Abstract
Hyperphosphorylated tau is the major protein subunit of neurofibrillary tangles in Alzheimer's disease (AD) and related tauopathies. It is not understood, however, why the neurofibrillary tangle-containing neurons seen in the AD brains do not die of apoptosis but rather degeneration even though they are constantly awash in a proapoptotic environment. Here, we show that cells overexpressing tau exhibit marked resistance to apoptosis induced by various apoptotic stimuli, which also causes correlated tau hyperphosphorylation and glycogen synthase kinase 3 (GSK-3) activation. GSK-3 overexpression did not potentiate apoptotic stimulus-induced cell apoptosis in the presence of high levels of tau. The resistance of neuronal cells bearing hyperphosphorylated tau to apoptosis was also evident by the inverse staining pattern of PHF-1-positive tau and activated caspase-3 or fragmented nuclei in cells and the brains of rats or tau-transgenic mice. Tau hyperphosphorylation was accompanied by decreases in β-catenin phosphorylation and increases in nuclear translocation of β-catenin. Reduced levels of β-catenin antagonized the antiapoptotic effect of tau, whereas overexpressing β-catenin conferred resistance to apoptosis. These results reveal an antiapoptotic function of tau hyperphosphorylation, which likely inhibits competitively phosphorylation of β-catenin by GSK-3β and hence facilitates the function of β-catenin. Our findings suggest that tau phosphorylation may lead the neurons to escape from an acute apoptotic death, implying the essence of neurodegeneration seen in the AD brains and related tauopathies.
Keywords: Alzheimer's disease, tau hyperphosphorylation, glycogen synthase kinase-3
Chronic neurodegeneration characterized by accumulation of hyperphosphorylated tau and formation of neurofibrillary tangles (NFTs) is a hallmark lesion in Alzheimer's disease (AD) and related tauopathies (1–4). Although the mechanism underlying neurodegeneration remains elusive, the idea that neurons undergo apoptosis in the course of neurodegeneration is supported by studies showing that AD-related toxic stimuli, such as β-amyloid, cause cell death as manifested by up-regulation of apoptotic markers (5, 6). However, apoptosis accounts for only a minor proportion of neurons lost in AD brains (7); most NFT-bearing neurons undergo chronic degeneration (8–13) rather than apoptosis, even though they are constantly exposed to apoptotic stimuli, suggesting that mechanism(s) exist enabling neurons to escape apoptosis.
Studies on postmortem AD brains have demonstrated that abnormally hyperphosphorylated tau is the major protein subunit of NFT (1–4), which suggests that hyperphosphorylation of tau may play a role in leading the neuronal cells to desert apoptosis. Tau is a microtubule-associated protein. The major function of tau is to promote microtubule assembly and maintain the stability of the microtubules. The roles of tau hyperphosphorylation and accumulation in the development of neurofibrillary degeneration seen in the AD brains (1–4) and related tauopathies (14) have been studied extensively for the last two decades. Most recently, it has been demonstrated in tau-transgenic mice that suppression of a mutant tau expression improved memory function (15, 16). However, formation of tau filaments seems neuroprotective (17, 18). Until now, the direct evidence for the role of tau phosphorylation in determining the neurons committing chronic degeneration versus acute apoptosis is still lacking.
Tau phosphorylation is regulated by protein kinases and protein phosphatases. Glycogen synthase kinase-3β (GSK-3β) is one of the most implicated tau kinases involved in Alzheimer-like tau hyperphosphorylation (19–21). GSK-3β phosphorylates not only tau but also β-catenin; the latter is a phosphoprotein involved in Wnt signaling (22, 23). In Wnt pathway, phosphorylation of β-catenin by GSK-3β facilitates its proteolysis, whereas the unphosphorylated β-catenin is stable and thus can be translocated into nuclei to promote cell survival. The relationship between tau and β-catenin phosphorylation is currently unknown.
In this work, we found that tau hyperphosphorylation rendered neurons antiapoptotic through stabilizing β-catenin in two different cell lines and in rat and tau-transgenic mouse models. These results provide an explanation for why tangle-bearing neurons in the brains of subjects with AD and related tauopathies do not die of apoptosis.
Results
Cells Overexpressing Tau Are More Resistant to Apoptotic Stimuli-Induced Apoptosis.
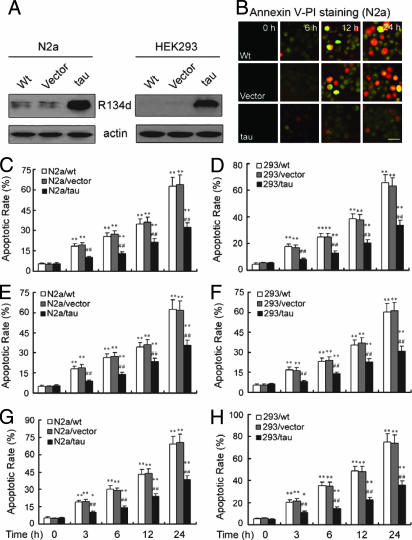
To study the role of tau in cell viability, we stably expressed the longest form of human tau (tau441) in mouse neuroblastoma N2a (N2a/tau) and human HEK293 (HEK293/tau) cells, the last of which did not express endogenous tau (Fig. 1A). The pcDNA (vector) was stably expressed as control. Then, we treated the cells with apoptotic inducers. Compared with the untransfected N2a or HEK293 cells, the apoptosis was much less severe in cells expressing high levels of tau protein after various durations of staurosporine treatment, as demonstrated by Annexin V–propidium iodide (PI) staining (Fig. 1B) followed by flow cytometric quantification of the apoptotic rate (Fig. 1 C and D). Cells expressing high levels of tau also showed significant resistance to apoptosis induced by camptothecin and H2O2 (Fig. 1 E–H), along with significantly increased cell viability as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) [supporting information (SI) Fig. 6 A–F] and lactate dehydrogenase assays (SI Fig. 6 G–L). To eliminate the possibility that the apoptosis resistance of the cell was induced by selecting for the stable cell lines but not by tau, we transiently transfected tau into N2a cells for 48 h and treated the cells with staurosporine for 6 h, and then we measured the apoptotic rate by flow cytometry. We observed that transient transfection of tau also made the cells more resistant to apoptosis (SI Fig. 7). These results suggest that tau may play an antiapoptotic role in the cells.
Fig. 1.
Cells overexpressing tau are more resistant to apoptotic stimuli-induced apoptosis. (A) Human tau441 (tau) or pcDNA (Vector) were stably expressed in mouse neuroblastoma N2a and human embryonic kidney HEK293 cells. Total tau was detected by Western blotting with antibody R134d. Actin serves as a loading control. (B) Cells untransfected (Wt) or transfected with pcDNA (Vector) or human tau441 (tau) were treated with 1 μM staurosporine for 0–24 h as indicated, and the number of apoptotic cells was determined by using Annexin V–PI staining. (Scale bar, 20 μm.) (C, E, and G) N2a cells were treated with 1 μM staurosporine or 1 μM camptothecin or 250 μM H2O2 for 0–24 h as indicated, and then the apoptotic rate was measured by flow cytometry for quantification. (D, F, and H) HEK293 cells were treated with 1 μM staurosporine or 1 μM camptothecin or 250 μM H2O2 for 0–24 h as indicated, and then the apoptotic rate was measured by flow cytometry to quantify. ∗, P < 0.05; ∗∗, P < 0.01 treated versus untreated controls; ##, P < 0.01, tau441-transfected versus vector or wild type at the same time points.
Hyperphosphorylation of Tau by GSK-3β Rescues Apoptotic Stimuli-Induced and GSK-3β-Potentiated Apoptosis.
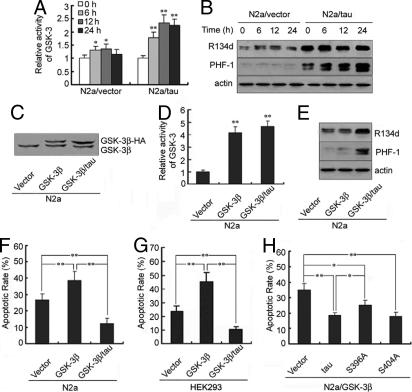
To explore the involvement of tau phosphorylation in the aforementioned antiapoptosis, we measured the phosphorylation status of tau and the activity of GSK-3. In N2a cells, apoptotic stimuli induced activation of GSK-3 (Fig. 2A) and concomitant increases in tau hyperphosphorylation at Ser396–Ser404 recognized by antibody PHF-1 in the presence of tau overexpression (Fig. 2B), suggesting that the antiapoptotic effect of tau involves tau phosphorylation and GSK-3. Of the two GSK-3 isoforms, GSK-3α and GSK-3β, GSK-3β is the major tau kinase functioning in AD pathology (19). To determine further the role of GSK-3β and tau phosphorylation in the antiapoptosis, we transiently transfected GSK-3β-HA into N2a cells with or without stable expression of tau441. The expression of GSK-3β-HA (Fig. 2C) along with significantly increased GSK-3 activity (Fig. 2D) and strong PHF-1 tau positivity (Fig. 2E) were confirmed by an in vitro 32P labeling kinase assay and Western blotting. Overexpression of GSK-3β in the absence of tau promoted staurosporine-induced apoptosis in both N2a (Fig. 2F) and HEK293 (Fig. 2G) cells. However, in the presence of high levels of exogenous tau, the apoptotic-promoting effect of GSK-3β was completely abolished, and cells were more resistant than control cells to staurosporine-induced apoptosis (Fig. 2 F and G). These data suggest that tau phosphorylation may rescue staurosporine-induced and GSK-3β-potentiated cell apoptosis.
Fig. 2.
Hyperphosphorylation of tau inhibits apoptotic stimuli-induced apoptosis. (A) GSK-3 activity was measured by a 32P-labeling assay using phospho-GS-peptide 2 as substrate (42) and expressed as relative level in N2a cells treated with 1 μM staurosporine for 0–24 h. ∗, P < 0.05; ∗∗, P < 0.01, versus vector at 0 h (mean ± SD, n = 5). (B) Tau was analyzed by Western blotting with R134d (to total tau) and PHF-1 (to Ser396–Ser404-hyperphosphorylated tau). (C) Exogenous GSK-3β-HA or its pcDNA was transfected into N2a with or without expression of exogenous tau, and the expression of GSK-3β was detected by using anti-GSK-3β antibody at 48 h after transfection. (D) GSK-3 activity measured by a 32P-labeling assay after its expression. ∗∗, P < 0.01 versus vector (mean ± SD, n = 5). (E) Tau phosphorylation at the PHF-1 epitope in N2a cells was measured at 48 h after GSK-3β expression. (F) The apoptotic rate in N2a cells transfected with GSK-3β-HA or pcDNA for 48 h and treated with 1 μM staurosporine for 6 h measured by flow cytometry. (G) Observations similar to F were shown in HEK293 cell, which does not express endogenous tau. (H) Wild-type tau or a tau construct bearing a Ser to Ala substitution at amino acid 396 (S396A) or 404 (S404A) was transfected simultaneously with GSK-3β into naïve N2a cells for 48 h, and cells were treated with staurosporine for 6 h. The apoptotic rate was determined by flow cytometry. ∗, P < 0.05; ∗∗, P < 0.01 as marked in F–H (mean ± SD, n = 3).
We next mutated tau amino acid residue Ser396, a critical target of GSK-3β phosphorylation, to Ala (S396A) and observed decreased ability of tau protein to protect cells from apoptosis (Fig. 2H), suggesting an important role for phosphorylation at this site in the antiapoptotic activity of tau. No obvious change was observed by a similar mutation at tau Ser404 (S404A), another GSK-3β site (Fig. 2H).
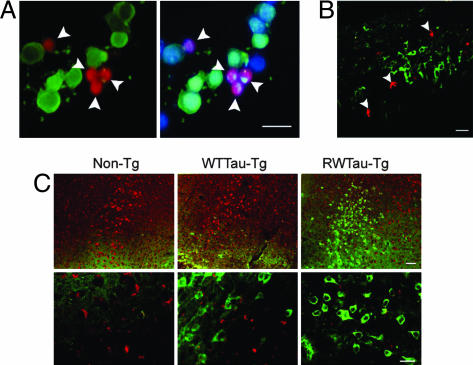
An antiapoptotic function of tau phosphorylation was supported further by mutually exclusive immunostaining of the hyperphosphorylated tau (green) and two major apoptotic markers, activated caspase-3 (red) and fragmented nuclei (blue), in N2a/tau cells transiently transfected with GSK-3β and treated with staurosporine (Fig. 3A). Furthermore, the idea that tau phosphorylation renders cells resistant to apoptosis was supported in vivo by two sets of experiments. First, we injected staurosporine into the rat hippocampus and observed the mutually exclusive distribution of PHF-1-positive tau and activated caspase-3 (Fig. 3B). We made similar observations in transgenic mouse lines overexpressing wild-type human tau (WTTau-Tg) or the FDTP mutant R406W tau (RWTau-Tg) (24); the latter is more readily phosphorylated (25) and in littermate controls (Non-Tg). Nonoverlapping PHF-1 and activated caspase-3 positivities were apparent in brains of 3-month-old mice after staurosporine treatment, and the phosphorylation of tau at PHF-1 epitope was most dramatic in RWTau-Tg mice (Fig. 3C). Overall, our data strongly suggest that tau phosphorylation mediated by GSK-3β blocks cells from responding to exogenous proapoptotic stimuli.
Fig. 3.
Segregation of hyperphosphorylated tau from apoptotic markers. (A) N2a/tau cells transfected with GSK-3β for 48 h were treated with 1 μM staurosporine for 6 h and triple-labeled with Hoechst stain, activated caspase-3 antibody, and PHF-1. (B) Staurosporine (500 μM, 3 μl) was injected into the rat hippocampus for 24 h, and brain sections were immunostained for activated caspase-3 and PHF-1 tau. (C) Staurosporine (500 μM, 1 μl) was injected into the hippocampus of nontransgenic littermates (Non-Tg), wild-type tau transgenic (WTTau-Tg), and R406W tau transgenic (RWTau-Tg) mice (24) for 24 h, and brain sections were immunostained for activated caspase-3 and PHF-1 tau. (Lower) Higher magnification. Merged images show mutually exclusive immunostaining pattern of Hoechst (blue) or/and activated caspase-3 (red) with PHF-1 tau (green). (Scale bar, 20 μm.)
Hyperphosphorylation of Tau Stabilizes β-Catenin.
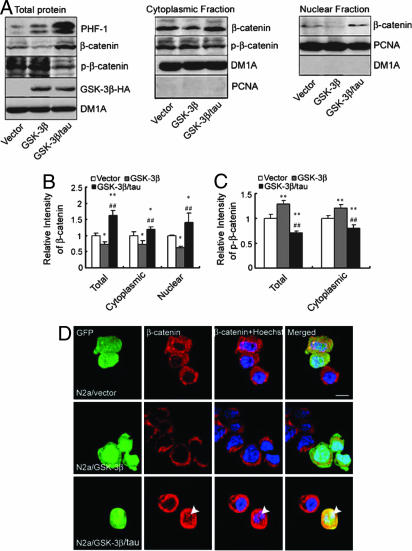
To determine the mechanism underlying the antiapoptotic effect of tau phosphorylation, we asked whether tau phosphorylation alters the level and localization of β-catenin, a well recognized cell survival factor and GSK-3β substrate. Expression of GSK-3β in N2a/vector cells resulted in a significant decrease in the total level of β-catenin (Fig. 4 A Left and B). However, when GSK-3β was expressed in N2a/tau cells, total levels of β-catenin significantly increased, but levels of the phosphorylated β-catenin (P-β-catenin) markedly decreased accompanied by concomitantly high levels of PHF-1-positive tau (Fig. 4 A Left and B and C). These data suggest that tau phosphorylation antagonizes phosphorylation of β-catenin by GSK-3β and maintains high levels of β-catenin. Because the survival-promoting function of β-catenin requires its nuclear translocation (26), we examined β-catenin levels in cytoplasmic and nuclear fractions. Compared with cells with negligible tau expression, levels of total β-catenin were elevated significantly in both cytoplasmic and nuclear fractions, whereas P-β-catenin was reduced in cytoplasmic fractions in cells expressing tau (Fig. 4 A Center and Right and B and C). Tau overexpression potentiates translocation of β-catenin into the nuclear fraction (Fig. 4 A Right and B), a result confirmed by immunofluorescence, indicating a clear nuclear β-catenin signal when tau was overexpressed (Fig. 4D, arrowheads).
Fig. 4.
Hyperphosphorylation of tau by GSK-3β stabilizes β-catenin and promotes nuclear translocation of β-catenin. (A) Exogenous GSK-3β-HA or its pcDNA was transfected into N2a with or without expression of exogenous tau for 48 h, and cells were treated with 1 μM staurosporine for 6 h. Cytoplasmic and nuclear fractions were obtained, and immunoblots for β-catenin and phosphorylated β-catenin (P-β-catenin) were measured. DM1A and PCNA were used for loading controls and markers for cytoplasmic and nuclear fractions, respectively. (B and C) Quantitative analysis of A. ∗, P < 0.05; ∗∗, P < 0.01 versus vector; ##, P < 0.01, GSK-3β/tau group versus GSK-3β group (mean ± SD, n = 3). (D) Naïve N2a cells were cotransfected transiently with GFP-labeled tau or pcDNA and GSK-3β (1:3) (50) for 48 h and then treated with 1 μM staurosporine for 6 h. β-Catenin immunostaining was carried out by using rhodamine red X-coupled second antibody (red) with Hoechst staining for cell nuclei (blue); translocation of β-catenin from the cytosol to the nucleus in tau-expressing cells (arrowhead) is shown. (Scale bar, 20 μm.)
β-Catenin Mediates the Antiapoptotic Function of Tau Phosphorylation.
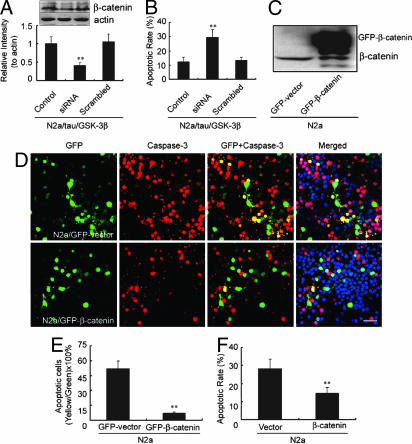
To explore the direct role of β-catenin in mediating the antiapoptotic effects of tau phosphorylation, we used siRNA to knock down β-catenin. Reduction of β-catenin (≈70%) by siRNA abolished the antiapoptotic effect of tau (Fig. 5 A and B). To confirm the antiapoptotic function of β-catenin, we overexpressed β-catenin in naïve N2a cells (Fig. 5C). After staurosporine treatment, both the number of apoptotic cells (Fig. 5 D and E) and the rate of apoptosis (Fig. 5F) were reduced significantly in β-catenin-transfected cells. These results strongly suggest that β-catenin up-regulation, likely resulting from a competitive phosphorylation between tau and β-catenin by GSK-3β, antagonizes apoptosis.
Fig. 5.
Knockdown of β-catenin eliminates the antiapoptotic effect of tau, and overexpression of β-catenin inhibits staurosporine-induced apoptosis. (A) N2a/tau cells were transfected with β-catenin siRNA or scrambled siRNA control for 48 h after prior transfection with GSK-3β-HA for 6 h. The level of β-catenin was measured by Western blotting, and it was calculated by defining the control level as 1 arbitrary unit. (B) Cells expressing GSK-3β-HA and different siRNAs described in A were further treated with 1 μM staurosporine for 6 h, and the apoptotic rate was quantified by flow cytometry. ∗∗, P < 0.01 versus control (mean ± SD, n = 3). (C) GFP-β-catenin or a GFP-containing vector was transiently expressed in naïve N2a cells, and β-catenin was measured by Western blotting at 48 h after the transfection. (D) Cells expressing GFP-β-catenin or GFP-vector described in C were treated with 1 μM staurosporine for 6 h and stained with antiactivated caspase-3 (red) and Hoechst (blue). The number of apoptotic cells (yellow) was significantly lower in β-catenin-positive cells than in vector control cells. (Scale bar, 20 μm.) (E) Quantitative analysis from three separate experiments in D. (F). The apoptotic rate was quantified by flow cytometry. ∗∗, P < 0.01 (mean ± SD, n = 3).
Discussion
Neurodegeneration characterized by intracellular accumulation of hyperphosphorylated tau and formation of neurofibrillary tangles is a hallmark lesion in AD and related tauopathies (1–4). However, it is still not understood why the neurons bearing hyperphosphorylated tau and tangles choose to undergo chronic degeneration rather than acute apoptosis even though they are often exposed to a proapoptotic environment, especially during the development of AD or related tauopathies. In the present study, we have found that tau hyperphosphorylation enables cells to escape apoptosis, a phenomenon that may explain why most NFT-bearing neurons survive proapoptotic insults and die chronically of degeneration. The involvement of tau in cell viability was also observed in cerebellar granule neurons (27), and it was further supported by recent histological analyses of neurons in transgenic mouse models expressing mutant and nonmutant forms of human tau (17, 28, 29). In both cases, the presence of tau filaments did not correlate directly with the death of individual neurons. We also observed that dephosphorylated tau at the Tau-1 epitope was colabeled with apoptotic markers (data not shown), which supports a previous report that dephosphorylation of tau renders the cells more vulnerable to apoptosis (30). Although a proapoptotic effect of tau pseudophosphorylation was also reported (31), we conceive that the antiapoptotic function of tau requires its phosphorylation. Because neurons in adults are rarely replenished, it is likely that neurons have evolved mechanisms to survive apoptotic assaults to maintain an optimal brain structure. We believe that one of these mechanisms is tau phosphorylation-induced abortive apoptosis as demonstrated in our study, which could allow neurons to survive apoptotic attack and wait for chances of self-repair.
Tau is a phosphoprotein containing normally 2–3 mol of phosphate per mol of tau protein. The levels of total tau in the AD brains were 4- to 5-fold higher than in the control cases, and the increases were in the form of abnormally phosphorylated tau (32). Phosphorylation of tau is catalyzed by a group of protein kinases; and among them, GSK-3β is one of the most prominent tau kinases (20). A previous study in SH-SY5Y cells, which express little tau protein, demonstrated that GSK-3β facilitated exogenously induced apoptosis (33). In the present study, we also observed that overexpression of GSK-3β alone potentiated staurosporine-induced apoptosis. However, simultaneous overexpression of tau eliminated the GSK-3β-potentiated apoptosis, suggesting that tau phosphorylation rescues GSK-3β-mediated cell death. In addition to tau, GSK-3β also phosphorylates β-catenin and thus regulates the proteolysis and cellular translocation of the protein (26). As a component of “Wnt/GSK-3/β-catenin,” a well characterized survival pathway in cancer, β-catenin is being more appreciated in the nervous system. Our results demonstrated that up-regulation of β-catenin during tau hyperphosphorylation plays a role in preventing the cells from apoptosis. Because both tau and β-catenin are favored substrates of GSK-3, we speculate that tau phosphorylation may alter phosphorylation/activity of β-catenin through a competitive mechanism. Indeed, overexpressing of β-catenin blocks nitric oxide-induced apoptosis in colonic cancer cells (34), whereas destabilization or down-regulation of β-catenin potentiates apoptosis (35, 36). These findings point to β-catenin as a key mediator in neuronal survival in AD and support the view that a loss of function of the Wnt signaling pathway may play a role in the progression of AD (37). Tau may also exert its antiapoptotic function through stabilizing microtubules. However, it is well known that hyperphosphorylated tau detaches from microtubules, which leads to disruption of microtubules. Because the tau in our study is hyperphosphorylated, this possibility can be ruled out.
Because hyperphosphorylated tau is the major protein subunit of NFTs in the degenerated neurons in AD brains, studies have been focusing on the detrimental effects of tau hyperphosphorylation; for instance, hyperphosphorylated tau is no longer competent in promoting the assembly of microtubules (38), and it sequesters normal tau and thus disrupts microtubules (39). In addition, neurons exhibiting tau hyperphosphorylation cannot maintain axonal transport, a sign of neurodegeneration (40). In the present work, we demonstrate an antiapoptotic function of tau phosphorylation. The protective effect of tau phosphorylation on oxidative stress has also been proposed recently (41). It seems that hyperphosphorylation of tau may prevent the brain from rapid loss of a majority of neurons by leading the neurons to escape apoptosis, but the neurons with tau hyperphosphorylation are “sick” and no longer competent for normal physiological functions, such as promoting microtubule assembly and maintaining normal axonal transport. Additionally, extended survival time of these sick neurons makes them less resistant to environmental/metabolic insults and also allows NFT to evolve from the hyperphosphorylated tau. These incompetent, sick neurons may be the origin of neurodegeneration. Thus, the formation of NFTs in AD brains may result from the ability of neurons to abort acute apoptotic cell death and enter pathways culminating chronic neurodegeneration. We propose that selection of this chronic death pathway in cells with tau hyperphosphorylation is under the tight regulation of multiple factors, which warrants further investigation. Taken together, the findings in this work and previous ones suggest that modulation of tau phosphorylation in different stages of AD and related tauopathies offers promising opportunities to rescue the cells from acute apoptotic death and to prevent degeneration.
Materials and Methods
Antibodies, Rats, and Transgenic Mice.
The detailed information for the antibodies used in this work is listed in Table 1. Male Sprague–Dawley rats (3 months old) were supplied by the Experimental Animal Central of Tongji Medical College. Transgenic mouse lines (3 months old) overexpressing wild-type and the FDTP tau mutant R406W [driven by the calcium/calmodulin-dependent kinase II (CaMKII)-TA promoter] and the littermate controls (24) were kind gifts of A Takashima (Institute of Physical and Chemical Research of Japan). All animal experiments were performed according to the “Policies on the Use of Animals and Humans in Neuroscience Research” revised and approved by the Society for Neuroscience in 1995, and the animal study was approved by the academic review board of our college.
Table 1.
Antibodies employed in the study
| Antibody | Specific | Type | WB | IF | Source |
|---|---|---|---|---|---|
| R134d | Total tau | pAb | 1:30,000 | K. Iqbal | |
| PHF-1 | Phosphorylated tau at Ser396–Ser404 | mAb | 1:500 | 1:300 | P. Davies |
| Anti-tau-1 | Nonphosphorylated tau at Ser198–Ser199–Ser202 | mAb | 1:20,000 | 1:200 | Chemicon |
| HA tag (6E2) | HA tag | mAb | 1:1,000 | Cell Signaling | |
| Cleaved caspase-3 (Asp175) antibody | Large fragment(17/19 kDa) of activated caspase-3 resulting from cleavage adjacent to Asp175 | pAb | 1:1,000 | 1:100 | Cell Signaling |
| GSK-3β | Total GSK-3β | mAb | 1:1,000 | 1:50 | Cell Signaling |
| Mouse anti-β-catenin | β-Catenin | mAb | 1:500 | 1:100 | Zymed |
| DM1A | α-Tubulin | mAb | 1:1,000 | Sigma | |
| Anti-phospho-β-catenin (pSer33/pSer37) | β-Catenin phosphorylated at Ser33 and Ser37 | mAb | 1:400 | Sigma | |
| Proliferating cell nuclear antigen (PCNA) | PCNA | mAb | 1:400 | eBioscience | |
| β-actin | β-Actin | mAb | 1:1,000 | Abcam | |
| Goat anti-mouse peroxidase | 1:5,000 | Pierce Chemical Co. | |||
| Goat anti-rabbit peroxidase | 1:5,000 | Pierce Chemical Co. | |||
| Oregon green 488 goat anti-mouse IgG (H + L) | 1:1,000 | Molecular Probes | |||
| Rhodamine red X | 1:1,000 | Molecular Probes | |||
| goat anti-mouse IgG (H + L) | |||||
| Rhodamine red X | 1:1,000 | Molecular Probes | |||
| goat anti-rabbit IgG (H + L) |
WB, Western blotting; IF, immunofluorescence; pAb, rabbit polyclonal antibody.
Establishment of Cell Lines Stably Expressing Tau or Its Vector.
The longest human tau construct (tau441) or pcDNA (vector) was transfected into murine neuroblastoma N2a or HEK293 cells with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, and the single clone was selected to establish cell lines stably overexpressing tau441 (namely, N2a/tau and HEK293/tau) or pcDNA (namely, N2a/vector and HEK293/vector). Cells were cultured in 50% DMEM/50% Opti-MEM supplemented with 5% FBS and 200 μg/ml G418. The culture medium was changed every 3 days.
Brain Injection in Rats and Transgenic Mice.
Brain injection was performed by using a stereotaxic instrument (SR-6N; Narishige Scientific Instrument Laboratory, Tokyo, Japan) (42). Staurosporine (3 μl of 500 μM) was injected into the rat's hippocampus at coordinates from bregma and dura of AP −4.0, L/R −2.0 and V −3.0 (in mm). After 24 h, the brain slices were prepared for immunofluorescence analysis. Transgenic mouse lines were injected in the hippocampal region with staurosporine (1 μl of 500 μM) at coordinates 1.9 mm/−1.0 mm/1.9 mm.
Induction of Apoptosis.
Before administration of apoptotic inducers, the cells were cultured in serum-free medium for 12 h to induce differentiation. To induce apoptosis, cells were treated with 1 μM camptothecin (43), 1 μM staurosporine (44), or 250 μM H2O2 (45) for 3, 6, 12, and 24 h in serum-free medium. Apoptosis was assayed by using an Annexin V–PI staining kit by following the manufacturer's procedure (Bender Medsystems, San Bruno, CA) (46), and the apoptotic rate was automatically quantified by flow cytometry with the standardized program of the instrument (FACSCalibur, BD Biosciences, San Jose, CA). Cell viability was also measured by MTT (47) and lactate dehydrogenase assays (48).
Assay of GSK-3 Activity.
The activity of GSK-3 in hippocampal extracts of the rats was determined by using phospho-GS peptide 2 (42). Briefly, 7.5 μg of protein was incubated for 30 min at 30°C with 250 μmol/liter peptide substrate and 200 μmol/liter [γ-32P]ATP (1,500 cpm/pmol ATP) in 30 mmol/liter Tris, pH 7.4/10 mmol/liter MgCl2/10 mmol/liter NaF/1 mmol/liter Na3VO4/2 mmol/liter EGTA/10 mmol/liter 2-mercaptoethanol in a total volume of 25 μl. The reaction was stopped with 25 μl of 300 mmol/liter o-phosphoric acid. The reaction mixture of 25 μl was applied in triplicate to phosphocellulose units. The filters were washed three times with 75 mmol/liter o-phosphoric acid, dried, and counted with a liquid scintillation counter. The relative kinase activity was expressed based on controls.
Double- or Triple-Labeling Immunofluorescence.
The rats or mice were killed by an overdose of chloral hydrate (1 g/kg) or CO2 and fixed in situ for 20 min by perfusion of 4% paraformaldehyde in 24 mM NaH2PO4/126 mM Na2HPO4, pH 7.2 at 4°C. Coronal brain sections of hippocampal tissue were cut at 35 μm with a freezing microtome (CM1900, Leica Microsystems, Wetzlar, Germany). Sections were then incubated overnight at 4°C with primary antibodies as indicated in each figure, and the immunoreactivity was probed with rhodamine red X- or Oregon green 488-conjugated secondary antibodies (see Table 1). For the triple-labeling studies, 1 μg/ml Hoechst 33258 (Sigma, St. Louis, MO) was used for the nuclear staining. For each primary antibody, three to five consecutive sections from each brain were used. The images were observed with a laser confocal microscope (FV500; Olympus, Tokyo, Japan) or a fluorescence microscopy (System Microscopy BX60; Olympus). For cell studies, cells were cultured on coverslips and fixed with 4% paraformaldehyde for 1.5 h at 4°C and then incubated for 12≈36 h at 4°C with primary antibodies overnight. The rest processes were the same as described above.
Western Blotting.
Western blotting was performed according to methods established in our laboratory (42). Briefly, the cell homogenates or brain extracts were mixed with sample buffer containing 50 mmol/liter Tris·HCl (pH 7.6), 2% SDS, 10% glycerol, 10 mmol/liter DTT, and 0.2% bromophenol blue and boiled for 5 min. The proteins were separated by 10% SDS/PAGE and transferred to PVDF membrane. Immunostaining was visualized with a chemiluminescent substrate kit and CL-XPosure Film and quantitatively analyzed by digital science 1D software (Eastman Kodak, Rochester, NY). Band intensity was measured as the sum optical density and expressed is as a level relative to each control. Tau phosphorylation levels were normalized relative to the total tau. Data were analyzed by using SAS 8.01 statistical software and expressed as mean ± SD. Differences among multiple groups and serial studies were tested by repeated-measures ANOVA. Mutant tau expression constructs containing the S396A or S404A mutations were gifts of F. Liu (New York State Institute for Basic Research, Staten Island, NY).
Subcellular Fractionation and Analysis of β-Catenin.
Subcellular fractionation was carried out according to the established methods (49). Roughly 2 × 106 cells were washed with 1× PBS and suspended in 100 μl of buffer A (10 mM Tris·HCl, pH 7.4/10 mM NaCl/3 mM MgCl2/0.03% Nonidet P-40) containing a mixture of protease inhibitors, i.e., 10 μg/ml each aprotinin and leupeptin and 100 μg/ml PMSF. The samples were incubated on ice until >95% of the cells could be stained by trypan blue. Samples were then centrifuged at 1,000 rpm (rotor Nr. 12154-H; Sigma) at 4°C for 5 min. The supernatant (cytoplasmic fraction) was collected and the pellet was washed with buffer B (50 mM NaCl, 10 mM Hepes, pH 8.0/25% glycerol/0.1 mM EDTA/0.5 mM spermidine/0.15 mM spermine), then resuspended in 100 μl of buffer C [350 mM NaCl/10 mM Hepes, pH 8.0/25% (vol/vol) glycerol/0.1 mM EDTA/0.5 mM spermidine/0.15 mM spermine] and rocked in 4°C for 30 min. Samples were centrifuged at 14,000 × g for 10 min. The resulting supernatant contains the nucleoplasm fractions.
To knock down β-catenin, we used N2a/tau cells transiently transfected with GSK-3β for 6 h. The β-catenin siRNA or control siRNA (20 nM) (Santa Cruz Biotechnology, Santa Cruz, CA) was then transfected by using siPORT Amine transfection agent (Ambion, Austin, TX) according to the manufacturer's instructions. After 48 h, the cells were either used for the measurement of β-catenin level by quantitative Western blotting or further treated with 1 μM staurosporine for 6 h for the apoptosis assay by flow cytometry.
Supplementary Material
Acknowledgments
We thank Drs. K. Iqbal, I. Grundke-Iqbal, C. X. Gong, and F. Liu at New York State Institute for Basic Research for antibody R134d and tau plasmids; Dr. A. Takashima at the Institute of Physical and Chemical Research of Japan for transgenic mice; Dr. P. Davies at Albert Einstein Medical School for antibody PHF-1; Dr. J. R. Woodgett at University of Toronto for GSK-3β-HA plasmid; and Dr. B. Vogelstein at Howard Hughes Medical Institute for β-catenin plasmids. This work was supported in part by National Natural Science Foundation of China Grants 30430270, 30472030, and 30328007 (to J.-Z.W. and S.-J.L.); National Science and Technology Committee of China Grants G1999054007 and 2006CB500703 (to J.-Z.W.); and by National Institutes of Health Grants R01 NS046673 (to H.X.) and R01 NS054880 (to F.-F.L.).
Abbreviations
- AD
Alzheimer's disease
- GSK-3
glycogen synthase kinase-3
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NFT
neurofibrillary tangles
- PI
propidium iodide.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609303104/DC1.
References
- 1.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ihara Y, Nukina N, Miura R, Ogawara M. J Biochem (Tokyo) 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- 4.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 5.Yuan J, Yankner BA. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 6.Dickson DW. J Clin Invest. 2004;114:23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jellinger KA. J Cell Mol Med. 2001;5:1–17. doi: 10.1111/j.1582-4934.2001.tb00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak E, Braak H, Mandelkow EM. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 9.Coleman PD, Yao PJ. Neurobiol Aging. 2003;24:1023–1027. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Raina AK, Hochman A, Ickes H, Zhu X, Ogawa O, Cash AD, Shimohama S, Perry G, Smith MA. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:251–254. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 11.Raina AK, Zhu X, Shimohama S, Perry G, Smith MA. Cell Biochem Biophys. 2003;39:249–255. doi: 10.1385/CBB:39:3:249. [DOI] [PubMed] [Google Scholar]
- 12.Perry G, Nunomura A, Smith MA. Nat Med. 1998;4:897–898. doi: 10.1038/nm0898-897. [DOI] [PubMed] [Google Scholar]
- 13.Perry G, Nunomura A, Lucassen P, Lassmann H, Smith MA. Science. 1998;282:1268–1269. doi: 10.1126/science.282.5392.1265h. [DOI] [PubMed] [Google Scholar]
- 14.Lee VM, Goedert M, Trojanowski JQ. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 15.Duff K, Planel E. Nat Med. 2005;11:826–827. doi: 10.1038/nm0805-826. [DOI] [PubMed] [Google Scholar]
- 16.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, et al. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. J Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso Adel C, Li B, Grundke-Iqbal I, Iqbal K. Proc Natl Acad Sci USA. 2006;103:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 20.Baum L, Seger R, Woodgett JR, Kawabata S, Maruyama K, Koyama M, Silver J, Saitoh T. Brain Res Mol Brain Res. 1995;34:1–17. doi: 10.1016/0169-328x(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 21.Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. J Neuropathol Exp Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, et al. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 23.Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. J Biol Chem. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- 24.Tatebayashi Y, Miyasaka T, Chui DH, Akagi T, Mishima K, Iwasaki K, Fujiwara M, Tanemura K, Murayama M, Ishiguro K, et al. Proc Natl Acad Sci USA. 2002;99:13896–13901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. J Biol Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 26.Yuan J, Zhang J, Wong BW, Si X, Wong J, Yang D, Luo H. Cell Death Differ. 2005;12:1097–1106. doi: 10.1038/sj.cdd.4401652. [DOI] [PubMed] [Google Scholar]
- 27.Amadoro G, Serafino AL, Barbato C, Ciotti MT, Sacco A, Calissano P, Canu N. Cell Death Differ. 2004;11:217–230. doi: 10.1038/sj.cdd.4401314. [DOI] [PubMed] [Google Scholar]
- 28.Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, et al. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT. Am J Pathol. 2006;168:1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rametti A, Esclaire F, Yardin C, Terro F. J Biol Chem. 2004;279:54518–54528. doi: 10.1074/jbc.M408186200. [DOI] [PubMed] [Google Scholar]
- 31.Fath T, Eidenmuller J, Brandt R. J Neurosci. 2002;22:9733–9741. doi: 10.1523/JNEUROSCI.22-22-09733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatoon S, Grundke-Iqbal I, Iqbal K. FEBS Lett. 1994;351:80–84. doi: 10.1016/0014-5793(94)00829-9. [DOI] [PubMed] [Google Scholar]
- 33.Bijur GN, De Sarno P, Jope RS. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, MacNaughton WK. Cancer Res. 2005;65:8604–8607. doi: 10.1158/0008-5472.CAN-05-1169. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, et al. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 36.Chen YZ. Apoptosis. 2004;9:415–422. doi: 10.1023/B:APPT.0000031447.05354.9f. [DOI] [PubMed] [Google Scholar]
- 37.De Ferrari GV, Inestrosa NC. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 38.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 39.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, et al. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 41.Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Trends Mol Med. 2005;11:164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Liu SJ, Zhang JY, Li HL, Fang ZY, Wang Q, Deng HM, Gong CX, Grundke-Iqbal I, Iqbal K, Wang JZ. J Biol Chem. 2004;279:50078–50088. doi: 10.1074/jbc.M406109200. [DOI] [PubMed] [Google Scholar]
- 43.Ropeleski MJ, Riehm J, Baer KA, Musch MW, Chang EB. Gastroenterology. 2005;129:170–184. doi: 10.1053/j.gastro.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Cooper JK, Schilling G, Peters MF, Herring WJ, Sharp AH, Kaminsky Z, Masone J, Khan FA, Delanoy M, Borchelt DR, et al. Hum Mol Genet. 1998;7:783–790. doi: 10.1093/hmg/7.5.783. [DOI] [PubMed] [Google Scholar]
- 45.Marques CA, Keil U, Bonert A, Steiner B, Haass C, Muller WE, Eckert A. J Biol Chem. 2003;278:28294–28302. doi: 10.1074/jbc.M212265200. [DOI] [PubMed] [Google Scholar]
- 46.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 47.Denizot F, Lang R. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 48.Tesco G, Latorraca S, Piersanti P, Sorbi S, Piacentini S, Amaducci L. Ann NY Acad Sci. 1992;673:149–153. doi: 10.1111/j.1749-6632.1992.tb27446.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 50.Jiang H, Guo W, Liang X, Rao Y. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.