Abstract
The ability to be synchronized by light–dark cycles is a fundamental property of circadian clocks. Although there are indications that circadian clocks are extremely light-sensitive and that they can be set by the low irradiances that occur at dawn and dusk, this has not been shown on the cellular level. Here, we demonstrate that a subset of Drosophila's pacemaker neurons responds to nocturnal dim light. At a nighttime illumination comparable to quarter-moonlight intensity, the flies increase activity levels and shift their typical morning and evening activity peaks into the night. In parallel, clock protein levels are reduced, and clock protein rhythms shift in opposed direction in subsets of the previously identified morning and evening pacemaker cells. No effect was observed on the peripheral clock in the eye. Our results demonstrate that the neurons driving rhythmic behavior are extremely light-sensitive and capable of shifting activity in response to the very low light intensities that regularly occur in nature. This sensitivity may be instrumental in adaptation to different photoperiods, as was proposed by the morning and evening oscillator model of Pittendrigh and Daan. We also show that this adaptation depends on retinal input but is independent of cryptochrome.
Keywords: circadian rhythm, dual, oscillator model, PERIOD, synchronization, TIMELESS
Endogenous circadian clocks prepare organisms according to the most reliable and predictable of environmental changes, the cycle of day and night. To function as reliable timers, circadian clocks themselves are synchronized to the 24-h cycle. This synchronization is accomplished mainly by light. Whereas light during the day has little effect on circadian clocks, they are most susceptible to light in the early and late night. Bright light pulses applied during the early night delay the phase of the clock; light pulses during the late night advance it (1). Stable synchronization occurs when the delaying and advancing effects of light on the clock in the early (at dusk) and late night (at dawn) are of equal strength. In nature, stable synchronization is a challenging task, because irradiances during dawn and dusk can vary largely from day to day because of the weather. Bünning (2) measured irradiances systematically throughout day and night and found that day-to-day fluctuations are smallest during early dawn and late dusk, when the irradiances are still <10 lux. Therefore, he proposed that organisms time their clocks to the very low irradiances occurring during early dawn and late dusk and thus must be very light-sensitive. Indeed, he found that bean plants synchronize to light of moonlight intensity (0.6–0.8 lux) and that artificial moonlight applied during the night phase shifted the rhythm of leaf movement (2). Bean plants lower their leaves during the night, and Bünning suggested that they need to do so to decrease the moonlight reaching the leaf surface to avoid their light-sensitive clocks interpreting the moonlight as the coming dawn (2).
In animals, it is under debate whether natural, dim, nocturnal light affects the endogenous clock, and, indeed, most studies in mammals neglect the possibility that the clock may be highly light-sensitive. For instance, activity was recorded under nocturnal dim light ranging from starlight to full-moonlight intensity, assuming that dim light has little influence on the circadian pacemaker. However, it has been shown that nocturnal light can affect activity levels as well as activity patterns (3–8).
We aimed to test the effect of nocturnal dim light in the fruit fly Drosophila melanogaster, which serves as important model organism for understanding the circadian clock at the behavioral, cellular, and molecular level. Recent studies showed that Drosophila is also suited to analyze the mechanisms that underlie seasonal adaptations of circadian clocks (9–11). As do many animals (12), fruit flies show bimodal activity patterns with pronounced morning (M) and evening (E) activity peaks. In Drosophila, these peaks are controlled by distinct groups of circadian pacemaker neurons in the brain, the so-called M and E cells (13–15). The M activity occurs earlier and the E activity occurs later under long summer days, showing a behavioral adaptation to seasonal changes in day length (9, 11). This finding is in accordance with the long-standing two-oscillator model of Pittendrigh and Daan (16) originally developed for rodents that predicts that the M cells should shorten the period of their clock in response to light, whereas the E cells should lengthen their period upon light. Indeed, the molecular clock in subsets of Drosophila's M and E cells shortens and lengthens its period, respectively, when the flies are exposed to constant light conditions (17). It is, however, completely unknown whether this adaptive behavior would also occur under synchronized conditions in response to the low irradiances in the early morning and evening.
In this study we exposed wild-type flies and two photoreceptor mutants first to conventional light–dark (LD) cycles and subsequently to light–“moonlight” (LM) cycles with nocturnal dim light of moonlight intensity. We studied the activity patterns of the flies as well as the molecular cycling of the clock proteins PERIOD (PER) and TIMELESS (TIM). We found that wild-type flies respond strongly to the moonlight by increasing activity levels and by changing the timing of their activity. Moonlight at night makes them advance their M activity into the late night and delay their E activity into the early night; thus, they appear to interpret LM as long day. This dramatic change in activity was paralleled by a prominent reduction of overall clock protein levels in all clock neurons and by an advance and a delay of the molecular clock in subsets of the M and E cells, respectively. Mutants that lacked functional cryptochrome, a blue light photopigment that works as one of the main circadian photoreceptors (18, 19), responded similarly to wild-type flies, but they needed higher moonlight intensities to show this response. Eyeless mutants did not shift their activity at all.
Our results show that the circadian clock of fruit flies is highly light-sensitive, and they suggest that fruit flies may indeed use dim, early morning and late evening light to time their clocks in nature. Apparently, they use compound eyes and not cryptochrome as main photoreceptors for this timing.
Results
Locomotor Activity Under Moonlight–Dark, LD, and LM Cycles.
In a first experiment we tested whether wild-type and two photoreceptor mutant flies were able to synchronize to LD cycles with dim light (0.03 lux, equivalent to light from a quarter moon). The photoreceptor mutants were cryb, lacking functional cryptochrome (19), and clieya, lacking compound eyes (20). We found that all flies, including the mutants, synchronized to dim light (Fig. 1).
Fig. 1.
Wild-type flies and the photoreceptor mutants cryb and clieya are able to synchronize to dim light. Eleven wild-type, 23 cryb, and 12 clieya flies were recorded for 10 days under LD cycles with a light intensity of 0.03 lux during the light phase. A typical actogram is shown for each genotype. Periodogram analysis revealed that all tested flies synchronized to the 24-h cycle [mean periods: 24.0 ± 0.02 h (wild-type); 24.0 ± 0.02 h (cryb); and 24.0 ± 0.03 h (clieya)].
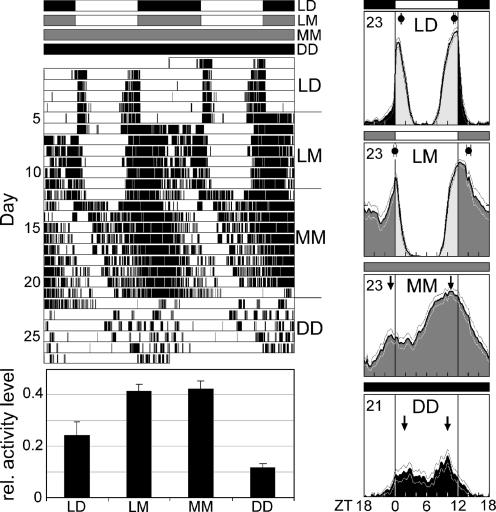
In a next experiment, wild-type flies were recorded sequentially under LD and LM cycles and under continuous moonlight (MM) and complete darkness (DD). Under LD cycles, the flies showed the well known bimodal activity patterns, consisting of M and E activity peaks, with little activity during midday and night (Fig. 2). The M activity peak started before lights-on, but activity increased to high levels only at lights-on. The latter reaction is known as the “lights-on effect” or “startle response” and is simply a shock response to light; in contrast, lights-off suppresses the activity of the flies. Both effects (activation by light and suppression by darkness) are independent of the endogenous clock and are called masking effects (11). Under LM conditions, the masking effects disappeared, and the flies became more active during the night (Fig. 2). They showed high activity levels even before lights-on and did not show the characteristic increase in activity at lights-on. Furthermore, activity was not suppressed by lights-off, but rather extended into the night. As a consequence, the midday activity break (sometimes called a siesta) became more prominent, and the midnight trough was less pronounced. On average, the M peak advanced by 1 h, and the E peak delayed by 3 h into the night; thus, the M–E interval became larger. We showed previously that higher continuous light intensities (≈0.5 lux, approximating full moonlight), led to free-run of M and E components with short and long periods, respectively (17). Moonlight of 0.03 lux may also shorten the period of the M component and lengthen that of the E component, but with period changes that are too small to provoke uncoupling of the M and E components. Rather, these changes may cause the two components to drift apart, resulting in a larger M–E interval. When the flies were subsequently placed in continuous low light (MM of 0.03 lux) and DD conditions, the flies free-ran with periods not significantly different from each other. Thus moonlight seems to have no effect on the overall speed of the clock; however, the flies were significantly more active and the M–E interval was larger under MM than under DD conditions (Fig. 2).
Fig. 2.
Activity patterns of wild-type flies recorded consecutively under LD cycles, LM cycles, MM, and DD. (Left) Double-plotted actogram of the locomotor behavior of an individual fly and the mean activity levels of all flies. (Right) Average activity profiles under LD, LM, MM, and DD of all 23 recorded flies. Under LD cycles, activity occurs in M and E, respectively. Under LM cycles, the M activity peak advances by 1.0 h (±0.2 h), whereas the E activity peak delays by 3.0 h (±0.2 h), making the fly nocturnal. The dots on top of the LD and LM profiles indicate the average time of M and E peaks (±SEM) calculated from the peak points of individual flies. Periodogram analysis revealed that all flies synchronized to LD and LM, showing mean periods of 24.0 ± 0.01 h and 24.0 ± 0.02 h, respectively. Under MM conditions, the flies free-ran, maintaining a large M–E interval (arrows). This interval was considerably smaller under DD conditions (arrows). Periods were not significantly different under MM (24.8 ± 0.1 h) and DD (24.7 ± 0.2 h), but activity levels were significantly higher under MM. This applies also for LM and LD. The error bars are SEMs.
The Effect of Moonlight on the Molecular Oscillations.
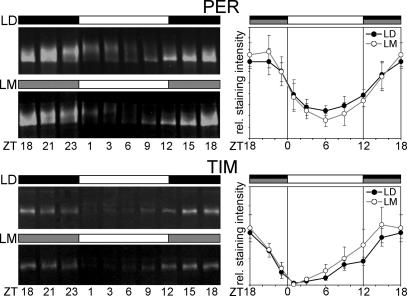
We next aimed to see the effect of moonlight on the molecular oscillations of the clock proteins in wild-type flies. As a first step, we compared head extracts of flies synchronized to LD cycles with that of flies synchronized to LM cycles. We did not find any significant differences in PER and TIM protein cycling between either condition, neither in the amount of the proteins nor in time courses of their oscillations (Fig. 3). Because PER and TIM are most abundant in the photoreceptor cells of the eyes, Western blots of head extracts reflect the time course of protein cycling in the compound eyes. We conclude that moonlight does not influence the peripheral clocks in the eyes.
Fig. 3.
Photoreceptor cells of wild-type flies show similar PER and TIM oscillations under LD and LM conditions. (Left) Western blots of whole-head extracts stained with anti-PER or anti-TIM. (Right) Mean staining intensity calculated for PER and TIM under LD and LM conditions for three independent blots, respectively. No significant differences occurred between LD and LM. The error bars are SEMs.
In a second step, we investigated the consequences of moonlight on the main circadian pacemaker cells in the brain, the so called lateral neurons. The lateral neurons consist of the LNd, l-LNv, and s-LNv cells, whereby the s-LNv cells can be subdivided into four cells that contain the neuropeptide pigment-dispersing factor (PDF) and a fifth cell that is PDF-negative (17). The LN cells are necessary and sufficient for the generation of robust adult locomotor rhythms in the absence of environmental time cues and for normal bimodal activity patterns under LD conditions (reviewed in ref. 21). Previous work showed that the M peak of activity is controlled by the four PDF-positive s-LNv cells, whereas the E peak is governed by the fifth PDF-negative s-LNv and some LNd cells (13, 14, 17). According to their function, the s-LNv cells are called M cells, whereas the combined fifth PDF-negative s-LNv and the LNd cells are called E cells.
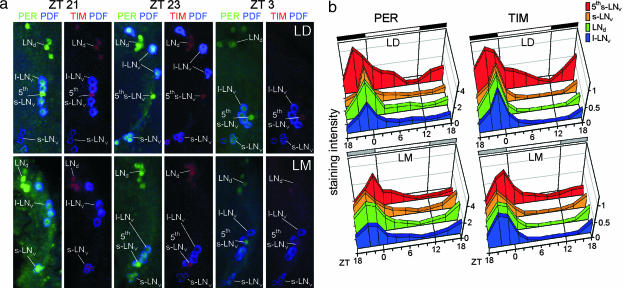
To judge the clock protein cycling in the different cell groups, we performed immunolabelings against PER, TIM, and a precursor of PDF on entire brains throughout the LD and the LM cycle (Fig. 4). Consistent with observations under LD conditions in ref. 22, we found that the s-LNv, LNd, and l-LNv cells peaked 1 h before lights-on [at Zeitgeber time 23 (ZT23)], with the s-LNv cells showing the sharpest clock protein peak. This finding contrasted with the fifth PDF-negative s-LNv cells. This cell reached maximal staining 3 h before lights-on (at ZT21) and showed a rather broad staining peak, such that considerable staining was apparent even after lights-on, when the other neurons were virtually devoid of these clock proteins.
Fig. 4.
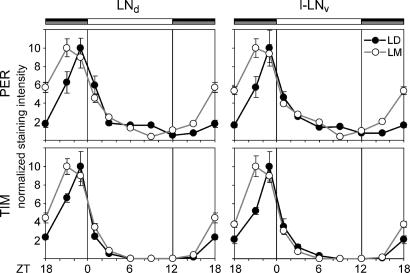
Moonlight induces temporal changes in PER and TIM immunoreactivity in the pacemaker neurons of wild-type flies. (a) PER and TIM staining in the different groups of lateral neurons under LD and LM conditions at ZT21, ZT23, and ZT3. PER is visualized in green, TIM in red, and PDF in blue. PDF labeling is essential for identifying the fifth s-LNv cell, which is located among the l-LNv cells but which lacks PDF. (b) Quantification of the staining results. The fifth s-LNv cell is maximally labeled at ZT21 under LD but at ZT23 under LM conditions. The s-LNv cells show the strongest labeling at ZT23 under LD but at ZT21 under LM conditions. As for the s-LNv cells, the l-LNv and LNd cells show the strongest labeling at ZT23 under LD and at ZT21 under LM conditions, but their peak becomes broader under LM and the phase advance appears less pronounced.
After transfer to LM conditions, significant phase changes of clock protein cycling occurred in all neurons. The phase of the s-LNv cells was phase-advanced by 2 h to ZT21, whereas the phase of the fifth PDF-negative s-LNv cells was delayed by 2 h and occurred at ZT23 (Figs. 4 and 5). Thus, the administration of low-intensity light during the dark phase of an LD cycle not only shifted the M and E activity peaks but also shifted the molecular cycling of clock proteins in the M cells (s-LNv) and in one E cell (fifth PDF-negative s-LNv). In contrast to previous findings, the LNd did not behave as E cells but rather as M cells, showing an advance in their phase of clock protein expression (Figs. 4 and 6). The same was true for the l-LNv cells (Figs. 4 and 6). However, the peaks in these two cell groups simultaneously broadened, suggesting that these groups of cells may be heterogeneous.
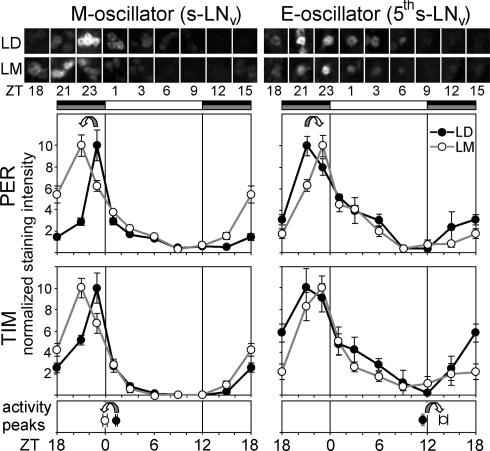
Fig. 5.
The molecular shift in the M (s-LNv) and E (fifth s-LNv) cells corresponds to the shift in activity peaks. (Left) M oscillator represented by the s-LNv. (Right) E oscillator represented by the fifth s-LNv. (Top) Representative stainings for PER. (Middle) LD and LM curves for PER and TIM. (Bottom) Phases of the M and E activity peaks of wild-type flies under LD and LM. Staining intensities for PER and TIM were normalized (maximal staining intensity set to 10) to better compare the peak points. Significant staining differences between LD and LM were found in the M cells at ZT18, ZT21, and ZT23 for PER and at ZT18 and ZT21 for TIM (P < 0.05). In the E cells, staining was significantly different at ZT21 for PER and ZT18 for TIM (P < 0.05). The M activity peak occurs immediately after the drop in PER and TIM levels in the M cells; M activity and protein peaks are both advanced under LM. The E activity peak occurs ≈14 h after peak levels in the fifth s-LNv; E activity and protein peaks are both delayed under LM. The error bars are SEMs.
Fig. 6.
The LNd and l-LNv cells respond as M oscillators. Significant staining differences between LD and LM were found in the LNd and l-LNv cells at ZT18 and ZT21 for PER and TIM (P < 0.05). Labeling is the same as in Fig. 5.
A second striking effect of moonlight was a reduction of the overall clock protein level in all pacemaker cells to ≈70% of the LD level (Fig. 4). This effect appears to be negatively correlated with the activity level, which was increased under moonlight (Fig. 2). This finding is consistent with a typical daily time course: Overall clock protein levels are high at night when the flies are inactive, but are low during the day when the flies show their main activity bout, the E peak. Similarly, the M activity peak begins as clock protein levels in the s-LNv cells are declining (Fig. 5).
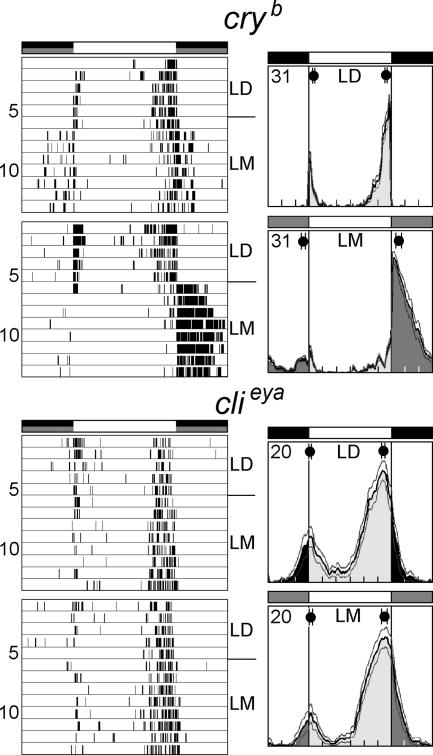
Which photoreceptors are responsible for mediating the moonlight effect? We then compared the activity patterns of clieya mutants and cryb mutants under LD and LM cycles. In clieya mutants, moonlight at night led to neither an advance nor a delay of M and E activity peaks, even when the moonlight intensity was increased to 0.5 lux (Fig. 7). In contrast, cryb mutants exhibited advances and delays of M and E peaks under LM conditions that were almost of the same magnitude as in wild-type flies when moonlight was increased to 0.5 lux (Fig. 7). These results indicate that it is mainly rhodopsins and not cryptochrome that mediate the responses to dim light at night under synchronized conditions.
Fig. 7.
Typical actograms of cryb and clieya mutants and average activity profiles under LD and LM conditions. The cryb fly shown in the topmost actogram was recorded under LM of 0.03 lux; all other flies were recorded under LM of 0.5 lux. The average activity profiles were calculated from flies recorded under LM of 0.5 lux. Periodogram analysis revealed 24-h rhythms for all flies under all conditions, showing that they synchronized to LD and LM (0.03 and 0.5 lux). cryb mutants shifted their activity peaks in a similar manner, as did wild-type flies, but clieya mutants did not respond at all to nocturnal moonlight. Labeling is the same as in Fig. 2.
Discussion
We show here that fruit flies respond strongly to nocturnal light of approximately quarter-moonlight intensity, at the behavioral level and at the level of the molecular clock. Nocturnal light provoked an advance of the M activity and a delay of the E activity into the night. Simultaneously, the midday trough broadened and the midnight trough diminished, making the flies nocturnal in a cycle of 12 h:12 h. In other words, they switched their temporal niche. Upon transfer to constant conditions, they reverted, with activity in MM always starting from the preceding light phase (see Fig. 2). A similar switch was observed in night-active white-fronted lemurs (Eulemur fulvus albifrons) (23). These animals switched from night-active to day-active after reduction of the nocturnal illumination below a certain threshold; but on release into constant conditions, free-running activity always started from the preceding dark phase. This switching was thought to be caused by direct effects of light on activity (masking effects) that do not interfere with the circadian clock. In other words, the animals have strong preferences for certain light conditions, and they accordingly avoid being active under both total darkness, because it precludes visual orientation, and in high irradiances, because it may damage their sensitive eyes. Studies on mice (Mus musculus) yield similar results (reviewed in ref. 24), with nocturnal animals becoming diurnal after mutations, genetic manipulations, or brain lesions that interfere with photoreceptor input to the circadian clock. One possible explanation is that mice with impaired photoreception simply prefer higher irradiances than wild-type mice.
Here we demonstrate that temporal niche switching may be caused by a change in the phasing of the endogenous clock as induced by dim light levels. Masking effects may still be involved, because the masking that is typically seen in LD is lost under LM conditions. Instead, the flies' activity appears to be promoted by dim light. Despite a contribution of masking effects, we have shown here that Drosophila's circadian pacemaker neurons are highly light-sensitive and respond to nocturnal light levels comparable to moonlight. The peripheral oscillators in the eye fail to do so. This finding is extremely interesting with respect to photoreceptor sensitivity. Whereas photoreceptors for visual image detection should quickly adapt to alterations in light intensity to ensure optimal vision, photoreceptors for the circadian clock should not adapt, at least not in the lower ranges. Otherwise, it would be impossible to measure increasing and decreasing irradiances during dawn and dusk. The observed clock protein oscillations in the photoreceptor cells of the compound eyes might regulate light sensitivity of the circadian system (25). If true, the clock protein levels and oscillations should not be altered by dim light, and the high sensitivity of the clock during early dawn and late dusk should be preserved. Indeed, we did not observe any alterations in PER and TIM protein levels under LM conditions. Consistent with this finding, we found that the compound eyes, not the photoreceptor cryptochrome, mediate the responses to moonlight. This finding is in line with previous observations showing that the compound eyes and, thus, rhodopsins are necessary for adaptation of activity times to long and short days (11). Interestingly, the insect rhodopsins are closely related to the mammalian melanopsin, which is critically involved in mouse circadian photoreception (26).
But then, what role remains for cryptochrome? Fig. 7 suggests that cryptochrome may contribute to the phase delay of the E peak under LM conditions, because cryb mutants show a less dramatic delay of this peak than wild-type flies (see Fig. 2). However, the E peak was not at all delayed in clieya mutants, suggesting that a phase-delaying effect of cryptochrome is either dependent on the compound eyes or is negligible. Cryptochrome is a blue-light photoreceptor and, thus, most appropriate to detect intensity changes in blue light. Furthermore, the proportion of blue light increases during dawn and decreases during dusk. Thus, cryptochrome appears particularly suited to distinguish dawn or dusk light transitions from moonlight, which does not change its spectrum over time.
We interpret our results as evidence for a differential action of dim light on the pace of M and E oscillators as was originally proposed in the dual-oscillator model for rodents (16) and recently verified for D. melanogaster (13, 14, 17). Because the M cells phase advance and a subset of the E cells phase delay their clock in response to dim light, these cells are optimally suited to adapt activity rhythms to seasonal changes in day length. In addition, the proposed role of the LNd cells as E cells (13, 14) is not supported by this study. This finding is in line with earlier work demonstrating that the LNd represent a heterogenous group of cells (27, 28) and that, putatively, only one LNd cell behaves as an E oscillator (17). It is quite possible that this LNd cell also phase delayed in the present study; but without a specific marker, we were not able to distinguish it from the other cells. In summary, our results are consistent with the involvement of the PDF-positive s-LNv cells and the PDF-negative fifth s-LNv cells on behavioral rhythmicity. We also show the relevance of the two-oscillator model under natural conditions.
Not all animals may be as light-sensitive as are fruit flies, but recent studies showed that even species such as hamsters and humans are more sensitive than supposed. The synchronization of Syrian and Siberian hamsters to different photoperiods was facilitated under dim night illumination (<0.005 lux) as compared with DD (29–31). Furthermore, the incidence of bimodal activity patterns and the interval between both components increased under LM conditions. In humans, dawn simulations at low light intensities were found to phase advance the circadian melatonin and the activity rhythm (32, 33). Together with the results presented here, these studies suggest that clock function in many species is conspicuously altered by nocturnal illumination as experienced under dim moonlight. This finding might go back to the ability of primordial marine animals to synchronize their reproduction to the lunar cycle (34, 35), an ability that is apparently lost in humans and other terrestrial animals. Presumably, many terrestrial organisms do not use their light sensitivity for moonlight detection, but for timing their clock to the increasing and decreasing irradiances during dusk and dawn. Further studies are necessary to reveal whether these animals hide at night from the moonlight so as not to confound their clocks, whether they switch to nocturnal activity (or become sleepless) during the full moon, or whether they use cryptochrome to distinguish moonlight from dawn and dusk.
Materials and Methods
Fly Strains.
The wild-type strain CantonS was used for all experiments. Activity was additionally recorded in cryb (19) (+/+; cryb rec9 ss1) and clieya mutants (20) (initially named eya1), both in a red-eyed background. cryb mutants carry a point mutation in the flavin-binding site of the protein and, thus, have no functional cryptochrome, whereas clieya mutants lack the compound eyes but retain cryptochrome, the ocelli, and the Hofbauer–Buchner eyelet.
Behavioral Analysis.
Locomotor activities of individual male flies were monitored and analyzed as described in refs. 36 and 37. Illumination during the light phase was achieved with halogen photoptic lamps (Xenophot, 12 V, 120 W; Osram, Berlin, Germany) equipped with a heat filter and adjusted to 500 lux. Moonlight illumination was obtained with white UV-free light-emitting diodes (Lumitronix LED-Technik, Jungingen, Germany) and adjusted to 0.03 lux with neutral density filters (ROSCO Laboratories, London, U.K.). cryb and clieya mutants were additionally recorded under a moonlight intensity of 0.5 lux. Phase angles, activity levels, and periods were tested for significant differences with a two-tailed paired t test.
Western Blot Analysis.
Flies were synchronized for 4 days under LD (500 lux:0 lux) and LM (500 lux:0.03 lux) cycles, respectively, and collected every 2–3 h. Head extracts and Western blot procedures were performed as in ref. 38 with the following alterations: Proteins were transferred to nitrocelluose by using a wet blot chamber (Amersham Biosciences Europe, Freiburg, Germany) for 3 h at 4°C. After transfer, membranes were washed in 1× TBS, blocked for 1 h in Li-COR Biosciences (Bad Homburg, Germany) Odyssey Blocking Buffer and incubated with anti-PER [1:10,000; raised against the entire PER protein (39)] or anti-TIM [1:5,000; raised against GST fusion proteins expressing the residues 222–577 of TIM (40)]. The secondary antibody was fluorochrome coupled [goat anti-rabbit IRdye800 (Rockland, Gilbertsville, PA) at dilution 1:5,000 in 5% dry milk in TBS with 0.1% Tween 20]. Detection was performed with an Odyssey Infrared Imaging System (LI-COR Biosciences), and analysis of staining intensity of the PER-band was performed by using Image J software, version 1.33u (http://rsb.info.nih.gov/ij), produced by Wayne Rasband (National Institutes of Health, Bethesda, MD).
Analysis of PER and TIM Content in the Lateral Neurons.
Flies were synchronized for 4 days under LD and LM conditions as indicated above. They were fixed according to the above schedule, and their brains were dissected and immunostained with anti-PER (39), anti-TIM (40), anti-nb33 (a monoclonal antibody that recognizes the precursor of PDF; ref. 28), and secondary fluorescent antibodies exactly as described in ref. 17. At least 10 brains were examined for each time point. Labeling of the lateral neurons was visualized by laser-scanning confocal microscopy (LSM 510 META; Carl Zeiss MicroImaging, Jena, Germany) and analyzed with Image J as done in ref. 17. Staining intensity was calculated by using the formula I = (N − B)/B, which gives the mean labeling in the neurons above background, where N is the average pixel intensity in the neuron, and B is the average pixel intensity in the region adjacent to the positive neuron. Staining intensity was tested for significant differences between LD and LM conditions separately for the different cell groups with a one-way ANOVA followed by a post hoc test (Systat 10; SPSS, Chicago, IL).
Acknowledgments
We thank Wolfgang Engelmann, Eva Grieshaber, Alois Hofbauer, Martha Merrow, and Ralf Stanewsky for discussions and critical comments on a previous version of the manuscript; Martha Merrow for improving the language and style of the paper; and Alois Hofbauer (University of Regensburg), Francois Rouyer (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France), Ralf Stanewsky (Queen Mary University, London, U.K.), and Michael Young (The Rockefeller University, New York, NY) for antibodies. Our work is supported by the Sixth Framework Project EUCLOCK. W.B. is funded by a fellowship in the Graduate Colleague of Natural and Artificial Photoreceptors from the Deutsche Forschungsgemeinschaft. D.R. is funded by Deutsche Forschungsgemeinschaft Grant Fo207/10-2 and by EUCLOCK.
Abbreviations
- DD
complete darkness
- E
evening
- M
morning
- MM
continuous moonlight
- LD
light–dark
- LM
light–“moonlight”
pigment-dispersing factor
- ZTn
Zeitgeber time n.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Pittendrigh CS. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Bünning E. Planta. 1969;86:209–217. doi: 10.1007/BF00386453. [DOI] [PubMed] [Google Scholar]
- 3.Erkert HG, Gburek V, Scheideler A. Physiol Behav. 2006;88:39–46. doi: 10.1016/j.physbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Erkert HG, Gröber J. Folia Primatol (Basel) 1986;47:171–188. doi: 10.1159/000156276. [DOI] [PubMed] [Google Scholar]
- 5.Kappeler P, Erkert H. Behav Ecol Sociobiol. 2003;54:359–369. [Google Scholar]
- 6.Erkert HG. Int J Chronobiol. 1976;4:125–138. [PubMed] [Google Scholar]
- 7.Erkert HG. Oecologia. 1974;14:269–287. doi: 10.1007/BF01039797. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Duque E, Erkert HG. Folia Primatol (Basel) 2006;77:123–138. doi: 10.1159/000089699. [DOI] [PubMed] [Google Scholar]
- 9.Majercak J, Sidote D, Hardin PE, Edery I. Neuron. 1999;24:219–230. doi: 10.1016/s0896-6273(00)80834-x. [DOI] [PubMed] [Google Scholar]
- 10.Majercak J, Chen WF, Edery I. Mol Cell Biol. 2004;24:3359–3372. doi: 10.1128/MCB.24.8.3359-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieger D, Stanewsky R, Helfrich-Förster C. J Biol Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 12.Aschoff C. Ecology. 1966;7:657–662. [Google Scholar]
- 13.Grima B, Chelot E, Xia R, Rouyer F. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 14.Stoleru D, Peng Y, Agosto J, Rosbash M. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 15.Stoleru D, Peng Y, Nawathean P, Rosbash M. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- 16.Pittendrigh CS, Daan S. J Comp Physiol A. 1976;106:333–355. [Google Scholar]
- 17.Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C. J Neurosci. 2006;26:2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 19.Stanewsky R, Kaneko M, Emery P, Beretta B, Wagner-Smith K, Kay SA, Rosbash M, Hall JC. Cell. 2000;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 20.Bonini NM, Leierson WM, Benzer S. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 21.Chang DC. Behav Processes. 2006;71:211–225. doi: 10.1016/j.beproc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Shafer OT, Rosbash M, Truman JW. J Neurosci. 2002;22:5946–5954. doi: 10.1523/JNEUROSCI.22-14-05946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erkert HG, Cramer B. Folia Primatol (Basel) 2006;77:87–103. doi: 10.1159/000089697. [DOI] [PubMed] [Google Scholar]
- 24.Mrosovsky N, Hattar S. J Comp Physiol A. 2005;191:1011–1024. doi: 10.1007/s00359-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen DM, Christianson JS, Sapp RJ, Stark WS. Vis Neurosci. 1992;9:125–135. doi: 10.1017/s0952523800009585. [DOI] [PubMed] [Google Scholar]
- 26.Peirson S, Foster RG. Neuron. 2006;49:331–339. doi: 10.1016/j.neuron.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Shafer OT, Helfrich-Förster C, Renn SCP, Taghert PH. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helfrich-Förster C, Shafer OT, Wülbeck C, Grieshaber E, Rieger D, Taghert P. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- 29.Gorman MR, Evans JA, Elliott JA. Chronobiol Int. 2006;23:245–250. doi: 10.1080/07420520500521905. [DOI] [PubMed] [Google Scholar]
- 30.Gorman MR, Elliott JA. J Comp Physiol A. 2004;190:631–639. doi: 10.1007/s00359-004-0522-7. [DOI] [PubMed] [Google Scholar]
- 31.Gorman MR, Kendall M, Elliott JA. J Biol Rhythms. 2005;20:38–48. doi: 10.1177/0748730404271573. [DOI] [PubMed] [Google Scholar]
- 32.Danilenko KV, Wirz-Justice A, Kräuchi K, Cajochen C, Weber JM, Fairhurst S, Terman M. Chronobiol Int. 2000;17:659–668. doi: 10.1081/cbi-100101072. [DOI] [PubMed] [Google Scholar]
- 33.Danilenko KV, Wirz-Justice A, Kräuchi K, Weber JM, Terman M. J Biol Rhythms. 2000;15:437–446. doi: 10.1177/074873000129001521. [DOI] [PubMed] [Google Scholar]
- 34.Neumann D. J Biol Rhythms. 1989;4:285–294. [PubMed] [Google Scholar]
- 35.Naylor E. Symp Soc Exp Biol. 1985;39:63–93. [PubMed] [Google Scholar]
- 36.Helfrich-Förster C. J Comp Physiol A. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 37.Helfrich-Förster C. J Biol Rhythms. 2000;15:135–154. doi: 10.1177/074873040001500208. [DOI] [PubMed] [Google Scholar]
- 38.Wülbeck C, Szabo G, Shafer OT, Helfrich-Förster C, Stanewsky R. Genetics. 2005;169:751–766. doi: 10.1534/genetics.104.036244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanewsky R, Frisch B, Brandes C, Hamblen-Coyle MJ, Rosbash M, Hall JC. J Neurosci. 1997;17:676–696. doi: 10.1523/JNEUROSCI.17-02-00676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers MP, Wagner-Smith K, Rothenfuh-Hilfiker A, Young MW. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]