 μg/ml) increased Akt phosphorylation in a time-dependent manner (2.3-fold increase at 120 min of incubation compared with the 0 time point; P < 0.05) (Fig. 1C). When the cells were treated with LA for 30 min before LPS stimulation, Akt phosphorylation was elevated initially (in agreement with the data in Fig. 1 A and B) and further increased steadily during the 2-h incubation with LPS (Fig. 1C). The levels of phospho-Akt were always significantly higher in the cells pretreated with LA compared with the cells treated with LPS only (P < 0.05). Preincubation with LY294002 abolished the LPS-induced phosphorylation of Akt independent of the presence of LA (SI Fig. 8).
Lipoic Acid Inhibits LPS-Induced Up-Regulation of MCP-1 and TNFα and NF-κB DNA Binding Activity in THP-1 Cells.
Because LA enhanced activation of the PI3K/Akt signaling pathway (Fig. 1), and because this pathway is known to negatively regulate NF-κB activation and inflammatory gene expression (15), we investigated the role of LA in LPS-induced NF-κB activation and MCP-1 and TNFα expression. Treatment of cells with LPS for 2 h caused a 6.2- and 13.5-fold increase in MCP-1 and TNFα mRNA levels, respectively. However, when the cells were incubated with 0.5 mM LA for 1 h before LPS stimulation, up-regulation of MCP-1 and TNFα was inhibited by 44 ± 4% and 26 ± 1%, respectively (P < 0.01; n = 3).
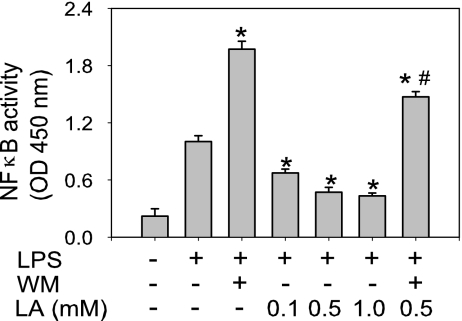
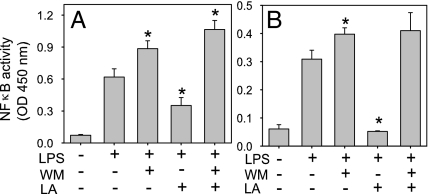
Furthermore, LPS induced a 3-fold increase in NF-κB DNA binding activity, which was further enhanced by the PI3K inhibitor wortmannin (WM) (P < 0.05 compared with LPS only) (Fig. 2). Pretreatment of cells with 0.1, 0.5, or 1.0 mM LA dose-dependently inhibited LPS-induced NF-κB activity by 32 ± 2%, 49 ± 3%, and 56 ± 3%, respectively (P < 0.05 compared with LPS only) (Fig. 2). However, when the cells were incubated with WM before treatment with 0.5 mM LA and LPS, the inhibitory effect of LA was reversed (Fig. 2). These data indicate that activation of the PI3K/Akt pathway by LA partially inhibited NF-κB activation and that the inhibitory effect of LA on LPS-induced up-regulation of MCP-1 and TNFα occurred through inhibition of NF-κB.
Fig. 2.
Lipoic acid inhibits LPS-induced NF-κB DNA binding activity in human monocytic THP-1 cells. Cells were serum-starved for 2 h and then incubated without or with 200 nM WM for 30 min, followed by incubation without or with the indicated concentrations of LA for 1 h and then 1 μg/ml LPS for an additional hour. After treatment, nuclear extracts were prepared and NF-κB (p65) DNA binding activity was quantified by ELISA. Data shown are means ± SEM of three independent experiments. *, P < 0.05 compared with cells treated with LPS only; #, P < 0.05 compared with cells treated with WM plus LPS.
Lipoic Acid Increases Phosphorylation of Akt and GSK3β in Blood Cells of LPS-Exposed Mice.
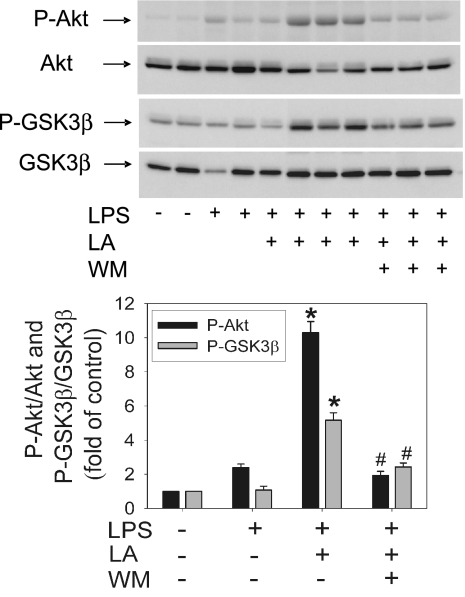
To assess the in vivo relevance of the above data, we investigated whether LA could enhance activation of the PI3K pathway in vivo by assessing phosphorylation of Akt and its direct downstream target, GSK3β, in cells derived from whole blood of LPS-exposed C57BL/6N mice. Treatment of mice with LA (100 mg/kg of body weight, i.p. injection) 1 h before LPS challenge (50 μg, i.p.) enhanced both Akt and GSK3β phosphorylation >4-fold compared with LPS treatment alone (P < 0.05) (Fig. 3). These LA-induced increases in Akt and GSK3β phosphorylation were significantly inhibited by WM (0.3 mg/kg of body weight, i.p.) (P < 0.05) (Fig. 3), indicating that they were mediated by activation of the PI3K pathway.
Fig. 3.
Lipoic acid increases phosphorylation of Akt and GSK3β in cells derived from whole blood of LPS-treated mice. Mice were treated with vehicle control, LPS (50 μg), LA (100 mg/kg of body weight) plus LPS, or WM (0.3 mg/kg of body weight) plus LA and LPS as described in Methods. One hour after treatment, the animals were killed, blood was collected, whole-cell lysates were prepared, and phospho-Akt and phospho-GSK3β were determined by Western blotting. The intensity of the phospho-Akt and phospho-GSK3β bands was quantitated by densitometry and, after normalization to total Akt or GSK3β protein, expressed as fold of control cells from untreated mice. Data shown are means ± SEM of two (control) or three animals per group. *, P < 0.05 compared with animals treated with LPS only; #, P < 0.05 compared with animals treated with LA plus LPS.
Lipoic Acid Inhibits the LPS-Induced Increase in Serum Soluble Adhesion Molecules in Mice.
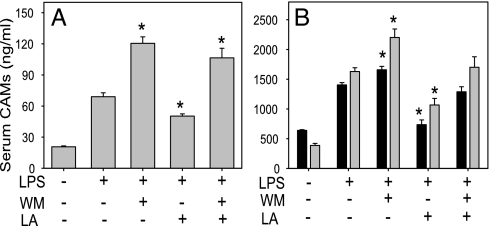
As shown in Fig. 4, treatment of mice with LPS increased serum concentrations of the soluble CAMs, sE-selectin, sVCAM-1, and sICAM-1, 2- to 4-fold compared with untreated control animals. Pretreatment with LA significantly inhibited these LPS-induced increases in CAMs (P < 0.01) (Fig. 4). In contrast, treatment with WM enhanced the LPS-induced increases in CAMs; however, when the animals were treated with WM before LA and LPS, LA's protective effect was diminished (Fig. 4). There were no statistically significant differences in the induction of CAMs between the animals treated with LPS and WM and those treated with LPS, WM, and LA. Therefore, these data indicate that the PI3K/Akt pathway plays a critical role in negatively regulating CAM expression in vivo and the protective effect of LA is mediated by activation of this pathway. In contrast, LA did not significantly inhibit the LPS-induced increase in serum levels of MCP-1 and TNFα.
Fig. 4.
Lipoic acid inhibits the LPS-induced increase in serum sE-selectin (A) and sVCAM-1 and sICAM-1 (B). Mice were treated with vehicle control, LPS (50 μg), WM (2 × 0.3 mg/kg of body weight) plus LPS, LA (100 mg/kg of body weight) plus LPS, or WM plus LA and LPS as described in Methods. Four hours after treatment, the animals were killed, blood was collected, and serum sE-selectin (A), sVCAM-1 (B, black bars), and sICAM-1 (B, gray bars) were measured by ELISA. Data shown are means ± SEM of five to seven animals per group. *, P < 0.05 compared with animals treated with LPS only.
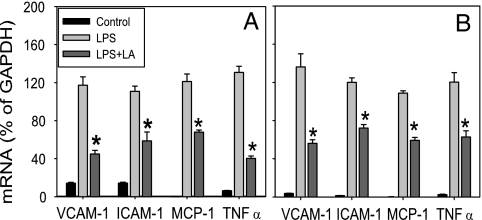
Lipoic Acid Inhibits LPS-Induced Inflammatory Gene Expression in Mouse Tissues.
To investigate whether LA inhibits acute inflammatory responses in tissues, we assessed gene expression of inflammatory mediators in lung, heart, and aorta of LPS-exposed mice, using real-time quantitative RT-PCR. Treatment of mice with LPS strongly up-regulated inflammatory gene expression in all tissues examined (Fig. 5 and SI Fig. 9), and pretreatment with LA inhibited the LPS-induced increases in lung mRNA levels of VCAM-1, ICAM-1, MCP-1, and TNFα by 70 ± 4%, 54 ± 10%, 44 ± 2%, and 73 ± 2%, respectively (P < 0.05 compared with animals treated with LPS only) (Fig. 5A). Very similar inhibitory effects of LA were observed in heart (Fig. 5B) and aorta (SI Fig. 9). LPS-induced IL-6 gene expression also was reduced, albeit nonsignificantly, by LA in all tissues examined.
Fig. 5.
Lipoic acid inhibits LPS-induced gene expression of adhesion molecules, MCP-1, and TNFα in mouse lung (A) and heart (B). Mice were treated with vehicle control, LPS (50 μg), or LA (100 mg/kg of body weight) plus LPS as described in Methods. Four hours after treatment, the animals were killed and total RNA was isolated from lung and heart. VCAM-1, ICAM-1, MCP-1, and TNFα mRNA levels were quantified by using real-time quantitative RT-PCR. After normalization to the internal control gene, GAPDH, the results for each target gene were expressed as percentage of GAPDH. Data shown are means ± SEM of four to seven animals per group. *, P < 0.05 compared with animals treated with LPS only.
Lipoic Acid Inhibits LPS-Induced NF-κB DNA Binding Activity in Mouse Tissues.
The DNA binding activity of the p65 subunit of NF-κB was detectable at low levels in all tissues examined of control animals and was increased ≈5-fold in animals treated with LPS (Fig. 6). LA treatment significantly suppressed LPS-induced NF-κB activity in lung, heart, and aorta by 49 ± 14%, 100%, and 53 ± 11%, respectively (P < 0.05 compared with animals treated with LPS only). Treatment of mice with WM further enhanced LPS-induced NF-κB activity in lung and heart (Fig. 6). When the animals were treated with WM before LA and LPS, the protective effect of LA on LPS-induced NF-κB activity was abolished (Fig. 6). There were no statistically significant differences between the animals treated with LPS and WM and those treated with LPS, WM, and LA. These data strongly suggest that PI3K plays a critical role in negatively regulating NF-κB activity and that the protective effect of LA is mediated by activation of the PI3K/Akt pathway.
Fig. 6.
Lipoic acid inhibits LPS-induced NF-κB DNA binding activity in mouse lung (A) and heart (B). Mice were treated with vehicle control, LPS (50 μg), WM (2 × 0.3 mg/kg of body weight) plus LPS, LA (100 mg/kg of body weight) plus LPS, or WM plus LA and LPS as described in Methods. Four hours after treatment, the animals were killed and nuclear extracts were isolated from lung and heart. DNA binding activity of NF-κB (p65) was quantified by ELISA. Data shown are means ± SEM of four to seven animals per group. *, P < 0.05 compared with animals treated with LPS only.
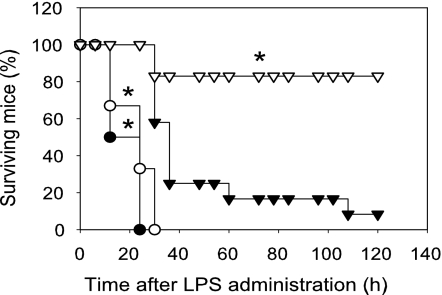
Lipoic Acid Improves Survival of Endotoxemic Mice.
Activation of the PI3K/Akt pathway improves survival and protects against septic death in experimental animals (17, 19, 20, 31). Therefore, we investigated whether LA could protect against LPS-induced endotoxemic shock and lethality. As shown in SI Fig. 10, treatment of mice with LPS induced lethality in a dose-dependent manner: LPS at a dose of 0.6 mg was not lethal, whereas 2 mg killed all animals within 48 h, and 1.5 mg caused 75% of mice to die within 84 h. In subsequent experiments, 1.5 mg of LPS was used to investigate the potential protective effect of LA. As shown in Fig. 7, i.p. injection of 100 mg of LA per kilogram of body weight 1 h before LPS challenge significantly improved survival (P < 0.01 compared with animals treated with LPS only). The animals initially showed signs of endotoxemia in both groups, including immobility, lethargy, piloerection, mild febrile shivering, and diarrhea. These signs in the animals treated with LA plus LPS were milder than the mice treated with LPS only. Most of the animals in the LA-treated group gradually recovered, and on the fifth day all surviving mice appeared normal.
Fig. 7.
Lipoic acid improves survival of endotoxemic mice. Mice (6–12 animals per group) were injected with 1.5 mg of LPS (filled triangles), 100 mg of LA per kilogram of body weight plus LPS (open triangles), 3 × 0.3 mg of WM per kilogram of body weight plus LPS (filled circles), or WM plus LA and LPS (open circles) as described in Methods. The animals were monitored for lethality three times daily for up to 5 days. *, P < 0.01 compared with animals treated with LPS only.
We also determined the role of the PI3K/Akt pathway in LPS-induced lethality. As shown in Fig. 7, treatment of animals with WM before LPS challenge significantly increased lethality compared with animals treated with LPS only (P < 0.001), consistent with previous data (16). When the animals were treated with WM before LA and LPS, the protective effect of LA on LPS-induced lethality was abolished (Fig. 7). There were no statistically significant differences in LPS-induced lethality between the animals treated with LPS and WM and those treated with LPS, WM, and LA. These data indicate that the protective effect of LA against septic shock and death occurred through activation of the PI3K/Akt pathway.
Discussion
The pathophysiology of endotoxic shock is characterized by the release of multiple proinflammatory cytokines and chemokines, expression of CAMs, and infiltration of neutrophils and monocytes into inflamed tissues. Because of the complexity of the pathophysiology of septic shock, major efforts have focused on identifying novel antiinflammatory drugs that prevent the proinflammatory process at the early stage of gene expression of key inflammatory mediators (32).
We have previously shown that LA inhibits TNFα- and LPS-induced expression of CAMs and MCP-1 in human aortic endothelial cells and consequent adherence of monocytes (11). Here we provide evidence that LA exhibits strong antiinflammatory activities in vitro and in vivo by attenuating LPS-induced activation of cultured monocytes and reducing NF-κB-dependent expression of CAMs, TNFα, and MCP-1 and endotoxic lethality in mice.
It is well documented that NF-κB plays a prominent role in LPS-induced transcriptional regulation of most inflammatory genes that contribute to the development of septic shock, multiple organ failure, and death (6–8, 33). Of clinical relevance, NF-κB binding activity was increased in patients with acute inflammation and sepsis and correlated with clinical severity and mortality (34, 35). In the present study we showed that LPS induced NF-κB DNA binding activity in human monocytic cells and in various tissues of mice, and LA treatment strongly suppressed NF-κB activation and NF-κB-dependent up-regulation of CAMs, TNFα, and MCP-1. Therefore, our data are consistent with previous findings that the thiol antioxidants, N-acetyl-l-cysteine and pyrrolidine dithiocarbamate, inhibit NF-κB activation and reduce the extent of microvascular injury, systemic hypotension, and multiple organ failure in septic rats (12, 13).
The PI3K/Akt pathway is a conserved family of signal transduction enzymes that are involved in regulating cellular proliferation and survival. A growing body of evidence suggests that the PI3K/Akt pathway plays an important role as a negative feedback regulator of excessive innate immune and Toll-like receptor-mediated proinflammatory responses (15–22). Hence, inhibition of PI3K/Akt signaling enhanced LPS-induced activation of NF-κB and AP-1 and gene expression of TNFα and tissue factor in cultured cells (15). Similar effects were observed in vivo, where inhibition of PI3K/Akt enhanced LPS-induced inflammation and lethality in mice (16) and, conversely, activation of PI3K/Akt increased survival (17, 20, 31). Furthermore, a recent study in genetically altered mice found that PI3Kγ negatively regulates the expression of CD47 and β3 integrins during Gram-negative sepsis, which was correlated with vascular injury in the lungs by polymorphonuclear leukocytes (36). These studies indicate that the PI3K/Akt pathway limits inflammation in endotoxemia and sepsis.
We found that LA activated the PI3K/Akt pathway and induced Akt phosphorylation in a dose- and time-dependent manner in human monocytic cells. These observations are consistent with other reports that LA activates PI3K/Akt in other cell types (37–39). Similar to LA, LPS also induced Akt phosphorylation in human monocytic cells. However, the levels of phospho-Akt increased only 1 h after the addition of LPS. These findings are consistent with those of Guha and Mackman (15), who showed that the LPS-induced activation of the PI3K/Akt pathway was slightly delayed relative to the activation of the MAPK pathways. Thus, it appears that the delay in PI3K/Akt activation initially allows LPS to activate MAPK and induce an acute inflammatory response, which is subsequently blunted and then shut down by LPS-induced Akt phosphorylation (15, 21). In contrast, when the cells were treated with LA before LPS stimulation, Akt phosphorylation was elevated initially and further increased during LPS exposure. Hence, the proinflammatory response to LPS was suppressed by LA from the beginning, resulting in inhibition of LPS-induced NF-κB activity and NF-κB-dependent TNFα and MCP-1 gene expression. These antiinflammatory effects of LA in monocytic cells were abolished by the PI3K inhibitor WM, indicating that they required PI3K/Akt activation.
Consistent with our findings in cell culture, we observed that inhibition of the PI3K/Akt pathway in vivo by WM significantly enhanced the LPS-induced increase in serum levels of CAMs, NF-κB DNA binding activity in lung and heart, and septic death of mice. Thus, our data provide evidence that the PI3K/Akt pathway plays a critical role in attenuating LPS-induced acute inflammatory responses in lung and heart and extend previous observations that this pathway serves as a negative regulator of LPS-induced NF-κB activation and expression of proinflammatory mediators in vivo (16, 35). Furthermore, we found that LA treatment significantly increased phosphorylation of Akt and its downstream target, GSK3β, in blood cells of LPS-treated mice, which was accompanied by effective attenuation of acute inflammatory responses in serum and various tissues.
Two striking findings of the present study were that LA significantly improved survival of septic mice, and inhibition of PI3K with WM abolished this protective effect of LA. Previous studies suggested that LA acts as an antiinflammatory agent in vivo and plays a role in cardiovascular protection (27–29), although LA's mechanism of action remained unclear. Our results suggest a mechanism by which LA activates the PI3K/Akt signaling pathway and consequently inhibits NF-κB activity, expression of proinflammatory mediators, and septic lethality.
Although it has been shown previously that LA activates the PI3K/Akt pathway in various cell types, e.g., 3T3-L1 adipocytes (37) and rat cortical neurons (38), most of these studies investigated the role of LA in PIK3/Akt-mediated insulin signaling, glucose transport, and cell survival. Our study demonstrates that LA activates the PI3K/Akt pathway and inhibits LPS-induced, NF-κB-dependent up-regulation of proinflammatory mediators in monocytes, which play a critical role in inflammation and sepsis. This is important because there are substantial differences among different cell types regarding the antiinflammatory role of the PI3K/Akt pathway. It has been reported that this pathway either acts as a positive (40–42) or negative regulator (15, 18, 19) of NF-κB activation and cytokine production, depending on the nature of the stimulus and the cell type.
Furthermore, our data show that LA activates the PI3K/Akt pathway in vivo and, hence, attenuates LPS-induced proinflammatory responses in serum and tissues and protects against septic death in mice. Although previous studies have found that LA exerts antiinflammatory effects in a mouse model of asthma (29), a rat model of angiotensin II-induced renal injury (43), and in patients with metabolic syndrome (28), no data have been published showing that LA inhibits LPS-induced acute inflammatory responses in vivo.
In summary, our results show that LA effectively attenuates LPS-induced acute inflammatory responses in vitro and in vivo by activating the PI3K/Akt signaling pathway. Therefore, LA might be useful in the prevention of sepsis and inflammatory vascular diseases.
Methods
Animal Studies.
Male C57BL/6N mice at 11–12 weeks of age weighing 22–24 g were purchased from Simonsen Laboratories (Santa Clara, CA). The animals were housed under specific pathogen-free conditions in a temperature- and humidity-controlled environment and given unlimited access to water and food (pelleted rodent nonpurified diet, Wayne Rodent BLOX 8604; Harlan Teklad, Madison, WI). Mice were acclimatized for 7 days before initiation of experiments. All animal procedures were approved by the Institutional Animal Care and Use Committee of Oregon State University.
Solutions of d,l-α-lipoic acid (Sigma–Aldrich, St. Louis, MO) were prepared immediately before use by dissolving 10 mg/ml LA in 120 mM Tris buffer, adjusting the pH to 7.4, and sterilely filtering the resulting solution. WM (Sigma–Aldrich) stock solutions were prepared in DMSO and further diluted into sterilized HBSS (Sigma–Aldrich). LPS (serotype 055:B5 from Escherichia coli; Sigma–Aldrich) stock solutions were prepared in HBSS.
Mice were randomly assigned to five experimental groups: control (i.p. injection of HBSS), LPS (i.p. injection of 50 μg of LPS), WM plus LPS [i.p. injections of 0.3 mg of WM per kilogram of body weight 90 min before and 90 min after i.p. injection of LPS (16)], LA plus LPS (i.p. injection of 100 mg of LA per kilogram of body weight 60 min before i.p. injection of LPS), or WM plus LA and LPS (i.p. injections of 0.3 mg of WM per kilogram of body weight 90 min before and, if applicable, 90 min after and 100 mg of LA per kilogram of body weight 60 min before i.p. injection of LPS). Each group consisted of 20–25 animals. One or 4 h after HBSS (control) or LPS (all other groups) administration, the animals were killed and blood and tissues were collected for analysis.
For survival experiments investigating the dose-dependent effect of LPS, animals were injected i.p. with 0.6, 1.0, 1.5, or 2.0 mg of LPS. To investigate the effect of LA, animals received an i.p. injection of 100 mg of LA per kilogram of body weight 60 min before i.p. injection of 1.5 mg of LPS. To investigate the effect of WM, animals received i.p. injections of 0.3 mg of WM per kilogram of body weight 90 min before and 90 and 360 min after i.p. injection of 1.5 mg of LPS (16) or the same injections of WM and an i.p. injection of 100 mg of LA per kilogram of body weight 60 min before injection of 1.5 mg of LPS. Each group consisted of 5–12 animals. The animals were monitored for endotoxemia and death three times daily for up to 5 days.
Cell Culture Studies.
Human monocytic THP-1 cells purchased from the American Type Culture Collection (Rockville, MD) were grown in RPMI medium 1640 (Sigma–Aldrich) containing 10% FCS (GIBCO, Grand Island, NY), 100 μg/ml streptomycin, 100 units/ml penicillin, 250 ng/ml fungizone, 1 mM glutamine, and 50 μM 2-mercaptoethanol (all from Sigma–Aldrich). To assess the effect of LA on Akt phosphorylation, cells were serum-starved for 16 h, washed once, and then incubated without or with 10 μM LY294002 (Sigma–Aldrich) for 30 min before incubation with 0.1 or 0.5 mM LA in serum-free medium for different times. Control cells were incubated with the vehicle DMSO (≤0.1%, final concentration). To assess the effect of LA on Akt phosphorylation in the presence of LPS, cells were serum-starved for 2 h, washed once, and then incubated without or with 10 μM LY294002 for 30 min followed by 0.1 or 0.5 mM LA for 30 min. Thereafter, cells were washed once and then incubated for different times with LPS (1 μg/ml) without or with 0.1 or 0.5 mM LA in culture medium containing 2% FCS. To assess MCP-1 and TNFα gene expression, cells were incubated with 0.5 mM LA or vehicle for 1 h before adding 0.1 μg/ml LPS and incubating for another 2 h. To assess NF-κB DNA binding activity, cells were incubated with 200 nM WM for 90 min and/or 0.1, 0.5, or 1.0 mM LA for 60 min or vehicle before addition of 1 μg/ml LPS and incubation for 1 h.
Statistical Analysis.
The data were calculated as means ± SEM and analyzed by ANOVA with Fisher's probable least-squares difference post hoc test. An unpaired Student t test was used when only two groups were compared. The survival of groups of animals was analyzed by log-rank test. Statistical significance was set at P < 0.05. Percent inhibition was calculated as follows: [1 − (value for LPS plus LA-treated animals − value for untreated control animals/value for LPS-treated animals − value for untreated control animals)] × 100%.
All other experimental details are contained in SI Methods.
Supplementary Material
Acknowledgments
This work was supported by National Center for Complementary and Alternative Medicine Center of Excellence Grant P01 AT002034 (to B.F., W.-J.Z., and T.H.) and National Institutes of Health Grant ES11542 (to W.-J.Z.).
Abbreviations
- Akt
protein kinase B
- CAM
cellular adhesion molecule
- ICAM-1
intercellular adhesion molecule 1
- LA
α-lipoic acid
- MCP-1
monocyte chemoattractant protein 1
- PI3K
phosphoinositide 3-kinase
- VCAM-1
vascular cell adhesion molecule 1
- WM
wortmannin
- GSK
glycogen synthase kinase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700305104/DC1.
References
- 1.Guha M, Mackman N. Cell Signalling. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 3.Parrillo JE. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 4.Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Christman JW, Holden EP, Blackwell TS. Crit Care Med. 1995;23:955–963. doi: 10.1097/00003246-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 7.Karin M, Liu Z, Zandi E. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Colavitti R, Rovira II, Finkel T. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 9.Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. J Clin Invest. 1993;92:1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber C, Erl W, Pietsch A, Strobel M, Ziegler-Heitbrock HW, Weber PC. Arterioscler Thromb. 1994;14:1665–1673. doi: 10.1161/01.atv.14.10.1665. [DOI] [PubMed] [Google Scholar]
- 11.Zhang WJ, Frei B. FASEB J. 2001;15:2423–2432. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. J Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- 13.Liu SF, Ye X, Malik AB. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 14.Victor VM, Rocha M, De la Fuente M. Int Immunopharmacol. 2003;3:97–106. doi: 10.1016/s1567-5769(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 15.Guha M, Mackman N. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 16.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 17.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, Walsh K, Koh GY. FASEB J. 2002;16:126–128. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 19.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 20.Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 21.Fukao T, Koyasu S. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 22.Martin MK, Rehani RS, Jope SM, Michalek Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Packer L, Witt EH, Tritschler HJ. Free Radical Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 24.Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Curr Med Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 25.Biewenga GP, Haenen GR, Bast A. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler D. Treat Endocrinol. 2004;3:173–189. doi: 10.2165/00024677-200403030-00005. [DOI] [PubMed] [Google Scholar]
- 27.Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G, et al. J Clin Gastroenterol. 2005;39:737–742. doi: 10.1097/01.mcg.0000174023.73472.29. [DOI] [PubMed] [Google Scholar]
- 28.Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 29.Cho YS, Lee J, Lee TH, Lee EY, Lee KU, Park JY, Moon HB. J Allergy Clin Immunol. 2004;114:429–435. doi: 10.1016/j.jaci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Jesudason EP, Masilamoni JG, Jesudoss KS, Jayakumar R. Mol Cell Biochem. 2005;270:29–37. doi: 10.1007/s11010-005-3301-z. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda N, Hayashi Y, Takahashi Y, Hattori Y. J Pharmacol Exp Ther. 2006;319:1348–1354. doi: 10.1124/jpet.106.109785. [DOI] [PubMed] [Google Scholar]
- 32.Awad SS. Am J Surg. 2003;186:23S–30S. doi: 10.1016/j.amjsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Liu SF, Malik AB. Am J Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 34.Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Mantel D, Bottiger BW, Stern DM, Waldherr R, Saeger HD, et al. J Clin Invest. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnalich F, Garcia-Palomero E, Lopez J, Jimenez M, Madero R, Renart J, Vazquez JJ, Montiel C. Infect Immun. 2000;68:1942–1945. doi: 10.1128/iai.68.4.1942-1945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong E, Gao XP, Predescu D, Broman M, Malik AB. Am J Physiol. 2005;289:L1094–L1103. doi: 10.1152/ajplung.00179.2005. [DOI] [PubMed] [Google Scholar]
- 37.Rudich A, Tirosh A, Potashnik R, Khamaisi M, Bashan N. Diabetologia. 1999;42:949–957. doi: 10.1007/s001250051253. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Xing GQ, Barker JL, Chang Y, Maric D, Ma W, Li BS, Rubinow DR. Neurosci Lett. 2001;312:125–128. doi: 10.1016/s0304-3940(01)02205-4. [DOI] [PubMed] [Google Scholar]
- 39.Muller C, Dunschede F, Koch E, Vollmar AM, Kiemer AK. Am J Physiol. 2003;285:G769–G778. doi: 10.1152/ajpgi.00009.2003. [DOI] [PubMed] [Google Scholar]
- 40.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 41.Thomas KW, Monick MM, Stabler JM, Yarovinsky T, Carter AB, Hunninghake GW. J Biol Chem. 2002;277:492–501. doi: 10.1074/jbc.M108107200. [DOI] [PubMed] [Google Scholar]
- 42.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 43.Mervaala E, Finckenberg P, Lapatto R, Muller DN, Park JK, Dechend R, Ganten D, Vapaatalo H, Luft FC. Kidney Int. 2003;64:501–508. doi: 10.1046/j.1523-1755.2003.00108.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.