Abstract
Invariant natural killer T (iNKT) cells are innate-like lymphocytes recognizing CD1d-restricted glycolipid antigens, such as α-galactosylceramide (αGC). We assessed whether iNKT cells help B lymphocyte responses and found that mice immunized with proteins and αGC develop antibody titers 1–2 logs higher than those induced by proteins alone. Activation of iNKT cells enhances protection against infections such as influenza and elicits higher frequencies of memory B cells and higher antibody responses to booster immunizations. Protein vaccination with αGC, but not with conventional adjuvants, elicits IgG responses in mice lacking MHC class II molecules, demonstrating that iNKT cells can substitute for CD4+ T cell help to B cells. Interestingly, the decay of circulating antibodies is faster in mice lacking iNKT cells. These findings point to a homeostatic role for iNKT cells on critical features of the antibody response such as immunity and B cell memory.
Keywords: α-galactosylceramide, B cell memory, protein vaccination, serological memory
The help of CD4+ T cells to B cells in secondary lymphoid organs leads to naïve B lymphocyte activation that results in the generation of both long-lived plasma cells (PCs) and memory B cells expressing high-affinity, somatically mutated, class-switched Ig. This interaction requires presentation of processed antigens by B cells to CD4+ T cells, signaling by costimulatory molecules such as CD40 on B cells, and production of cytokines by activated T cells (1).
It has been shown that generation of T-dependent antigen-specific antibody responses also requires the participation of molecular and cellular components of the innate immune system. For instance, Toll-like receptors are molecules of the innate immune system that sense microbial infection and induce both dendritic cell maturation (2, 3) and direct B cell activation (4, 5). Similarly, NK cells, which are effector cells of the innate immune system, can activate B cells to produce IgG (6, 7).
Invariant natural killer T (iNKT) cells express a peculiar TCRα chain encoded in mice and humans by the homologue invariant Vα14-Jα18 and Vα24-JαQ rearrangements, respectively. The invariant TCRα chain pairs with variable TCRβ chains that, however, use a restricted repertoire of V regions, comprising Vβ8.2, Vβ7, and Vβ2 in mice and Vβ11 in human (8, 9). This TCR recognizes CD1d (10, 11), a non-MHC-encoded class I-like molecule expressed on cells of haematopoietic (e.g., monocytes, dendritic cells, B and T lymphocytes), and nonhaematopoietic (e.g., thymic epithelial cells, keratinocytes, hepatocytes) origin (12, 13). iNKT cells can be activated by exogenous glycosphingolipids, such as α-galactosylceramide (αGC, isolated from marine sponges) (14, 15), α-glycuronosylceramides, or diacylglycerol (isolated from the Gram-negative bacteria Sphingomonas and Borrelia burgdorferi spirochete, respectively), which specifically bind CD1d (16–19). Furthermore, iNKT cells exhibit a substantial intrinsic autoreactivity that can be explained with the CD1d-restricted recognition of mammalian endogenous (glyco)lipids such as isoglobotrihexosylceramide (iGB3) (20). iNKT cells are regarded as actors of the innate immune system because they display a constitutive effector-memory phenotype with immediate effector functions and express a semiinvariant T cell receptor (TCR) together with NK cell receptors (21–23).
In vivo administration of αGC into mice and human rapidly activates iNKT cells to release T helper 1 (Th1) and Th2 cytokines that in turn contribute to the activation of NK cells, dendritic cells, and T lymphocytes (13, 24). Indeed, αGC-activated iNKT cells have been reported to potentiate T cell responses against infectious agents (25–27) or tumors (28–30). It has been demonstrated that administration of αGC alone in mice results in increased levels of serum IgE (31). We have previously shown that human iNKT cells efficiently help B cell proliferation and antibody production in a CD1d-restricted manner in vitro (32).
Here, we have addressed whether iNKT cells can help specific antibody responses induced in mice by protein subunit vaccines. We found that coadministration of αGC and protein vaccines significantly enhances critical features of the antibody response, such as protection from infections and B cell memory.
Results
Activation of iNKT Cells Enhances Antibody Responses to Protein Antigens in Vivo.
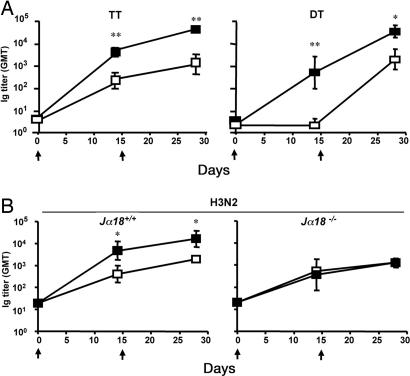
We have recently demonstrated that human iNKT cells can help B lymphocytes to proliferate and produce immunoglobulins in vitro. To determine the in vivo relevance of this finding, we immunized C57BL/6 mice i.m. with bacterial [tetanus toxoid (TT) and diphtheria toxoid (DT)] or viral (the hemoagglutinin/neuroaminidase subunits from the human A/Panama/2007/99 influenza virus, H3N2) proteins with or without the iNKT cell-specific glycolipid αGC, and assessed serum titers of protein-specific antibodies at various time points. Fig. 1A shows that, independently of the antigen used, mice immunized with proteins and αGC displayed antibody titers 1–2 logs higher than titers of mice immunized with proteins alone. Comparable results were obtained in BALB/c, CD1, and C3H/HeJ mice or when immunizations were performed i.m., i.p., s.c., or i.v., indicating that the adjuvant activity is not strain-specific and is independent of Toll-like receptor 4 activation and largely independent of the route of administration [see supporting information (SI) Fig. 7 and SI Fig. 8]. Unlike previous reports (33), we never observed consistent antibody responses after intranasal immunization with proteins admixed with αGC (see SI Fig. 8).
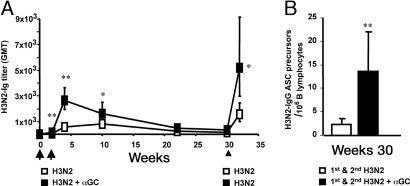
Fig. 1.
Activated iNKT cells help antibody responses to bacterial and viral proteins in vivo. Groups of four to six mice were immunized twice (arrows) with the indicated proteins, given alone (□) or with αGC (■). (A) GMTs (and 95% C.I.) of circulating antigen-specific antibodies in C57BL/6 WT mice immunized with TT or DT with or without αGC. (B) GMTs and 95% C.I. of antigen-specific antibodies in sera from C57BL/6 mice bearing (Jα18+/+) or lacking (Jα18−/−) iNKT cells, immunized with H3N2 or H3N2 plus αGC. Results are from one experiment of six (TT), two (DT), or four (H3N2). Asterisks indicate statistically different values between mice immunized with the protein alone and mice immunized with the protein and αGC (∗, P < 0.05; ∗∗, P < 0.01).
To prove that the adjuvant activity of αGC was caused by iNKT cell activation, mice lacking iNKT cells (Jα18−/−) and their WT littermates (Jα18+/+) were immunized with H3N2 alone or αGC. As shown in Fig. 1B, all mice immunized with H3N2 alone developed comparable antibody responses, regardless of the presence of iNKT cells. However, when immunization was done with H3N2 plus αGC, mice bearing iNKT cells showed a significant enhancement in titers of H3N2-specific antibodies, whereas mice lacking iNKT cells failed to do so. Comparable results were obtained in mice lacking CD1d (CD1d−/−), the restriction element that presents αGC to iNKT cells (data not shown).
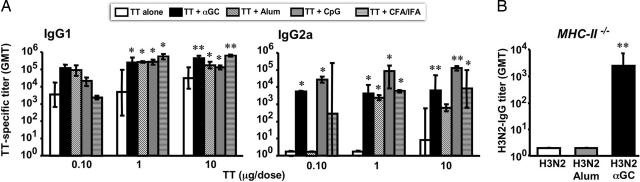
To compare αGC activity with that of more conventional adjuvants, mice were immunized with increasing doses of TT given alone, admixed with αGC, or with an optimal dose of one of the following adjuvants: complete Freund's adjuvant (one of the strongest adjuvants used in mice; ref. 34), CpG (a strong Th0/Th1 immune-stimulator currently being tested in human; ref. 35), and Alum (an adjuvant licensed for human use, considered a Th0/Th2 inducer; ref. 36). As shown in Fig. 2A, αGC is overall as potent as the best benchmark adjuvants in helping Ig production, which is particularly evident at the lowest antigen dose. Moreover, αGC does not polarize the Th response but elicits a “balanced Th0” production of IgG1 and IgG2a antibodies.
Fig. 2.
Activated iNKT cells help T-dependent antibody responses like conventional adjuvants and can substitute for MHC-II-restricted T lymphocytes. (A) IgG1 and IgG2a subclass distribution of circulating TT-specific antibodies in C57BL/6 mice 2 weeks after two immunizations with increasing doses of TT alone, TT plus αGC, TT plus Alum, TT plus CpG, TT plus complete Freund's adjuvant (CFA)/incomplete Freund's adjuvant (IFA). (B) GMTs and 95% C.I. of H3N2-specific IgG in sera from C57BL/6 MHC-II−/− mice 2 weeks after two immunizations with H3N2 alone, H3N2 plus Alum, or H3N2 plus αGC. Results are from one experiment representative of three, in which 3–10 mice per group were tested. Asterisks indicate statistically different values between mice immunized with the protein alone and mice immunized with the protein and the given adjuvant (∗, P < 0.05; ∗∗, P < 0.01).
An important feature for an adjuvant is that it can be used for several immunizations. As it has been reported that iNKT cells are hyporesponsive to a second systemic injection of αGC (37, 38), we investigated whether repeated injections of αGC would affect the adjuvant activity of iNKT cells in vivo. To this aim, mice were immunized with H3N2 and 0.1 μg of αGC followed 2 weeks later by another immunization with TT and 0.1 μg of αGC. The results in SI Table 1 show that IgG titers to TT in BALB/c mice immunized i.m. with TT and αGC are significantly higher than in mice immunized with TT alone, regardless of the previous immunization with an unrelated protein (H3N2) and αGC. This finding demonstrates that repeated i.m. injections of low doses (0.1 μg) of αGC do not result in the iNKT cell hyporesponsiveness observed in mice that had received systemically high doses (1–25 μg) of αGC (37, 38).
Finally, we assessed whether antibody responses to protein antigens could develop with the help of iNKT lymphocytes in the absence of conventional CD4+ helper T cells, a situation where other adjuvants fail to help antibody responses. Thus, mice that lack MHC class II molecules (MHC-II−/−) were immunized twice with H3N2, given alone, with Alum, or with αGC. As expected, MHC-II−/− mice immunized with H3N2 alone or with Alum did not develop antigen-specific antibodies (Fig. 2B). Instead, MHC-II−/− mice immunized with H3N2 plus αGC remarkably mounted detectable H3N2-specific IgG antibodies.
Altogether, these results demonstrate that iNKT cells activated in vivo by αGC potentiate antibody responses to protein antigens in a manner comparable to that of the best conventional adjuvants. Furthermore, at a variance with conventional adjuvants, αGC does not require MHC-class II–restricted CD4+ T lymphocytes to elicit IgG responses.
iNKT Cells Help Humoral Immunity.
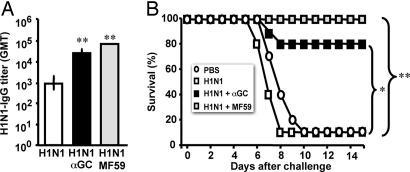
Having demonstrated that αGC enhances antibody responses to pathogen-derived proteins, we addressed the quality of the response and asked whether these antibodies were capable of protecting mice from infections. To this end, we compared the adjuvant effect of αGC with that of MF59 (a squalene-in-oil emulsion adjuvant licensed for routine use in human with flu vaccines; ref. 39) in a mouse model of vaccination against influenza virus infection, where protection depends mainly on the induction of neutralizing antibodies against the hemoagglutinin/neuroaminidase subunits (40). Three groups of mice were immunized twice with H1N1 proteins (from the human influenza virus A/NewCaledonia/20/99) alone, with H1N1 plus MF59, or with H1N1 plus αGC. Two weeks after the last immunization, mice were challenged with 100 LD50 of the mouse-adapted A/WS/33(H1N1) flu virus, and their survival was followed up for 14 days. As shown in Fig. 3A, 1 day before challenge, mice immunized with H1N1 and either αGC or MF59 exhibit comparable antibody titers, which were significantly higher than those displayed by mice immunized with the protein alone. Consequently, 2 weeks after virus challenge, 80% of mice immunized with H1N1 plus αGC, and 100% of those immunized with H1N1 plus MF59 were alive, whereas only 10% of mice immunized with H1N1 alone survived (Fig. 3B).
Fig. 3.
iNKT cell activation enhances the protective efficacy of a subunit influenza vaccine. (A) GMTs and 95% C.I. of circulating H1N1-specific IgG in C57BL/6 mice 2 weeks after two immunizations with H1N1 alone, H1N1 plus αGC, or H1N1 plus MF59. (B) Survival curves of the mice described in A and control mice injected with PBS alone after intranasal challenge with the influenza virus A/WS/33. Results are from one experiment representative of two in which 10 mice per group were tested. Asterisks indicate statistically different values between mice immunized with H1N1 alone and mice immunized with H1N1 plus αGC or H1N1 plus MF59 (∗, P < 0.05; ∗∗, P < 0.002).
Altogether, these results demonstrate that αGC-dependent iNKT cell activation enhances the efficacy of vaccines against infectious diseases.
iNKT Cells Spontaneous Activity Contributes to Sustain Serological Memory.
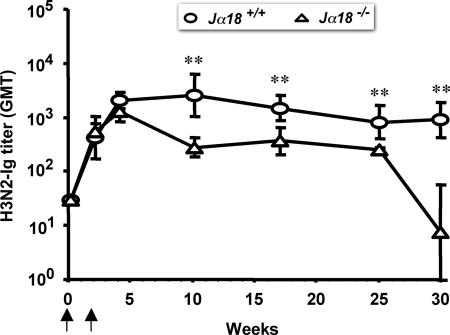
To investigate the role of iNKT cells in the regulation of serological memory, we assessed the persistence of circulating antigen-specific antibodies in mice bearing (Jα18+/+) or lacking (Jα18−/−) iNKT cells immunized twice with a protein antigen alone (H3N2). In both groups of mice, titers of antigen-specific antibodies peaked at comparable levels 2 weeks after the last immunization. However, the decay of antibody titers was faster in Jα18+/+ mice as compared with Jα18−/− mice (Fig. 4). As this phenomenon was observed in the absence of exogenous αGC, we conclude that iNKT cell-dependent spontaneous activity (18, 20) should influence the survival of PCs, which is the critical parameter regulating the half-life of circulating antibodies in mice (41). Since starting from 3 weeks after the second immunization with H3N2 alone the number of H3N2-specific PCs in the bone marrow (BM) was below the detection limit of ELISPOT assays, this conclusion could not be formally demonstrated (data not shown).
Fig. 4.
iNKT cells sustain serum antibody titers. Decay of serum antibodies in C57BL/6 mice bearing (Jα18+/+) or lacking (Jα18−/−) iNKT cells immunized (arrows) with H3N2 alone. Results are from one experiment representative of three in which five mice per group were tested. Asterisks indicate statistically different values (P < 0.03) between the two groups of mice.
All together, these results demonstrate that iNKT cells play a homeostatic role in maintaining circulating antibody levels and suggest that they may do so by regulating PCs survival.
iNKT Cell Activation by αGC Enhances Recall Antibody Responses and Contributes the Maintenance of B Cell Memory.
A key feature of the adaptive immune system is the ability to mount a quicker recall response to previously encountered antigens. To assess whether iNKT cell activation by αGC influenced the recall antibody response, mice were immunized twice, at weeks 0 and 2, with H3N2 or with H3N2 plus αGC. A third (recall) immunization with H3N2 alone was then given to all mice at week 30. Fig. 5A shows that, after the first two doses, antibody titers in mice immunized with H3N2 plus αGC were significantly higher than those from mice injected with H3N2 alone. In the following weeks, H3N2-specific antibodies decayed over time with similar rates, reaching background levels in both groups at ≈ week 30. Interestingly, after a boost with H3N2 alone given at week 30, mice that received H3N2 plus αGC in the first two immunizations developed significantly higher antibody titers than mice that always received the protein alone (Fig. 5A). Consistent with these results, we found that at week 30 (just before the boost) the frequency of H3N2 antibody-secreting cell (ASC) precursors was significantly higher in the spleen of mice immunized twice with H3N2 plus αGC than in the spleen of mice immunized twice with H3N2 alone (Fig. 5B).
Fig. 5.
Activated iNKT cells enhance B cell memory. (A) GMTs and 95% C.I. of H3N2-specific antibodies in C57BL/6 WT mice before and after two immunizations (arrows) with H3N2 or H3N2 plus αGC and a subsequent boost (arrowhead) with H3N2 alone. (B) Numbers (average ± SD) of H3N2-IgG ASC precursors per 106 B lymphocytes found on week 30 in the spleens from two groups of mice primed as in A. Results are from one experiment representative of two, in which seven mice per group were analyzed. Asterisks indicate statistically different values (∗, P < 0.05; ∗∗, P < 0.01) between the two groups of mice.
Collectively, these findings demonstrate that iNKT cell activation results in a higher antibody response to a recall immunization because of an increased expansion of the antigen-specific memory B cell pool.
Mechanisms of iNKT Cells Help Antibody Responses.
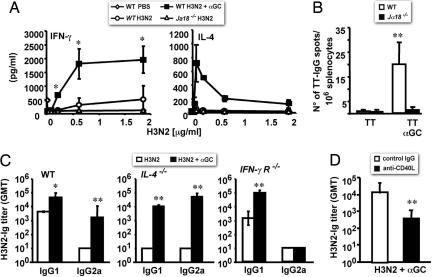
We envisaged that αGC-activated iNKT cells could enhance antibody responses by influencing either the induction of antigen-specific CD4+ T cells, which are important to help antibody production, or the differentiation of B cells to PCs, which are the cells that do actually produce antibodies, or both. To address the first point, WT and Jα18−/− C57BL/6 mice were immunized once with H3N2 or H3N2 plus αGC, and killed 10 days later to assess the capability of MHC-II-restricted CD4 T cells in their spleen to produce IFN-γ and IL-4 in response to increasing doses of H3N2 in vitro. We found that splenocytes from WT, and not those from Jα18−/− mice, secreted consistent amounts of cytokines in response to H3N2 in vitro (Fig. 6A). Notably, only splenocytes from WT mice immunized with H3N2 plus αGC produced high amount of IL-4 (Fig. 6A Right), which demonstrates that aGC-activated iNKT cells potentiate the generation of antigen-specific CD4+ T cells after vaccination.
Fig. 6.
CD40/CD40L interactions are required for iNKT cell help to antibody responses, whereas IL-4 and IFN-γR are dispensable. (A) IFN-γ and IL-4 released in response to H3N2 in vitro by splenocytes obtained from WT and Jα18−/− C57BL/6 injected with PBS, H3N2, or H3N2 plus αGC. Shown are average values (±SD) obtained by analyzing three mice per group. (B) Numbers (average ± SD) of PCs producing TT-specific IgG in the BM from WT and Jα18−/− C57BL/6 mice (six mice per group) 8 weeks after the second immunization with TT alone or TT plus αGC. (C) IgG1/IgG2a subclass distribution of H3N2-specific serum antibodies in WT C57BL/6 mice or in congenic mice lacking either IL-4 (IL-4−/−) or the IFN-γR (IFN-γR−/−), 2 weeks after two immunizations with H3N2 or H3N2 plus αGC. Shown are GMTs with 95% C.I. obtained analyzing three mice per group. (D) GMTs with 95% C.I. of H3N2-specific antibodies found on day 14 in sera from C57BL/6 WT mice (five mice per group) treated on days −1, 0, and +1 with saturating amounts of a neutralizing anti-CD40L mAb or control IgG and immunized on day 0 with H3N2 plus αGC. Results from one experiment representative of two are shown. Asterisks indicate statistically different values (∗, P < 0.05; ∗∗, P < 0.01) between groups of mice of the same genetic background immunized with the protein alone or the protein plus αGC.
To determine the effects of aGC-activated iNKT cells on the generation of antigen-specific PCs, we immunized WT and Jα18−/− mice twice with TT or TT plus αGC. Eight weeks after the second immunization, frequencies of specific PCs in the BM of immunized mice were determined by a TT-specific-IgG ELISPOT assay ex vivo. We found detectable numbers of TT-specific IgG producing PCs only in mice immunized with TT plus αGC, whereas mice immunized with TT alone had no detectable TT-specific PCs in their BM (Fig. 6B). This finding demonstrates a key effect of aGC-activated iNKT cells on PC generation and/or survival.
To address the role of cytokines in iNKT cells' help to B cells, we analyzed the adjuvant effect of αGC in C57BL/6 WT mice and congenic mice lacking IL-4 or the receptor for IFN-γ (IFN-γR). Fig. 6C Left shows that in WT mice immunization with H3N2 alone induced a Th2 response as indicated by the presence of IgG1 in the absence of IgG2a, whereas immunization with H3N2 plus αGC elicited a Th0 response with both IgG1 and IgG2a. Mice lacking IL-4 did not develop any antibody response when immunized with H3N2 alone, whereas when immunized with H3N2 plus αGC they mounted a Th0 response, although with an inverted ratio between IgG1 and IgG2a with respect to their WT littermates (Fig. 6C Center). Mice lacking IFN-γR (Fig. 6C Right), although unable to produce IgG2a antibodies, after immunization with H3N2 plus αGC displayed significantly higher IgG1 titers than mice immunized with H3N2 alone. Altogether, these findings suggest that IL-4 can be replaced by other cytokines (e.g., IL-13) to obtain the Th2-like component of αGC-dependent iNKT cells' help to B lymphocytes, while IFN-γ is essential for the Th1-like component of the iNKT cell effector function and therefore for a balanced Th0 helper effect of iNKT cells.
Finally, we asked whether CD40/CD40L interactions were required for the αGC-dependent iNKT cell help in vivo. We therefore assessed antibody responses to H3N2 in mice treated with a neutralizing anti-CD40L mAb. As shown in Fig. 6D, after immunization with H3N2 plus αGC, mice treated with the anti-CD40L mAb displayed significantly lower H3N2-specific antibody titers than mice treated with control IgG.
Discussion
We have shown that immunization with proteins and αGC activates iNKT cells to help protein-specific B cells to produce antibodies. This adjuvant effect leads not only to increased primary and memory antibody responses, but also to protective immunity against pathogens. Furthermore, αGC induces antibody titers as high as those obtained by classical adjuvants. Our results represents a substantial increase of what is known about the adjuvant activity of αGC-activated iNKT cells (33, 42) and clarifies crucial aspects of the interaction between NKT and B cells by providing clues on the role played by iNKT cells in regulating critical features of the adaptive antibody response, such as protection from infections and generation of the memory B cell pool. Depending on the cytokine pattern they promote, adjuvants can be categorized as Th1 (e.g., CpG) or Th2 (e.g., Alum) and in mice this pattern correlates with the prevalent production of antibodies of the IgG2a and IgG1 isotype, respectively. αGC does not polarize the Th response, rather it elicits a balanced Th0 production of IgG1 and IgG2a antibodies.
Although hyporesponsiveness of iNKT cells has been reported for a second systemic injection of high doses of αGC in mice (37, 38), we did not observe a similar phenomenon after two immunizations with protein antigens and αGC. The lack of iNKT cell hyporesponsiveness in our model could be related to either the dose of injected αGC or its route of administration. The dose of αGC we used (0.1 μg) is 2- to 20-fold lower then those used to induce iNKT cells anergy (37, 38). Indeed, it has been reported that after two systemic injections of 0.2 μg of αGC, the in vitro production of IL-4 by iNKT cells is unaffected, whereas the production of IFN-γ is reduced only 2-fold (37), suggesting that the iNKT cell hyporesponsiveness phenomenon is dose-dependent. The route of administration of αGC used in the present work (i.m. or s.c.) is different from the one (i.p.) reported to induce anergy in iNKT cells (37, 38), and it might provide a slower distribution of the compound as compared with systemic injection, possibly resulting in a milder activation of iNKT cells and no subsequent hyporesponsiveness.
Efficient CD4+ T cell help to B cells requires cognate interaction and bystander stimuli such as CD40 engagement and cytokines. INKT cell help to B cells in vivo also depends on CD40L-CD40 interaction, as demonstrated by the reduction of antibody responses to proteins and αGC in the presence of neutralizing anti-CD40L antibodies. iNKT cells can help B cells in mice lacking either IL-4 or IFN-γR. However, these KO mice mount antibody responses that are quantitatively normal but qualitatively deficient in the IgG isotype specifically induced by either cytokine. Indeed, in the absence of IL-4, immunization with protein and αGC results in an inversed polarization, with a predominance of Th1-associated antibodies (IgG2a) and a lower Th2 response (IgG1). In mice lacking the IFN-γR, polarization is more dramatic as IgG2a antibodies are undetectable while IgG1 antibodies are normally induced.
These results are consistent with those obtained in the same KO mice after different antigenic challenges (43, 44) and indicate that iNKT cells provide help to B cells through the combined action of CD40–CD40L interactions and cytokines.
αGC-activated iNKT cells elicit a stronger CD4+ T cell response against the coadministered protein antigen, suggesting that their adjuvant effect on the antibody response is also related to an increased availability of MHC-II restricted T cell help to B cells (28, 29). We have confirmed that in mice knockout for MHC class II, due to the absence of conventional CD4+ Th cells, there is no B cell response toward T-dependent antigens admixed with conventional adjuvants such as alum (45). Remarkably, MHC II−/− mice vaccinated with a protein antigen and αGC, have detectable levels of protein-specific IgG, demonstrating that iNKT cells can, at least in part, replace conventional CD4+ Th functions.
In mice lacking iNKT cells, effector antibody responses after two protein immunizations are similar to those observed in WT mice, demonstrating that the absence of these cells does not influence the amount of antibodies produced at the peak of the immune response. Interestingly, in mice genetically lacking iNKT cells we have observed a significant decrease in the duration of serum antibody titers elicited by protein vaccination as compared with WT mice. Since ≈8 weeks after the second immunization (when levels of antigen-specific Ig titers starts to diverge between WT and Jα18−/− mice) the number of antigen-specific PCs in the BM is below the detection limit of ELISPOT assays, we could not formally prove the relation between iNKT cells and PCs. However, we have assessed PC frequency in WT mice immunized with proteins and aGC and found an increased frequency of BM-resident specific PCs. Therefore, it is likely that PC survival, which is the most relevant parameter accounting for serological memory in mice (41), is under the influence of a spontaneous homeostatic activity of iNKT cells, possibly triggered by the recognition of an endogenous glycolipid, such as iGB3 (18, 20). Further experiments are warranted to formally clarify the mechanisms underlying the spontaneous iNKT cell help to serological memory.
Taken together, our results underscore the relevance of the interplay between innate and adaptive effectors for the development of efficient antibody responses and reveal iNKT cells as players involved in this interaction.
Methods
Mice and Protocols for Experiments in Vivo.
Mice.
WT CD1 and C57BL/6 mice were from Charles River (Calco, Italy). WT BALB/c, C3H/HeJ, B6.129P2-Il4tm1Cgn/J IL-4-deficient (44), and B6.129S7-Ifngtm1Ts/J IFN-γR-deficient (43) mice were from Jackson Laboratories (Bar Harbor, ME). B6.129-Tcra-Jtm1Tg mice Jα18-deficient (46) and B6.129-CD1tm1Gru CD1d-deficient mice (47) were kindly obtained from M. Taniguchi (RIKEN, Yokohama, Japan) and H. R. MacDonald (Ludwig Institute for Cancer Research, Lausanne, Switzerland) respectively, and were backcrossed 12 times onto C57BL/6 at the San Raffaele Scientific Institute. B6.129S2-Igh-6tm1Cgn MHC-II-deficient (AB0, referred to as MHC-II−/−) (48), were from the European Mouse Mutant Archive (Orleans, France).
Immunization.
TT (0.1–10 μg per dose), DT (3 μg per dose), and H3N2 and H1N1 (3 μg per dose, from the human influenza viruses A/Panama/2007/99 and A/New Caledonia/20/99, respectively) were from Novartis Vaccines. Unless specified otherwise, immunization were performed on days 0 and 15, and groups of 3–10 mice, 6–8 weeks old, were injected i.m. with antigens in 50 μl of PBS alone or with αGC (0.1 μg per dose; Alexis, Lausen, Switzerland), Alum (0.1 mg/dose, Pierce, Rockford, IL), MF59 (50%; Novartis Vaccines), CpG (25 μg per dose; 5′-tccatgacgttcctgacgtt-3′; Primm, Milan, Italy), or 50% complete Freund's adjuvant/incomplete Freund's adjuvant. To block in vivo CD40–CD40L interactions, 400 μg of the MR1 antiCD40L mAb (Taconic Europe A/S, Ejby, Denmark) were given i.p. at days −1, 0, and +1.
Influenza infection.
Two weeks after the second immunization with H1N1 alone, H1N1 plus αGC, or H1N1 plus MF59, 10 mice per group were challenged intranasally with 10 μl of PBS containing 100 LD50 of the influenza virus A/WS/33(H1N1). Animal survival was assessed daily for 14 days. Mice were housed and treated according to procedures reviewed and approved by the Animal Ethical Committees of our institutes.
Measurement of Antigen-Specific Antibody Titers, PCs, and Memory B Cell Frequencies.
Circulating antigen-specific antibodies were titrated by endpoint ELISA. Serial 1:3 dilution of sera was plated onto ELISA plates (Nunc, Roskilde, Denmark) coated with each given antigen. Bound antibodies were detected with alkaline phosphatase-conjugated goat anti-mouse IgGAM(H+L), IgG, IgG1, IgG2a, or IgG2c (Southern Biotechnology Associates, Birmingham, AL), followed by the pNPP substrate (Sigma/Aldrich, St. Louis, MO). Antibody titers are expressed as reciprocal dilutions giving an OD405 > mean blank OD405 plus 3 SD. Blanks consistently displayed OD405 < 0.1 and <10% variability.
The frequency of PCs in the BM from WT and Jα18−/− mice (five mice per group) was determined 8 weeks after the second immunization with TT or TT and αGC. Single-cell suspensions were incubated overnight at 2 × 106 per well, in RPMI medium 1640 (Gibco/Life Sciences, Carlsbad, CA) 5% FCS (HyClone, Logan, UT), into TT-coated MultiScreenHTS ELISPOT plates (Millipore, Milan, Italy). Spots were revealed with an HRP-conjugated goat-anti-mouse IgG antiserum followed by the AEC substrate (Sigma/Aldrich) and enumerated by using computer-assisted video image analysis Axioplan 2 (Zeiss, Thornwood, NY).
ASC precursor frequencies were determined by culturing splenocytes in limiting dilution as described (49). Eight replicates for each cell dilution were plated into 96-well plates in 0.2 ml of RPMI medium 1640, 5% FCS ± CpG (5 μg/ml; Primm, Milan, Italy) and IL-2 (1,000 units/ml; Novartis). Antigen-specific IgGs in 10-day supernatants were detected by ELISA as described above. The splenocyte dilution containing one H3N2-ASC precursor was determined by applying the Reed and Muench formula (50) to the distribution of wells, giving an OD405 higher than the mean blank OD405 plus 3 SD. Frequencies of preexisting H3N2-specific ASC in the spleen were <2/106 B cells.
Antigen-Specific Cytokine Production in Vitro.
Splenocytes were obtained from WT and Jα18−/− mice (three mice per group) 10 days after a single s.c. injection of PBS, H3N2, or H3N2 and αGC, and were cultured in duplicate, at 2 × 106 per well, in 0.2 ml of RPMI medium 1640, 5% FCS, and 0–2 μg of H3N2. IFN-γ and IL-4 in 48-h supernatants were measured by ELISA (BD Pharmingen).
Statistics.
For statistical analysis antibody titers were log10-transformed and tested for normal distribution by using the Kolmogorov–Smirnov test for continuous variables. Geometric mean antibody titers (GMTs) and the 95% C.I. were derived from the anti-log10 of the mean, and of the mean ± SD, of the log10 titer transformations. Comparisons between two groups were done with the two-tailed Student t test for unpaired samples, applying correction for unequal variances when required. Multiple comparisons were done by ANOVA with the LSD correction. Survival rates after flu challenge were compared with the Mann–Whitney U test (two-tailed). For all tests, a value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro (G.C.), the Italian Ministry of Health (P.D.), and Italian Ministry of University and Research Grant FIRB-RBNE017 (to G.C. and P.D.). P.P and E.T. are supported by fellowships from the Ph.D. program in Molecular Medicine, Vita-Salute University, San Raffaele, Italy.
Abbreviations
- αGC
α-galactosylceramide
- iNKT
invariant natural killer T
- ASC
antibody-secreting cell
- PC
plasma cell
- BM
bone marrow
- TT
tetanus toxoid
- DT
diphtheria toxoid
- TCR
T cell receptor
- Th
T helper
- GMT
geometric mean antibody titer.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700191104/DC1.
References
- 1.Rajewsky K. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 3.Kaisho T, Akira S. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- 4.Pasare C, Medzhitov R. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 5.Ruprecht CR, Lanzavecchia A. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 6.Yuan D, Wilder J, Dang T, Bennett M, Kumar V. Int Immunol. 1992;4:1373–1380. doi: 10.1093/intimm/4.12.1373. [DOI] [PubMed] [Google Scholar]
- 7.Gray J, Horwitz D. J Immunol. 1995;154:5656–5664. [PubMed] [Google Scholar]
- 8.Lantz O, Bendelac A. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendelac A, Lantz O, Quimby M, Yewdell JW, Bennink JR, Brutkiewicz R. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 11.Exley M, Garcia J, Balk SP, Porcelli S. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcelli SA, Modlin RL. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 13.Brigl M, Brenner MB. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 14.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 15.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, Wilson IA, Wong CH. Proc Natl Acad Sci USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard, Wang V, Gao Y, Yin N, et al. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 19.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, et al. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 22.Bendelac A, Rivera MN, Park SH, Roark JK. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 24.Van Kaer L. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, Taniguchi M, Bendelac A, Van Kaer L, Koezuka Y, Tsuji M. Proc Natl Acad Sci USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. J Ex Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skold M, Beha SM. Infect Immun. 2003;71:5447–5455. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. J Immunol. 2003;171:5140–5514. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 30.Smyth MJ, Crowe NY, Takeda K, Yagita H, Godfrey DI. Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 31.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. J Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 32.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 34.Claassen E, de Leeuw W, de Greeve P, Hendriksen C, Boersma W. Res Immunol. 1992;143:478–483. doi: 10.1016/0923-2494(92)80057-r. [DOI] [PubMed] [Google Scholar]
- 35.Klinman DM, Currie D, Gursel I, Verthelyi D. Immunol Rev. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 36.Lindblad EB. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 37.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang C-R, Joyce S, Van Kaer L. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podda A, Del Giudice G. Exp Rev Vaccines. 2003;2:197–203. doi: 10.1586/14760584.2.2.197. [DOI] [PubMed] [Google Scholar]
- 40.Higgins DA, Carlson JR, Van Nest G. Vaccine. 1996;14:478–484. doi: 10.1016/0264-410x(95)00240-2. [DOI] [PubMed] [Google Scholar]
- 41.Manz RA, Hauser A, Hiepe F, Radbruch A. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 42.Lang GA, Exley MA, Lang ML. Immunology. 2006;119:116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn R, Rajewsky K, Muller W. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 45.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 46.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 47.Smiley ST, Kaplan MH, Grusby MJ. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 48.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernasconi NL, Traggiai E, Lanzavecchia A. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 50.Reed LJ, Muench H. Am J Hyg. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.